Fatal arterial hemorrhage after pancreaticoduodenectomy:How do we simultaneously accomplish complete hemostasis and hepatic arterial flow?
2021-05-08
Yasuyuki Kamada,Tomohide Hori,Hidekazu Yamamoto,Hideki Harada,Michihiro Yamamoto,Masahiro Yamada,Takefumi Yazawa,Ben Sasaki,Masaki Tani,Asahi Sato,Hikotaro Katsura,Ryotaro Tani,Ryuhei Aoyama,Yudai Sasaki,Masazumi Zaima,Department of Surgery,Shiga General Hospital,Moriyama 524-8524,Shiga,Japan
Abstract BACKGROUND Although arterial hemorrhage after pancreaticoduodenectomy (PD) is not frequent,it is fatal. Arterial hemorrhage is caused by pseudoaneurysm rupture,and the gastroduodenal artery stump and hepatic artery (HA) are frequent culprit vessels. Diagnostic procedures and imaging modalities are associated with certain difficulties. Simultaneous accomplishment of complete hemostasis and HA flow preservation is difficult after PD. Although complete hemostasis may be obtained by endovascular treatment (EVT) or surgery,liver infarction caused by hepatic ischemia and/or liver abscesses caused by biliary ischemia may occur. We herein discuss therapeutic options for fatal arterial hemorrhage after PD.AIM To present our data here along with a discussion of therapeutic strategies for fatal arterial hemorrhage after PD.METHODS We retrospectively investigated 16 patients who developed arterial hemorrhage after PD. The patients’ clinical characteristics,diagnostic procedures,actual treatments [transcatheter arterial embolization (TAE),stent-graft placement,or surgery],clinical courses,and outcomes were evaluated.RESULTS The frequency of arterial hemorrhage after PD was 5.5%. Pancreatic leakage was observed in 12 patients. The onset of hemorrhage occurred at a median of 18 d after PD. Sentinel bleeding was observed in five patients. The initial EVT procedures were stent-graft placement in seven patients,TAE in six patients,and combined therapy in two patients. The rate of technical success of the initial EVT was 75.0%,and additional EVTs were performed in four patients. Surgical approaches including arterioportal shunting were performed in eight patients.Liver infarction was observed in two patients after TAE. Two patients showed a poor outcome even after successful EVT. These four patients with poor clinical courses and outcomes had a poor clinical condition before EVT. Fourteen patients were successfully treated.CONCLUSION Transcatheter placement of a covered stent may be useful for simultaneous accomplishment of complete hemostasis and HA flow preservation.
Key Words: Pancreaticoduodenectomy; Endovascular treatment; Stent-graft; Covered stent; Transcatheter arterial embolization; Arterioportal shunting
INTRODUCTION
The mortality rate after pancreaticoduodenectomy (PD) is currently < 5%[1-8]because surgical procedures and perioperative management techniques have been well established[1,9-11]. However,postoperative complications still remain a matter of concern[1,2,4,6,8,11-13]. Although arterial hemorrhage after PD is not frequent,it is fatal. Its mortality rate reportedly ranges from 10% to 60%[1,2,4,7,12,14-23],and it easily results in shock and coagulopathy[1,18,23]. Arterial hemorrhage is mainly caused by pseudoaneurysm rupture of a splanchnic artery[18,24],and the gastroduodenal artery (GDA)stump,common hepatic artery (CHA),and proper hepatic artery (PHA) are the most frequent culprit vessels[1-3,6,18,25-28]. Diagnostic and treatment strategies should be decided on a case-by-case basis[18,28,29].
Arterial flow,especially in the liver,is modified after PD (Figure 1). Briefly,the hepatopetal flow of the hepatic artery (HA) depends on the blood supply from the celiac artery (e.g.,the CHA and PHA),not from the superior mesenteric artery (SMA) [e.g.,the inferior pancreaticoduodenal artery (IPDA) and retrograde-flowing GDA] and collateral circulation (e.g.,hepatopetal collateralsviathe inferior phrenic artery)[1,28,30,31].This leads to a simple question:How do we simultaneously accomplish complete hemostasis and HA flow preservation? Endovascular treatment (EVT) [e.g.,transcatheter arterial embolization (TAE) and stent-graft placement] are currently available[1,6,13,16,18,19,32-40],and surgical arterioportal shunting has therapeutic potential for the arterial blood supply[41,42].
TAE provides complete hemostasis[1,2,13,19,20,23,39,43],although this approach increases the risk of severe complications associated with liver infarction caused by hepatic ischemia[1,13,18,19,23,30,32,33,39]and/or liver abscesses caused by biliary ischemia[6,34,35]. In contrast,transcatheter placement of a stent-graft (bare or covered stent) preserves HA flow[1,13,16,18,36-38,40],although technical failure of hemostasis may rarely occur[1]. From the viewpoint of cost-effectiveness,EVT is more advantageous than conventional surgery[29].
Complete hemostasis of fatal hemorrhage and preservation of HA flow should be simultaneously obtained; however,this may be difficult after PD because the hepatopetal arterial supply has been modified (Figure 1). In the present study,we retrospectively investigated our treatments for fatal arterial hemorrhage after PD and evaluated our own results. We also herein discuss the safety and feasibility of transcatheter stent-graft placement and especially validate the therapeutic potential of using a covered (not bare) stent for simultaneous accomplishment of complete hemostasis and HA flow preservation.
MATERIALS AND METHODS
Patients
This study focused on the postoperative state after PD (Figure 1); therefore,patients who underwent other surgeries (e.g.,distal pancreatectomy or gastrectomy) were excluded from further analysis. During a 14-year period (from January 2007 to December 2020),291 PDs were performed in our institution. Fatal arterial hemorrhage occurred in 16 patients who underwent PD,and these patients were enrolled in this study. The patients’ mean age at the time of PD was 73.4 ± 7.7 years,and the patients comprised 11 men and 5 women. The types of PD and postoperative complications are summarized in Table 1. The median follow-up duration after PD was 1.34 years[range,14 d (death) to 9.55 years].
The clinical features,management strategy,and outcome of arterial hemorrhage were evaluated.
Surgical procedures of PD
The surgical procedures of PD have been described in detail elsewhere[9,44]. Lymphadenectomy and nerve dissection were performed in patients with malignancies in accordance with the Japanese guideline[45]. Briefly,the GDA from the celiac artery and IPDA from the SMA were cut after double ligation using a locking loop knot. Inherent reconstructions during subtotal stomach-preserving PD were performed by the modified Child’s method with Braun’s anastomosis. During pancreaticojejunostomy,an intraductal lost stent (pancreatic duct tube,5 Fr,burled,MD41515; Sumitomo Bakelite Co.,Ltd.,Tokyo,Japan) was placed,and duct-to-jejunal anastomosis was performed with interrupted polydioxanone sutures (4-0 PDS II,violet,RB-1,Z712D;Ethicon,Inc.,Cincinnati,OH,United States). Adequate approximation of the pancreatic stump and jejunal wall was ensured with interrupted polyvinylidene fluoride sutures (4-0 ASSP504-0IIN,ASFLEX,75 cm; Kono Seisakusho Co.,Ltd.,Ichikawa,Chiba,Japan). choledochojejunostomy was performed with interrupted polydioxanone sutures. A linear stapler was employed for gastrojejunostomy,and the entry hole was closed by hand suturing in a layer-to-layer fashion. Braun’s anastomosis was also performed by hand suturing in a layer-to-layer fashion.
Liver infarction caused by hepatic ischemia
Liver infarction was mainly diagnosed by imaging findings. A sudden increase in the serum aspartate aminotransferase concentration or a gradual increase in the total bilirubin concentration was used as supporting data[1].
Pancreatic leakage
Pancreatic leakage was diagnosed according to the criteria established by the International Study Group of Pancreatic Surgery[46].
TAE
TAE was performed as the EVT procedure in this study. We intend to arrest fatal
hemorrhage by placement of microcoils (Deltaplush; Codman & Shurtleff,Inc.,Raynham,MA,United States) in the pseudoaneurysm and/or culprit artery.

Table 1 Patients characteristics
Stent-graft placement
The EVT procedures involved transcatheter placement of a stent-graft. In general,procedures of stent-graft placement were performed under local anesthesia. The target artery was dilated by a balloon catheter (Graftmaster; Abbott Laboratories,Chicago,IL,United States). Balloon catheter pressures was increased in manner of 2 atm per 5 s,and the maximum of intracatheter pressure was 15 atm (1520 kPa). A covered stent(Graftmaster; Abbott Laboratories),not a bare stent,was placed at the culprit artery.The size and length of covered stent was carefully decided on a case-by-case basis,based on angiographic findings after balloon dilation. We aimed to simultaneously obtain complete hemostasis of fatal hemorrhage and preservation of HA flow. The second overlapping stent-graft was implanted in an overlapping fashion,if needed.The actual procedure is shown in Figure 2.
Arterioportal shunting
An arterioportal shunt was surgically created (Figure 3). The ileocecal vein and artery were anastomosed in a side-to-side fashion using polypropylene suture. Thereafter,the hepatopetal flow of the portal vein (PV) was well oxygenated.
Ethical approval
This retrospective study was approved by the ethics review committee for clinical studies of our institution. The study was performed in accordance with the ethical guidelines of the Declaration of Helsinki. All patients involved in this study provided written informed consent authorizing the use and disclosure of their protected health information.
Statistical analysis
All results are shown as mean ± SD or median (range). Survival rates were calculated using the Kaplan-Meier method. All calculations were performed using statistical software (SPSS Inc.,Chicago,IL,United States).
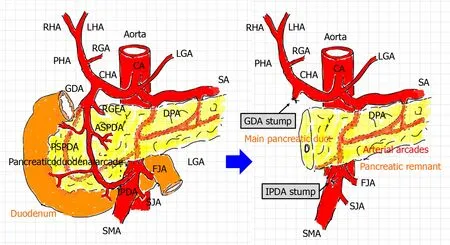
Figure 1 Arterial flow before and after pancreaticoduodenectomy. During pancreaticoduodenectomy (PD),the gastroduodenal artery (GDA) from the celiac artery and inferior pancreaticoduodenal artery (IPDA) from the superior mesenteric artery are ligated and then cut. Additionally,the pancreaticoduodenal arcade is resected. Hence,arterial flow to the liver is modified after PD. A hepatopetal blood supply from the GDA and IPDA via the pancreaticoduodenal arcade can no longer be expected. The hepatic artery flow depends on the celiac artery. Lymphadenectomy and nerve dissection for treatment of malignancies might render visceral arteries vulnerable to postoperative wall injuries. Arterial arcades still remain in the pancreatic remnant. ASPDA:Anterior superior pancreaticoduodenal artery; CA:Celiac artery; CHA:Common hepatic artery; DPA:Dorsal pancreatic artery; FJA:First jejunal artery; GDA:Gastroduodenal artery; HA:Hepatic artery;IPDA:Inferior pancreaticoduodenal artery; LGA:Left gastric artery; LHA:Left hepatic artery; PHA:Proper hepatic artery; RGA:Right gastric artery; RGEA:Right gastroepiploic artery; RHA:Right hepatic artery; PSPDA:Posterior superior pancreaticoduodenal artery; SA:Splenic artery; SJA:Second jejunal artery; SMA:Superior mesenteric artery.
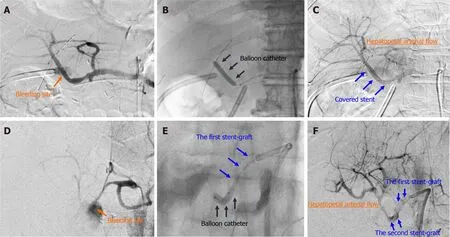
Figure 2 Actual procedures of transcatheter placement of covered stent. Actual procedures in patient 7 and patient 16 are shown. A:Patient 7,diagnostic angiography clearly detected the bleeding sites; B:Patient 7,the target artery was dilated by a balloon catheter; C:Patient 7,a covered stent was placed at the culprit artery; D:Patient 16,diagnostic angiography clearly detected the bleeding sites; E:Patient 16,the target artery was dilated by a balloon catheter; F:Patient 16,the second overlapping stent-graft was implanted in an overlapping fashion. The hepatopetal arterial flow resumed (C and F). Hence,complete hemostasis and preservation of hepatic artery flow were simultaneously obtained.
RESULTS
Institutional frequency of arterial hemorrhage after PD
The overall frequency of arterial hemorrhage after PD was 5.5% (16 of 291 patients who underwent PD in our institution).

Figure 3 Actual finding of arterioportal shunting. The ileocecal vein and artery were anastomosed in a side-to-side fashion. In a patient in whom the initial endovascular treatment failed (patient 14),hemostasis was completed by additional transcatheter arterial embolization,and liver infarction subsequently occurred.Therefore,an arterioportal shunt was surgically created to oxygenate the portal vein flow. In this case,arterioportal shunting minimized progression to fatal liver infarction due to hepatic ischemia and refractory liver abscess due to biliary ischemia. PV:Portal vein.
Patients’ characteristics and PD procedures
The primary diseases and surgical procedures are summarized in Table 1. Thirteen(81.3%) patients had malignancies. Lymphadenectomy and/or nerve dissection was performed in 12 (75.0%) patients.
Associated pancreatitis and pancreatic leakage
Associated pancreatitis occurred in seven patients,and nine (56.3%) patients had a soft pancreatic remnant (i.e.,pancreatic remnant without associated pancreatitis) (Table 1).Pancreatic leakage was observed in 12 (75.0%) patients (Table 1).
Hemorrhage onset,symptoms,and patients’ conditions before EVT
The clinical characteristics at hemorrhage onset are summarized in Table 1.Hemorrhage onset occurred at a median of 18 d (range,6-58 d) after PD. Sentinel bleeding was observed in 5 (31.3%) patients. Arterial hemorrhage was externalized through the intraperitoneal drain or wound in 13 (81.3%) patients and through the digestive tract in 2 (16.7%) patients. Sepsis,shock (including an unstable hemodynamic state),and liver infarction were observed in 9 (56.3%),11 (68.8%),and 2(16.7%) patients,respectively. Notably,four patients with poor clinical courses after EVT (Patients 13-16) had a poor clinical condition before EVT (Table 1).
Bleeding site,image findings,and definitive diagnosis
The most common and second most common sites of bleeding were the GDA stump (7 patients,43.8%) and HA (4 patients,25.0%),respectively (Table 2). Computed tomography (CT) angiography was the diagnostic modality in 13 (81.3%) patients. The imaging findings of CT angiography and angiography are summarized in Table 2. The median time from hemorrhage onset to definitive diagnosis and the median time from hemorrhage onset to EVT were 0 d (range,0-1 d) and 0 d (range,0-14 d),respectively(Table 2).
Actual EVT procedures,technical success of EVT,and long-term results after EVT
The treated arteries and ranges are summarized in Table 3. The initial EVT procedures were stent-graft placement in 7 (43.8%) patients,TAE in 6 (37.5%) patients,and combined therapy involving stent-graft placement and TAE in 2 (16.7%) patients(Table 3).

Table 2 Definitive diagnosis
The initial EVT failed and/or was incomplete in 4 (25.0%) patients,and the rate of technical success of the initial EVT was 75.0% (Table 3). The reasons for failed and/or incomplete EVT were stenosis in 2 patients,and subtle bleeding in one patient,and difficulty in packing in 1 patient (Table 3). Additional EVTs were performed in 4(25.0%) patients (Table 3). Antiplatelet and/or anticoagulation agents were administered to 5 (31.3%) patients (Table 3),and these 5 patients continuously received medications even after discharge from our hospital.
Recanalization did not occur (0.0%) throughout the long-term follow-up after TAE(Table 3). Collateral circulation was observed in 2 (25.0%) of eight patients who underwent TAE (Table 3). Additionally,all implanted stent-grafts (100.0%) maintained their patency throughout the long-term follow-up after stent-graft placement (Table 3).
Surgical approaches including arterioportal shunting
Surgical approaches were utilized in eight patients and are summarized in Table 3. In one patient who underwent failed EVT (patient 10),hemostasis and ligation of the CHA were surgically performed under laparotomy. In one patient in whom the initial EVT failed (patient 14),hemostasis was completed by additional TAE,and liver infarction subsequently occurred. Therefore,an arterioportal shunt was surgically created to oxygenate the PV flow (Figure 3). In this case,arterioportal shuntingminimized the patient’s progression to fatal liver infarction due to hepatic ischemia and a refractory liver abscess caused by biliary ischemia.

Table 3 Endovascular treatment

1The second stentgraft was implanted in overlapping fashion.2The day number from the initial endovascular treatment (EVT).3Findings in the latest dynamic image studies (time from the initial EVT).4Subtle bleeding was observed at the end of EVT,but thereafter,complete hemostasis was fainally obtained.5Shunt creation between the iliocecal artery and vein. CA:Celiac artery; CHA:Common hepatic artery; EVT:Endovascular treatment; GDA:Gastroduodenal artery; LHA:Left hepatic artery; RHA:Right hepatic artery; PHA:Proper hepatic artery; RGA:Right gastric artery; SA:Splenic artery; SMA:Superior mesenteric artery; TAE:Transcatheter arterial embolization.
Liver infarction due to hepatic ischemia
Liver infarction after EVT was observed in 2 (12.5%) patients (patients 13 and 14),and these patients underwent TAE (Tables 3 and 4). Complete hemostasis was obtained by TAE,but hepatopetal arterial flow was completely lost (Figure 4). Liver infarction due to hepatic ischemia subsequently occurred (Figure 4). In these patients,the serum aspartate aminotransferase concentration clearly increased after EVT (Figure 5).Fortunately,both patients successfully recovered from arterial hemorrhage after PD and liver infarction after TAE (Table 4).
Clinical course and outcome after EVT
The patients’ clinical courses and outcomes after EVT are summarized in Table 4.Three patients (patients 2,15,and 16) died during hospitalization,and the actual survival curves after PD and EVT are shown in Figure 6. The mean hospital stay after PD was 66.8 ± 27.7 d among 13 patients who achieved hospital discharge. Fourteen(87.5%) patients were successfully treated because the cause of death in 1 patient(patient 2) was unrelated to arterial hemorrhage (cancer-related death).
Two patients (patients 15 and 16) had a poor outcome even after successful EVT.These 2 patients had a poor clinical condition before EVT (Table 1). One patient(patient 15) had sepsis,shock,and disseminated intravascular coagulation before EVT and died of these conditions even after successful stent-graft placement (Tables 1 and 4). The other patient (patient 16) had sepsis,shock,and liver infarction before EVT(Table 1 and Figure 5) and finally died of liver failure even after successful stent-graft placement (Table 4).
DISCUSSION
In general,visceral artery pseudoaneurysms are rare but fatal[1,13,18,19,21,23,29,30]. The HA (i.e.,the CHA,PHA,and lobular branches) is the second most frequent site of visceral pseudoaneurysms,and the splenic artery is generally the most common[47].Pseudoaneurysms of the HA are usually iatrogenic[2,16,30,44]but may also be associated with localized infection or trauma[30]. Possible causes of intraoperative pseudoaneurysms include direct vascular injury during dissection or retraction,clamp injury to the vessel,or thermal injuryviaelectrocautery[17,30]. Lymphadenectomy and/or nerve dissection for malignancy renders visceral arteries more vulnerable to further wall injuries[2,16,17,30,39,44,48]. Complications following PD commonly consist of localized infection,anastomotic failure,delayed gastric emptying,and gastrointestinal bleeding[2,6,8,9,29,30]. Although arterial hemorrhage after PD is not frequent,it is fatal[1,2,4,7,12,14-23]. The GDA stump is the most common site of arterial hemorrhage,and the CHA and PHA are the next most common sites[16,18,19,21,22,27]. Arterial hemorrhage of the SMA after PD has also been reported[49,50].

Table 4 Clinical course and outcome after endovascular treatment
Pancreatic leakage compromises the arterial wall[2,6,16,17,19,22,24,29,30,39,44,51]. Pancreatic juice or localized infection gradually causes arterial wall erosions,resulting in pseudoaneurysms[2,6,16,17,22,24,29,39,44,51]. Pseudoaneurysm rupture causes sudden-onset,massive,and active hemorrhage[18]. Studies have shown a trend toward a higher prevalence of a soft pancreatic remnant in patients with arterial hemorrhage[2,6,44]. Leaving approximately 1 cm of the GDA stump,spreading an omental flap,and winding the HA by the round ligament of the liver have been suggested to minimize direct contact of pancreatic juice with adjacent vessels[18,22,51-53].

Figure 4 Liver infarction and abscess after transcatheter arterial embolization. Actual findings in patient 13 and patient 14 are shown. A:Patient 13,the bleeding sites were detected; B:Patient 13,complete hemostasis was obtained by transcatheter arterial embolization,but the hepatopetal arterial flow was completely lost (dotted orange arrows); C:Patient 13,the patient subsequently developed liver infarction due to hepatic ischemia (dotted yellow circles); D:Patient 14,the bleeding sites were detected; E:Patient 14,complete hemostasis was obtained by TAE,but the hepatopetal arterial flow was completely lost (dotted orange arrows); F:Patient 14,the patient subsequently developed liver infarction due to hepatic ischemia (dotted yellow circles) and a liver abscess due to biliary ischemia(dotted orange circle).
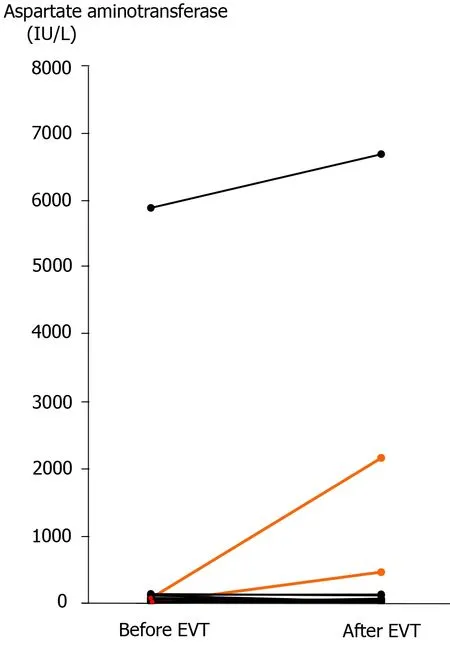
Figure 5 Serum aspartate aminotransferase concentration before and after endovascular treatment. Actual changes before and after endovascular treatment (EVT) are shown. In two patients who developed liver infarction after transcatheter arterial embolization (patients 13 and 14),the serum aspartate aminotransferase concentration was clearly elevated after EVT (orange lines). A high serum aspartate aminotransferase concentration was observed in a patient who had liver infarction before EVT (patient 16),and this patient finally died of liver failure even after successful stent-graft placement. EVT:Endovascular treatment.

Figure 6 Short-term survival after pancreaticoduodenectomy and endovascular treatment. Three patients (patients 2,15,and 16) died during hospitalization,and the actual survival curves after pancreaticoduodenectomy (PD) and endovascular treatment (EVT) are shown. Fourteen (87.5%) patients were successfully treated because the cause of death in one patient (patient 2) was unrelated to arterial hemorrhage (cancer-related death). A:PD; B:EVT. EVT:Endovascular treatment; PD:Pancreaticoduodenectomy; POD:Postoperative day.
Diagnostic procedures and imaging modalities are associated with certain difficulties[6,7,17,18,28,54]. Even based on laparotomy findings,definitive diagnosis may be difficult[54]. Bleeding from the digestive tract or intraperitoneal drain should be considered a warning because it is an important prelude to massive and active hemorrhage. The term “sentinel bleeding” was first coined in 1989 by Shankar and Russell[55]and was further discussed by Brodsky and Turnbull in 1991[34]. The incidence of sentinel bleeding is approximately 30% to 80%[2,18]. Pseudoaneurysms can be detected by CT in patients with sentinel bleeding[6,7]. Sentinel bleeding should be regarded very seriously[2,4,6,18],even in asymptomatic patients with conservatively treated pancreatic leakage[6,56,57]. An accurate definitive diagnosis should be made immediately,before the patient’s unstable hemodynamic state deteriorates[6,18,29].Diagnostic digestive endoscopy delays adequate treatment in hemodynamically unstable patients because of pseudoaneurysm rupture[7,58]. The diagnostic potential of CT angiography[6,16,19,28,43]and diagnostic angiography[6,7,17,18,29,39,54]have been established.Diagnostic angiographic findings include extravasation of contrast medium,pseudoaneurysm formation,non-smooth arterial intima,local vascular spasm,stenosis,and distal arterial branch expansion[20,54]. Diagnostic angiography should be considered even in patients with suspected hemorrhage[6,7,17,18,29,39,54],and subsequent EVT should be adequately performed if necessary[1,7,16-18,30,54].
EVT represents the first-line treatment for arterial hemorrhage after PD[2,7,17,40,59-61].Arterial hemorrhage easily results in unstable hemodynamic state[1,6,16,18,23,29,62],sepsis[2,6,7,13],and hepatic ischemia[6,28,39,63]. Prolonged hemorrhage leads to shock and coagulopathy[1,16,18,23],and further hemorrhage results in disseminated intravascular coagulation[1,16,18,23]. Complicated homeostasis is associated with a poor prognosis even after successful EVT[1,16,18],and the patient’s condition before EVT is strongly associated with complications after EVT[1,16,29]. In fact,our two patients who had a poor clinical condition before EVT (e.g.,sepsis,shock,and liver infarction) finally died even after successful EVT (Tables 1 and 4). If a patient shows any signs of a suspected hemorrhage,EVT should be performed as soon as possible before the development of complicated homeostasis[1,2,16,18,23,30]. Concern exists regarding the placement of foreign bodies (i.e.,coils and stent-grafts) in the setting of infection or inflammation[40].Intravascular stent infection can be a devastating complication,but it is very rare[40,64-66]. In fact,stent-grafts have been used to repair infected pseudoaneurysms[67,68].Though pancreatic juice-related localized infection may associate with pseudoaneurysm and arterial wall erosion[2,6,16,17,22,24,29,39,44,51],we consider that stent-grafts can be placed even in suspicious infectious site.
Arterial hemorrhage after PD usually occurs after at least 1 d[24],and delayed hemorrhage generally occurs after 1 wk[6,58]. In one study,one-third of arterial hemorrhages occurred 1 mo after PD[21]. Hence,delayed hemorrhage is common after PD[2,6,7-19,21,22,28,58,62],and the median or mean time point of hemorrhage onset ranges from 18 d to 21 d after PD[2,7,21,28]. Pancreatic leakage is a possible cause of delayed arterial hemorrhage[2,6,44],and delayed hemorrhage after PD carries a significantly higher mortality rate[2,6,7,21,28].
Because the GDA and IPDA were ligated and the pancreaticoduodenal arcade was resected during PD in the present study,hepatopetal blood supplyviathese arteries could no longer be expected (Figure 1). EVT may lead to severe complications (e.g.,hepatic ischemia,liver abscess formation,and PV stenosis)[1]. The EVT technique should be decided on a case-by-case basis[18,28,29]. Notably,the EVT procedure is strongly associated with complications after EVT[1,16]. Although TAE is technically easier than stent-graft placement[19],liver infarction secondary to hepatic ischemia frequently occurs[6,34,35]. Even a subtle ischemic change in the biliary tree results in intractable liver abscesses[6,34,35]. We also experienced a case of a refractory liver abscess due to biliary ischemia (patient 14) (Figure 4F). PV stenosis easily disturbs the hepatic parenchymal perfusion,resulting in liver infarction with a poor prognosis[23,69]. The rates of mortality and serious hepatic complications after EVT are approximately 20%to 50% and 20% to 80%,respectively[1,18,19,21,23,31,35,70-72].
TAE is advantageous for ensuring complete hemostasis[2,13,20,23,43,54,62,73-77],and the hemostatic rate is reportedly > 90%[17,20,23,54,71]. TAE is technically user-friendly at the most frequent site (i.e.,the GDA stump)[19,43],although both the proximal and distal sides of the GDA should be completely embolized[19]. To prevent recanalization and rebleeding,all arterial flows to the pseudoaneurysm should be completely interrupted[19,44,47,48]. Although the pancreaticoduodenal arcade is removed during PD,arterial arcades remain in the pancreatic remnant (Figure 1)[29,47]. Recanalizationviathe collateral circulation has been reported after TAE[29]; however,transcatheter techniques(e.g.,isolation,packing,and embolization) are available for various forms of pseudoaneurysms[29]. Notably,TAE is occasionally associated with serious hepatic complications caused by hepatic ischemia[1,16,23,30,32,33,69,70]. The liver has many potential collateral pathways that communicate with the adjacent arterial system[16,19,23,29,78,79],and a sudden complete block of HA flow immediately after surgery may induce an ischemic insult to the liver parenchyma[16,29,78,79]. Whether extrahepatic arteries (e.g.,the inferior phrenic artery and left gastric artery) provide sufficient hepatopetal collateral circulation to avoid fatal hepatic ischemia after TAE remains unclear[1,19,23,32].Additionally,the liver can tolerate considerable TAE without significant liver infarction because it has a dual blood supply from the HA and PV[19,20,23,29]. TAE may cause liver infarction in patients with poor collateral circulation because of their postoperative status[29]. Approximately 30% to 80% of patients develop hepatic ischemia after TAE[69,71],and approximately 20% to 40% of patients progress to liver infarction[23,70,71]. The reported mortality rate ranges from 30% to 50%[19,31,35,72].
Simultaneous accomplishment of complete hemostasis and HA flow preservation is difficult[1,7,13,16,18,28,59]. Transcatheter placement of a covered stent may be of value in maintaining the patency of adjacent arteries,and stent-graft placement is an ideal technique to preserve HA flow[1,6,13,16,17,21,27-31,40,54,61,63,80-82]. If necessary,a second overlapping stent-graft can be implanted[40,83]. Actually,we placed a second stent in two patients (Table 3). Some researchers have described patients who underwent this EVT technique[30,31,63,80-82,84],and others have documented such cases in published case series[13,36,38,85]. The success rate of stent-graft placement reportedly ranges from 75% to 80% because the target arteries require a specialized stent size and/or exhibit narrowness and tortuosity[16,37,38,59]. The overall mortality and clinical outcomes are affected by the patients’ conditions before stent-graft placement[16,29]. The use of antiplatelet agents or heparinization after stent-graft placement in the HA is still controversial[13,16,36,37,40,85]. Some clinicians do not use such agents in patients with an unstable hemodynamic state after arterial hemorrhage[37]. However,stent-graft placement in a patient with arterial hemorrhage after surgery carries a high risk of thrombosis because the damaged wall of the HA is surrounded by localized infection and/or massive hematoma,and the HA diameter is very small[16,40]. Hence,some clinicians use these agents after stent-graft placement[13,16,36,40,85].
Stent grafting is a technically difficult procedure and requires adaptation to vessels of various sizes[18,40]. However,this EVT technique is considered the most appropriate treatment method in patients with a favorable vascular anatomy. Stent-graft placement may fail for anatomical reasons (e.g.,tortuosity or variation)[13,17,40,59,84,86]or because of catheter-induced vasospasm or spontaneous thrombosis within the aneurysmal wall[17,87-89]. Actual reasons for failed or incomplete EVT in our institution were summarized in Table 3. Since stent-graft placement may technically failed due to tortuosity,variation,stenosis,vasospasm or thrombosis at the culprit artery[13,17,40,59,84,86-89],preliminary dilatation by balloon catheter is indispensable even for self-expandable covered stent. The interventional radiologist who performs the procedure must have adequate experience and skill[59,62].
If EVT fails or is incomplete,the next management option for arterial hemorrhage is still a surgical approach[2,7,62]. Surgical exploration and complete hemostasis are difficult and hazardous because of postoperative adhesions and the patient’s critical condition[17,18,48]. Surgical treatment is usually associated with a high mortality rate(29%-58%)[2,17-19,48]. Hepatopetal flow of the PV can be well oxygenated by creation of an arterioportal shunt,and some reports have described such cases[41,42]. The impact of a surgical approach involving arterioportal shunting on the prevention of liver infarction has been documented[90,91]. Although the clinical decision and optimal timing for the surgical approach of arterioportal shunting are still controversial,we consider that the presence of subtle clinical signs of progressive liver infarction after EVT is a clear indication for arterioportal shunting.
To our knowledge,most reports to date are limited to case reports or small series[13,30,31,36,38,63,80-82,84,85]. We acknowledge that this study has several limitations. The main limitation is that this was a retrospective study with a small number of patients from a single center. Of course,we have demonstrated our individual-tailored approach. Potential limitations due to bias and a small sample size are inherent to this type of study. This represents our experience in a single institution and our views may be affected by various biases. Hence,we understand that our conclusions must be drawn with extreme caution. However,we believe that transcatheter placement of a covered stent has therapeutic advantages for arterial hemorrhage after PD,with simultaneous accomplishment of complete hemostasis and HA flow preservation.
Actual therapeutic strategies for our patients who caused arterial hemorrhage after PD were summarized in Figure 7. We currently have an institutional therapeutic strategy for arterial hemorrhage after PD based on our own experiences:(1) CT angiography is performed if general condition is stable; (2) Diagnostic angiography is immediately performed even in a suspicious patient; and (3) Covered stent is subsequently placed at the culprit artery as the first line treatment.
CONCLUSION
In conclusion,transcatheter placement of a covered stent may be a powerful tool for simultaneous accomplishment of complete hemostasis and HA flow preservation,although arterial hemorrhage after PD is generally fatal.
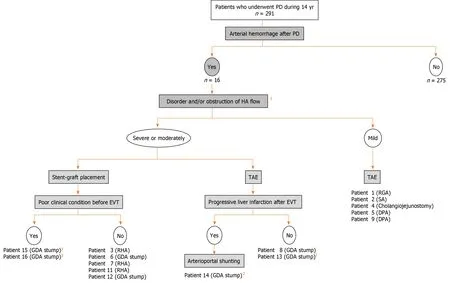
Figure 7 Flowchart of endovascular treatments and arterioportal shunting. Actual flowchart of our patients who caused arterial hemorrhage after pancreaticoduodenectomy was shown. 1Image findings of computed tomography angiography and/or diagnostic angiography. 2Patients with liver infarction after transcatheter arterial embolization. 3Patients with poor outcome even after successful stent-graft placement. PD:Pancreaticoduodenectomy; HA:Hepatic artery; TAE:Transcatheter arterial embolization; EVT:Endovascular treatment; GDA:Gastroduodenal artery; RHA:Right hepatic artery; SA:Splenic artery; DPA:Dorsal pancreatic artery.
ARTICLE HIGHLIGHTS
Research background
Arterial hemorrhage after pancreaticoduodenectomy (PD) is fatal.
Research motivation
This hemorrhage is caused by pseudoaneurysm rupture,and the gastroduodenal artery stump and hepatic artery are frequent culprit vessels.
Research objectives
Simultaneous accomplishment of complete hemostasis and hepatic artery flow preservation is difficult after PD. Although complete hemostasis may be obtained by transcatheter arterial embolization or surgery,liver infarction and/or abscesses may occur.
Research methods
Arterial hemorrhage after PD is fatal. This hemorrhage is caused by pseudoaneurysm.
Research results
We here evaluate our experience including actual treatments (transcatheter arterial embolization,stent-graft placement,or surgery),and discuss therapeutic strategies.
Research conclusions
Transcatheter placement of a covered stent is useful for simultaneous accomplishment of complete hemostasis and hepatic arterial flow preservation.
Research perspectives
Therapeutic options for fatal arterial hemorrhage after PD is shown.
杂志排行
World Journal of Hepatology的其它文章
- Pathologic and molecular features of hepatocellular carcinoma:An update
- Infantile giant cell hepatitis with autoimmune hemolytic anemia
- Long-term albumin infusion in decompensated cirrhosis:A review of current literature
- Bile acid indices as biomarkers for liver diseases I:Diagnostic markers
- Elderly patients (≥80years) with acute calculous cholangitis have similar outcomes as non-elderly patients (<80years):Propensity score-matched analysis
- Retrospective analysis of complications related to endoscopic retrograde cholangio-pancreatography in patients with cirrhosis vs patients without cirrhosis
