Orthobiologics in the treatment of hip disorders
2021-04-28AndrVinciusSaueressigKruelLucasLeiteRibeiroPauloDavidGusmStephanyCaresHuberJosbioSantosDuarteLana
André Vinícius Saueressig Kruel, Lucas Leite Ribeiro, Paulo David Gusmão, Stephany Cares Huber, José Fábio Santos Duarte Lana
André Vinícius Saueressig Kruel, Department of Orthopedics, Regenesis Clínica Médica, Bento Gonçalves, RS 95700-066, Brazil
Lucas Leite Ribeiro, Department of Orthopedics, Instituto Médico Salus, São Paulo, SP 01308-050, Brazil
Paulo David Gusmão, Department of Orthopedics, the Bone and Cartilage Institute, Porto Alegre, RS 90570-020, Brazil
Stephany Cares Huber, Department of Hematology, University of Campinas, Campinas, SP 13334-170, Brazil
José Fábio Santos Duarte Lana, Department of Orthopedics, the Bone and Cartilage Institute, Indaiatuba, SP 13334-170, Brazil
Abstract Orthobiologics are biological materials that are intended for the regeneration or healing of bone, cartilage and soft tissues.In this review we discuss the use of orthobiologics for hip disorders providing an update.The orthobiologics included in this article are hyaluronic acid, platelet rich plasma, bone marrow, adipose tissue and expanded mesenchymal stem cells.We explain the concepts and definitions of each orthobiological product, and the literature regarding its use in the hip joint.The paucity of guidelines for the production and characterization of the biological products leads to uneven results across the literature.Each biologic therapy has indications and benefits; however, noteworthy are the characterization of the orthobiologics, the application method and outcome analysis for further improvement of each technique.
Key Words: Orthobiologics; Hip disorders; Platelet-rich plasma; Mesenchymal stem cells; Bone marrow; Adipose tissue
INTRODUCTION
Orthobiologics can be defined as biological materials used in the regeneration and repair of conditions affecting bone and adjacent soft tissues[1,2].They can be implanted, injected or administered into the patient in order to increase the natural healing potential of the injured musculoskeletal tissue[3].Recently, a variety of orthobiologics has arisen and each type has peculiarities and often present various mechanisms of action.The theoretical advantages of orthobiologics are minimal invasiveness (compared to more austere open or endoscopic forms of traditional orthopedic surgery), greater healing potential (than mere exercise or physical therapy, for instance), faster recovery and reduced cost opposed to surgery, making it a viable alternative[4].
Pathologies affecting the hip joint can be divided into three arbitrary layers: intraarticular, periarticular and intra-osseous.This segmentation has arisen with the help of better imaging methods such as magnetic resonance imaging (MRI) and other procedures such as hip arthroscopy, where labral, chondro-labral junction, Teres ligament lesions or subchondral cystic lesions have been diagnosed and more conservatively treated.Arthritic hip joints have also been managed, although results have been less predictable[5].Hip avascular necrosis (AVN) is especially amenable to orthobiological therapy and enduring results have been shown, sometimes paired with adjunctive non-arthroplastic options like core-decompression[6].Core-decompression treatment consists of tunnel drilling and removal of the necrotic segment from the femoral head[7], providing an opportunity for new pooling of cells to be implanted in the site.
Subchondral bone-marrow edema, a less understood pathology leading to pain or hip arthritis, is also a good candidate for such treatments, considering its biomechanical and biological background and continuous development of imageguided procedures such as ultrasound oriented intra-osseous hip injection[8].Periarticular hip pathology includes chronic tendonitis, as well as deep gluteal space disorders.The latter express themselves often in conjunction with subtle or even overt peripheral nerve entrapments[9].Such tendinopathies hold special interest for their common prevalence and their difficult management in remitting cases.The aim of this review is to provide an update on orthobiologics that can be used for the treatment of hip ailments, with a special focus on the protocols of obtainment (when appropriate, along with our specific protocols), relationship to surgical aspects and clinical evidence of relevance.
ORTHOBIOLOGICAL TREATMENT S
Hyaluronic acid
Hyaluronic acid (HA) is a high viscosity polysaccharide naturally produced by B cells in the synovial membrane.It may also be manufactured from animal sources (typically avian) or bacteria in laboratory (biological) fermentation.Biochemically, it is part of the glycosaminoglycan group[10], acting as a salt under physiological conditions.It is also called sodium hyaluronate, or hyaluronan[11].Intra-articular hyaluronan preparations have been shown to relieve osteoarthritis-derived pain more than conventional treatments such as physical therapy, exercise, nonsteroidal antiinflammatory drugs (NSAIDs), intra-articular corticosteroids or even arthroscopic lavage[12].
The physicochemical properties of HA are determined by molecular mass and spatial conformation.It can also be classified as high, medium or low molecular weight.Normal (non-osteoarthritic) autologous HA in joints weighs around 5 to 7 million Daltons, whereas osteoarthritic joint HA weighs an average of 1 million Daltons.High molecular weight HA molecules intertwine to form a high viscosity solution, which serves both as a lubricant and shock absorber, and has biomolecular properties compatible with and favorable to cell growth[13].
HA is the major non-protein hydrodynamic component of synovial fluid.This substance, biological or synthetic, forms a layer around the cells where it interacts with pro-inflammatory mediators and binds to cellular receptors, modulating cell proliferation and migration, as well as gene expression[14].It is a collagen stimulator, capable of promoting tissue recovery and maintaining cellular integrity[15].These properties make HA useful for healing or even regeneration of tendon and chondral tissues in some cases.
Intra-articular (IA) injection of HA is considered a local treatment with no reports of systemic adverse events observed after the administration of other types of IA injections, such as corticosteroids, or even with oral administration of NSAIDs[16].This therapy represents an alternative treatment, especially for patients with comorbidities.
This alternative approach has shown beneficial effectsin vitro.It is suggested that the extracellular matrix (ECM) has an influence on cell metabolism, especially on osteoarthritic subchondral bone osteoblasts.HA can reverse its abnormal synthetic activity[17].
An IA injection of HA has been shown to reduce chondrocyte apoptosis whilst increasing its proliferation[18].This therapy has been shown to promote chondroprotection due to the HA binding to CD44 receptors.This event inhibits the expression of IL-1β, which leads to a decrease in the biosynthesis of MMP-1, 2, 3, 9, and 13[19-21].This process avoids catabolic enzyme activity within the joint cartilage[22].It is our experience, in human patient treatment, that it is important to use medium or high molecular weight HA, similar to the molecular weight that is normally produced in the body.Also, it is important to use HA derived from biological synthesis, to avoid undesired side effects.Another advantage of HA is the possibility of its combination with other products, biological or not.For example, the combination of calcitonin[23], sorbitol[24], platelet-rich plasma (PRP)[25,17]and/or bone marrow aspirate concentrate (BMAC)[26,27]have been postulated.Clinical trials reporting the use of HA for hip disorders are listed in Table 1.
PRP
PRP is an autologous non-immunogenic therapy, produced through the concentration of platelets in a small plasma volume (typically 3-6 times above baseline)[28].PRP therapy has advantages such as rapid preparation and technical simplicity; it is also a point-of-care procedure, and can be carried out in-office due to its minimal invasiveness, permitting intra-articular, intra-tendinous or even intra-osseous injection.As a result of its autologous origin, it exhibits a unique safety profile, lacking many drug-related side effects or interactions[29].In osteoarthritis (OA), PRP can interfere in the catabolic and inflammatory cascade, in order to promote anabolic responses.The bioactive molecules in PRP can act in inflammatory modulation, ECM synthesis and vessel remodeling in order to improve the natural healing of the tissue[30,31].One of the greatest problems regarding PRP is the lack of standardization.Important variables such as the number of platelets, buffy-coat content, exogenous activation and number of centrifugations are lacking in many publications, resulting in heterogeneous results, which therefore cannot be compared.In order to minimize this problem, some PRP classifications have been proposed.Recently, Lanaet al[32]published a thoughtful classification incorporating all these variables.Besides the aforementioned variables, light activation, image guidance method and red blood cell content were included.All these parameters are important for a standardization protocol.
PRP was used alongside hip arthroscopy surgery for a variety of pathologies[33].The technique consists of the administration of 4.5 mL of PRP into the repaired hip joint capsule in the peripheral compartment through the arthroscopic cannula, as well as 10 mL of PRP in the surrounding soft tissues as suggested by Marc Philippon and Robert Laprade’s group.Growth factors present in PRP in addition to the aforementionedhealing potential also aid in postoperative hemostasis[33].Regarding the use of leukocytes, it has been reported that leukocyte-rich PRP is effective in the initiation of a proinflammatory healing response to stimulate vascular tissue regrowth[34].However, for the treatment of hypovascular tissues, it was reported that it may induce profibrotic tissue formation, which reduces the quality of the tissue itself[33].PRP can also be used in combination with different orthobiologics, such as HA and bone marrow preparations[23-27].Also, even if PRP does not lead to cartilage regeneration, it may still offer symptomatic and functional benefitsviamodulation of inflammation and direct analgesia[29].The description of clinical trials for hip disorders is summarized in Table 1.PRP has also been compared to surgery, regarding gluteus medium tendonitis in a recent meta-analysis[35]and has been deemed effective and safer than endoscopic surgical procedures.In addition, in another recent metaanalysis, PRP was compared to HA and steroid injections and was considered to achieve the highest rank for pain relief at 6 mo in patients suffering from OA[36].
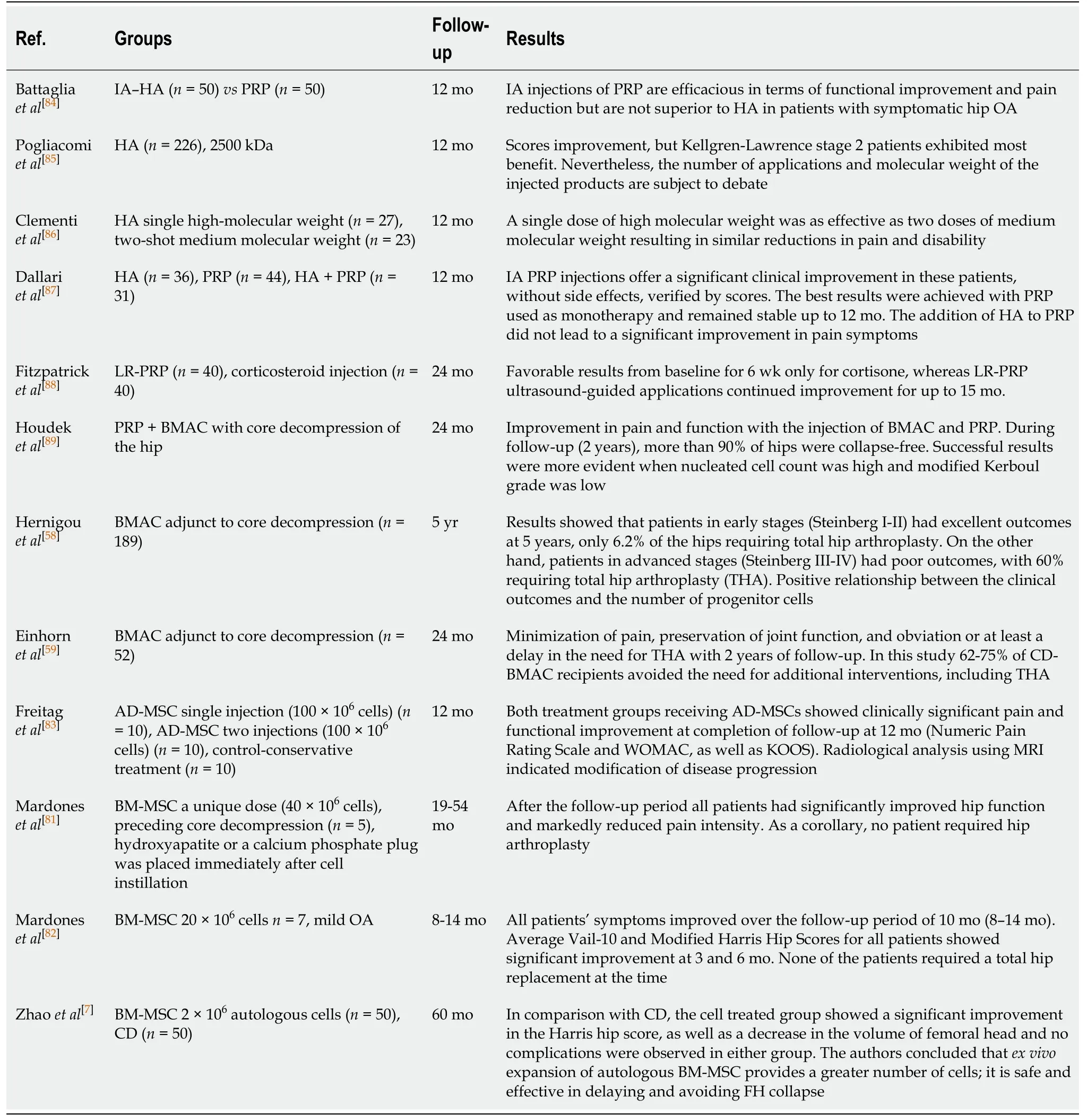
Table 1 Clinical studies regarding the use of orthobiologics for hip disorders
In our clinical practice, after a standardization protocol for PRP therapy, we opted for a handmade PRP preparation, with double centrifugation, enriched with buffy-coat (i.e., leukocyte-rich PRP) using the modified protocol of Amableet al[37]and Huberet al[38].With this technique, we obtained a 3-5-fold increase in platelet concentration and a 2-4 fold increase in white blood cells from baseline (therefore leukocyte rich).
Bone marrow derivatives
Bone marrow has been studied in the context of regeneration for many years.It encompasses erythroblasts, neutrophils, eosinophils, basophils, mononuclear cells [monocytes and mesenchymal stem cells (MSCs)], lymphocytes, megakaryocytes, and plasma.The erythroblasts, eosinophils and neutrophils are present in various stages of differentiation[39].Especially relevant are the bone marrow-derived mesenchymal stem cells (BM-MSCs), hematopoietic stem cells (HSCs) and other progenitors.The use of BM-MSCs has demonstrated benefits in the regeneration process.The HSCs are thought to be the true drivers for enhancing cartilage and bone regeneration, with an important role in the direct conversion to stromal MSCs and orchestration of the bone formation[40]as described by Omsted-Davis in 2003, who pointed out that a side population of marrow stem cells could regenerate the hematopoietic compartment of lethally irradiated mice when transplanted and could also differentiate to osteoblasts through a mesenchymal intermediate[41].This effect was also demonstrated clinically in the study by Marxet al[42]in 2014 where they devised a study with 40 adults undergoing craniomandibular reconstruction.Those patients attaining higher CD34+ counts registered a 100% regeneration of implantable bone, as opposed to 40% of this effect, when CD34+ counts were much lower[42].Pettine also demonstrated improved results in a study on intradiscal injection of bone marrow concentrate (BMC) with a three-year follow-up, and found that HSC (CD34+) also played an immunomodulatory role similar to MSCs and reported that patients who received greater concentrations of progenitor cells [colony forming unit-fibroblast, (CFU-F), and CD34+ lineage] experienced faster and greater pain reduction.The authors also claimed the following: ”This is the first study to link a clinical improvement to CFU-F and CD34+ cell concentrations in BMC[43]”.
MSCs were first discovered in the bone marrow, presenting an important paracrine effect.MSCs thus exhibit a secretory function, comprising anti-inflammatory, proangiogenic, immunomodulatory, anti-apoptotic, anti-fibrotic and wound healing properties, including a proliferative role[44].MSCs represent 0.01% to 0.001% of total bone marrow nucleated cells.Due to this low number, there is a trend for cellular expansion in order to obtain higher cell counts for clinical applications[45].However, the use of non-cultured cells presents some advantages that go beyond cost and good manufacturing practice and regulation.One of the major advantages is that the direct extraction allows the maintenance of its regenerative capacity and avoids senescence[44].Expansion techniques risk cells with variable differentiation capacity, increased senescence markers and tumor degeneration liability.Also, these cells must be cultured for two or three weeks - a slow and expensive process that in clinical practice demands a two-staged tissue implantation surgery.One important issue is that the use of pure, cultured MSCs do not contain HSCs, cytokines and growth factors as concentrated bone marrow does[46].The outcomes of concentratedvscultured cells should be assessed more carefully, taking into account the costs, time expenditure and clinical practice suitability[46].
Previous studies showed variables that could influence the number of MSCs, such as anatomic harvest site, aspirated volume, age and sex of the patient.Traditionally, MSCs could be predictably obtained from the iliac crest[47].It is also regarded that the aspiration of larger volumes decreases the concentration of osteoblast progenitors due to the dilution of bone marrow with peripheral blood[48].Regarding the anatomic site of harvesting, Pieriniet al[49]published a study comparing bone marrow collection from both the anterior and posterior iliac crest of twenty-two patients.The yield of colony-forming connective-tissue progenitors was 1.6 times greater in the posterior compared to the anterior iliac crest.In relation to the volume aspirated, Hernigouet al[50]published a study evaluating the aspiration of bone marrow from iliac crest using 10 mL and 50 mL syringes, and compared MSCs and progenitor cell content.It was verified that the aspirate performed with a 10 mL syringe had a greater concentration of MSCs and 300% more progenitor cells in comparison with the 50 mL syringe aspiration.
In order to increase the relative number of MSCs per volume of injectate for therapeutical purposes, the aspirate of bone marrow can be concentrated, thus the term BMAC.
BMAC
BMAC is the concentration of whole bone marrow aspirate that can be manufactured in different ways in order to concentrate nucleated cells.Common techniques used to achieve this concentration are the use of Ficoll density gradients, closed centrifugation systems and in-house preparations[51].It is believed that increased cell concentration would also enhance the amount of MSCs, which could then provide local microenvironment improvement in order to achieve healing and regeneration[52,53].Potentially, these MSCs are able to provide a direct cell source for repair.In addition, the nucleated cells may have a significant paracrine effect, releasing and delivering a myriad of cytokines and growth factors to orchestrate tissue repair processes[54,55].In comparison to PRP, there is a significant variation in the final products achieved.Fortieret al[56]evaluated the constituents of PRP and BMAC, showing a reduction in platelet content and an increase in white blood cell content in BMAC.The differences between these products could represent a different mechanism of action[56].Ziegler also performed a comparison between these products and concluded that BMAC had a significantly higher IL-1 receptor antagonist and therefore offers a more relevant source of anti-inflammatory therapy for OA[57].
The majority of studies have focused on BMAC for osteonecrosis of the femoral head (ONFH).Hernigouet al[58]described a technique for injecting BMAC as an adjunct to core decompression (CD).The beneficial effect is attributed not only to disruption of the necrotic zone, but also to the introduction of osteogenic progenitor cells into the femoral head.This study evaluated 189 decompressed hips using BMAC harvested from the iliac crest.The results showed that patients in early stages (Steinberg I-II) had excellent outcomes at 5 years, with only 6.2% of the hips requiring total hip arthroplasty.On the other hand, patients in advanced stages (Steinberg III-IV) had poor outcomes, where 60% required total hip arthroplasty (THA).In addition, it was reported that superior outcomes were verified in patients that presented increased numbers of progenitor cells, suggesting a relationship between the clinical outcomes and their count[58].Similar results were obtained in the study by Einhornet al[59], aimed at patients with symptomatic ONFH stages I-II.The researchers observed minimization of pain, preservation of joint function, and obviation or at least delayed need for THA within 2 years of follow-up.In this study 62%-75% of CD-BMAC recipients successfully avoided the need for additional interventions, including THA.
The use of BMAC has been suggested to restore joint harmony and minimize further chondral deterioration.One advantage of this orthobiologic is that MSCs are ideal for the chondrolabral junction, since these cells are able to differentiate into both fibrocartilage and hyaline-like tissue products[60].Clinical trials registered in the literature demonstrate the efficacy and safety of BMAC in treating arthritic cartilage symptoms and focal chondral lesions[61,62].Additionally, significant cartilage growth, for instance, was also demonstrated with the administration of BMAC in association with other treatment modalities such as microfracture and biologic scaffolds[63].The use of BMAC for femoroacetabular impingement and OA of the hip is scarce.In a case report of a professional soccer player, after two arthroscopies, the patient presented remitting hip pain 3 mo after the second surgery.He was treated with 3 PRP and 2 BMAC injections and was able to return to full activities[64].Mardoneset al[45]published a technique for the treatment of chondral hip lesions using BMAC and PRP clot.In this technique, hip arthroscopy was performed and microfracture was carried out for full thickness chondral lesions.Bone marrow was harvested and processed, as well as PRP, which was exogenously activated in order to obtain a clot.To finalize the arthroscopic procedure, the fibrin clot was placed over the microfracture site using a cannula.BMAC was inserted under the PRP clot.This technique was used in 13 patients.As a result, the authors reported improvement in the symptoms for a mean of 8 mo of follow-up[45].Table 1 shows clinical results of the use of bone marrow derivatives for hip disorders.
The results of BMAC in bone, cartilage and tendon injuries are encouraging.We believe that cell characterization is necessary for outcome optimization, and larger randomized controlled trials are highly warranted in order to further support these results.On the other hand, the use of BMAC especially in the early stages of hip diseases resulted in positive results for pain and function, and could represent a promising contemporary treatment strategy.
ADIPOSE-DERIVED TREATMENTS
Adipose tissue has a heterogeneous cell population including endothelial cells, endothelial progenitors, pericytes, fibroblasts, mesenchymal stromal cells, macrophages and adipocytes[65].Harvested fresh adipose tissue can undergo minimum manipulation processing or enzymatic digestion and mechanical separation, yielding a final product called the stromal vascular fraction (SVF).The benefits of the use of SVF in pre-clinical andin vitrostudies have been demonstrated by other authors[66,67].
In 2001, Zuket al[68]identified a stem cell population within human lipoaspirates with the use of collagenase, producing stable growth and proliferation kinetics in the culture.Much like BM-MSCs, such adipose-derived cells differentiatedin vitrotoward osteogenic, myogenic and chondrogenic clusters when treated with lineage specific factors.Thus, this indicates the actual presence of multipotent stem cells with multilineage potential[68,69].BM-MSCs have been used as a cellular therapeutic option for the treatment of AVN of the femoral head, but there is still dispute over its clinical success[70].Wyleset al[71]demonstrated that adipose-derived MSCs (AD-MSCs) outperformed BM-MSCs in growth rate and bone differentiation potential in the setting of AVN, suggesting that they could provide a more potent regenerative therapeutic strategy.Periarticular samples of adipose tissue and BM from the femoral canal were obtained from 15 patients undergoing hip replacement for late-stage osteonecrosis.MSCs were isolated from both sources and taken through a standardized cell division protocol to establish cumulative cell count.Proliferation capacity was increased by 4-fold in AD-MSCs in comparison with BM-MSCs after 20 d in culture[71].However, clinical evidence is still limited to case reports, such as the one published by Paket al[72]in 2011, where two patients were successfully treated for AVN with AD-MSCs (as fresh centrifuged graft) along with HA and PRP.With regard to veterinary medicine, Cuervoet al[73]demonstrated the safety and effectiveness of a single intra-articular injection of AD-MSCs in dogs with hip OA.Functional limitation, range of motion and other scores progressively improved during the 6-mo follow-up period.Additionally, better results were obtained in dogs treated with AD-MSCs than in dogs treated with PRP.Similar results were obtained by Vilaret al[74]where ADMSC therapy also improved limb function in dogs suffering from hip OA.Table 1 summarizes the results of the clinical trials using adipose-derived treatments for hip disorders.
Clinically, minimally manipulated (microfragmented) fresh adipose tissue has been employed in the treatment of chondral lesions, especially associated with arthroscopic procedures[75]or even for hip OA, but documented in short-follow-up case series[76].The microfragmented adipose-derived fraction (MFAT) is a mechanical technique that reduces the size of the adipose tissue clusters to eliminate oil and blood residue.The presence of MSCs in significant numbers was demonstrated.Navaet al[77]evaluated thein vitrosurvival and content of MSCs and anti-inflammatory activity of lipoaspirate and MFAT.It was noted that MFAT exhibited higher amounts of CD31 positive cells-an endothelial marker, and higher numbers of MSCs in comparison to lipoaspirate.The release of cytokines was similar in the first week of culture.However, the total amount secreted by lipoaspirate decreased much more rapidly than that produced by MFAT after 28 d of culture.When the MFAT culture medium of early (3-7 d) or late culture (28 d) was added to a monocyte culture it strongly inhibited the inflammatory pattern.The authors concluded that MFAT presents a long-lasting effect due to the anti-inflammatory activity attributed to their MSC content.These cells release cytokines that modulate the inflammatory cascade by a variety of mechanisms[77].
In summary, the use of adipose tissue has shown promising results as a viable option for hip disorders, including cultured (AD-MSC) or SVF cells.It is relatively easier to obtain SVF compared to cultured cells.Because of regulatory requirements, availability of approved Good Manufacturing Practice (GMP) facilities, associated costs, cell dosage, processing time or even the number of procedures for the patient, the use of fresh cells is becoming more attractive.
EXPANDED MSCS
MSCs were named almost 30 years ago as a class of cells that could be isolated from a variety of tissues, including bone marrow, adipose tissue, dental pulp and umbilical cord.These cells can be expanded in culture maintaining theirin vitroability to induce a variety of mesodermal phenotypes and tissues, including differentiation into bone, cartilage and fat-showing their multi-potent potential[78].Dominiciet al[79], 2006 published an article showing that the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy proposed minimum criteria to define human MSCs.These criteria include: Plastic adherence of MSCs when maintained in culture conditions; positivity for markers CD105, CD73 and CD90, and lack of expression of CD45, CD34, CD14, CD11b, CD79, CD19 and HLA-DR; Differentiation in osteoblasts, adipocytes and chondroblastsin vitro[79].In 2011, Caplan proposed a change in the name of these cells to medicinal signaling cells (MSC), which might actually be their main role-the ability to secrete bioactive factors, acting as immunomodulatory and trophic factors and showing the great importance of their paracrine effect.Thus, tissue-specific resident stem cells are responsible for the construction of new tissue, stimulated by bioactive factors released by exogenous MSC[78,80].
In 2012, Zhaoet al[7]published a paper with 100 patients treated with core decompression in comparison with patients treated with autologous implantation of cultured BM-MSC.The follow-up period was 60 mo after the procedure.As a result, the authors showed a protective effect in the BM-MSC group regarding the progression of osteonecrosis; thus, the cell group presented significantly fewer patients with osteonecrotic stage progression in comparison to CD (core decompression) alone.A decreased necrosis volume was also verified in those patients treated with cells in comparison to CD.The authors concluded that BM-MSC administration is a safe, reliable and highly effective procedure for the treatment of early-stage ONFH[81].In 2019, Mardoneset al[82]published a study evaluating the safety and efficacy of intraarticular infusion ofex vivoexpanded autologous BM-MSC in patients with OA of the hip.Ten patients were injected, each one with a dose of 60 × 106cells in three consecutive weekly doses.The follow-up period was 16-40 mo.Patients exhibited a significant improvement in the score evaluation.The radiographic score in general did not change with exception of one improved patient.The authors concluded that three consecutive injections of expanded BM-MSC proved to be a safe and clinically effective treatment in restoration of the range of motion and function of the hip plagued with OA[83].
Table 1 summarizes clinical studies regarding expanded MSCs, mainly from bone marrow origin.
The reviewed clinical studies suggest that intra-articular MSC therapies are safe when used to treat OA, focal chondral defects or even femoral head AVN.However, the efficacy of these therapies cannot be determined until more standardized level 1 clinical evidence is available.Improving study methodology and standardizing cell harvesting, processing, characterization, and delivery techniques will be necessary before the efficacy of intra-articular MSC therapies can be determined.Future randomized double blinded multi-arm clinical studies aimed at determining optimal cell source and count, ideal patient population target as well as optimal method of intra-articular delivery are still required.It is also important to consider the current limiting costs of cultured cells, which may cost up to 30000 euros[6]and may represent a major hindrance to their application.
CONCLUSION
Although many promising alternatives for degenerative hip pathology have been presented, definitive clinical evidence is still lacking in some treatments.Notwithstanding, cell therapies using mononuclear cells derived from bone marrow for femoral head AVN, as well as leukocyte-rich PRP for gluteal tendinopathy have been deemed successful by long-term quality randomized controlled trials.However, more promising therapies aimed at reducing pain and improving function await better clinical scrutiny by further investigation.
杂志排行
World Journal of Stem Cells的其它文章
- Stem cell therapy for heart failure: Medical breakthrough, or dead end?
- Calcium channels and their role in regenerative medicine
- Patient-specific induced pIuripotent stem ceIIs as “disease-in-adish”modeIs for inherited cardiomyopathies and channeIopathies -15 years of research
- Hypoxia-inducibIe factor-1α-mediated upreguIation of CD99 promotes the proIiferation of pIacentaI mesenchymaI stem ceIIs by reguIating ERK1/2
