Previously unknown behavior in parasitic cuckoo females: male-like vocalization during migratory activity
2021-04-19SwetlanaMeshcheryaginaandAlexeyOpaev
Swetlana G. Meshcheryaginaand Alexey Opaev
Abstract Background: In the last decade, enigmatic male-like cuckoo calls have been reported several times in East Asia.These calls exhibited a combination of vocal traits of both Oriental Cuckoo (Cuculus optatus) and Common Cuckoo(Cuculus canorus) advertising calls, and some authors therefore suggested that the enigmatic calls were produced by either Common × Oriental Cuckoo male hybrids or Common Cuckoo males having a gene mutation. However, the exact identity of calling birds are still unknown.Methods: We recorded previously unknown male-like calls from three captive Oriental Cuckoo females, and compared these calls with enigmatic vocalizations recorded in the wild as well as with advertising vocalizations of Common and Oriental Cuckoo males. To achieve this, we measured calls automatically. Besides, we video-recorded captive female emitting male-like calls, and compared these recordings with the YouTube recordings of calling males of both Common and Oriental Cuckoos to get insight into the mechanism of call production.Results: The analysis showed that female male-like calls recorded in captivity were similar to enigmatic calls recorded in the wild. Therefore, Oriental Cuckoo females might produce the latter calls. Two features of these female calls appeared to be unusual among birds. First, females produced male-like calls at the time of spring and autumn migratory activity and on migration in the wild. Because of this, functional significance of this call remained puzzling.Secondly, the male-like female call unexpectedly combined features of both closed-mouth (closed beak and simultaneous inflation of the ‘throat sac’) and open-mouth (prominent harmonic spectrum and the maximum neck extension observed at the beginning of a sound) vocal behaviors.Conclusions: The Cuculus vocalizations outside the reproductive season remain poorly understood. Here, we found for the first time that Oriental Cuckoo females can produce male-like calls in that time. Because of its rarity, this call might be an atavism. Indeed, female male-like vocalizations are still known in non-parasitic tropical and apparently more basal cuckoos only. Therefore, our findings may shed light on the evolution of vocal communication in avian brood parasites.
Keywords: Closed-mouth vocal behavior, Cuckoo call, Cuculus optatus, Female song, Male-like vocalization
Background
In cuckoos, vocal data are widely used to resolve the taxonomy and for species identification (Payne 2005). For example, Oriental Cuckoo (Cuculus optatus
) and Himalayan Cuckoo (Cuculus saturatus
) are often regarded as separate species, because the advertising calls of the two differ in the presence/absence of introductory note and the number of notes (King 2005; Payne 2005; Lindholm and Linden 2007; Xia et al. 2016). In the last decade, enigmatic cuckoo calls have been reported in eastern Asia: in South Korea (Moores 2013,2015) and in Primorski Krai of Russian Far East (Lastukhin 2015; own data SM). The photos of three calling bird have been taken. On these photos, however, traits necessary for exact species and/or sex identification were poorly seen (Moores 2015).It was noticed that the enigmatic calls exhibited a combination of vocal traits of both Oriental Cuckoo and Common Cuckoo (Cuculus canorus
). Based on this, Lastukhin (2015) suggested that Common × Oriental Cuckoo hybrids might produce these calls. Indeed, calls and songs can be intermediate in hybrid individuals due to genetical or cultural transmission or both (Payne 1980; Cadena et al. 2007; Marova et al. 2017). In parasitic cuckoos, however, hybrids are still unknown (Payne 2005; McCarthy 2006). The absence of hybridization in parasitic cuckoos could be at least partly due to their innate stereotypical sounds preventing mistakes in mate recognition (Lack 1968; Graves 1992; Payne 2005). Other authors who reviewed the existing evidence of birds’ hybridization in Russia Far East, also doubted about the cuckoos’ hybridization (Gluschenko and Korobov 2016; Gluschenko et al. 2016). Concerning South Korean recordings, Clive F. Mann, who is one of the authors of the bookCuckoos of the World
(Erritzøe et al. 2012), assumed that the calls were produced by Common Cuckoo having a gene mutation (Moores 2013). Other authors, however, argued that enigmatic calls apparently were not caused by a single rare mutation, as they have been recorded and/or heard several times (Gluschenko et al. 2016).All the aforementioned authors suggested that cuckoo males produced enigmatic calls. Indeed, the most commonly heard female call, that is, the bubbling call (Payne 2005; Erritzøe et al. 2012), differs considerably from both the male call and enigmatic call. InCuculus
, bubbling calls differ among species in time and frequency parameters, and in the number of notes, although overall structures are similar with each other (Kim et al. 2017b). There are also other call types in female repertoires. However,their structure and functional significance are poorly understood (Cramp 1985). By contrast, the advertising‘cu-coo’ call of Common Cuckoo male is especially well studied (Fuisz and De Kort 2007; Jung et al. 2014; Wei et al. 2015; Kim et al. 2017a; Li et al. 2017; Moskát et al.2017, 2018; Zsebök et al. 2017; Benedetti et al. 2018; Tryjanowski et al. 2018; Deng et al. 2019a; Xia et al. 2019),including its rare aberrant version ‘cu-kee’ (Møller et al.2016; Moskát et al. 2021). It is well known, however, that female birds can produce song and/or male-like advertising vocalization in several species (Odom et al. 2014;Boeme and Goretskaia 2016), including non-parasitic tropical cuckoos (Brumm and Goymann 2017), and duets and chorus are especially characteristic for such species(Tobias et al. 2016). Besides, in some species females have been found to sing when the male disappeared or when the testosterone level experimentally enhanced (Garamszegi et al. 2007).In this study, we recorded captive Oriental Cuckoo females and compared their calls with enigmatic vocalizations, and with typical advertising calls of both Common and Oriental Cuckoos. We found that the enigmatic calls were produced by Oriental Cuckoo females during migratory activity. Thus, it appeared to be unusual in terms of its functional significance.
A striking feature of adult male of several cuckoo species is the concentration of its acoustic energy into a low frequency. In birds, low-frequency sound is usually favored by closed-mouth vocal behavior due to the resonance condition generated by that behavior (Riede et al.2016). Apart from a few exceptions, closed-mouth vocalization is employed by advertising males. The usage of this behavior by female birds is poorly studied. Male-like female cuckoo call is rather low-frequency sound. Therefore, we additionally analyzed the possible mechanisms of call production by female, and then compared them with those of males.
Methods
Data on individuals that produced the enigmatic calls in the wild and in captivity
In the wild, the enigmatic calls were reported 22 times,in eastern Asia only (Fig. 1a; Table 1). In the majority (13 out of 22) of the aforementioned cases, the calling individual had not been seen. In the one case, both Common Cuckoo and Oriental Cuckoo (grey morph) were observed in the locality, where the enigmatic calls were just heard. In five cases, it was possible just to understood that the calling bird was cuckoo. Photos and videorecording of three calling individuals have been taken on Baekryeong Island. Back view as well as distance to photographer and optical effects prevent exact species identification in these cases (Moores 2015).
In total, from 2011 onwards, we held in captivity six Oriental Cuckoo females at different times, but up to three females simultaneously. Three (F1, F2 and F3) out of six females produced male-like vocalizations that resembled closely the enigmatic cuckoo calls. All females held in a single heated room (15 m) having the window on the south side and artificial UV-light in winter.They lived in group and moved freely around the room.There were many perches in the room used by cuckoos. The room connected to the loggia (7.5 m), where females spent a time readily during spring and summer months. This room simultaneously acts as an office for the researcher. Diet of females consisted of insects and protein substitutes.
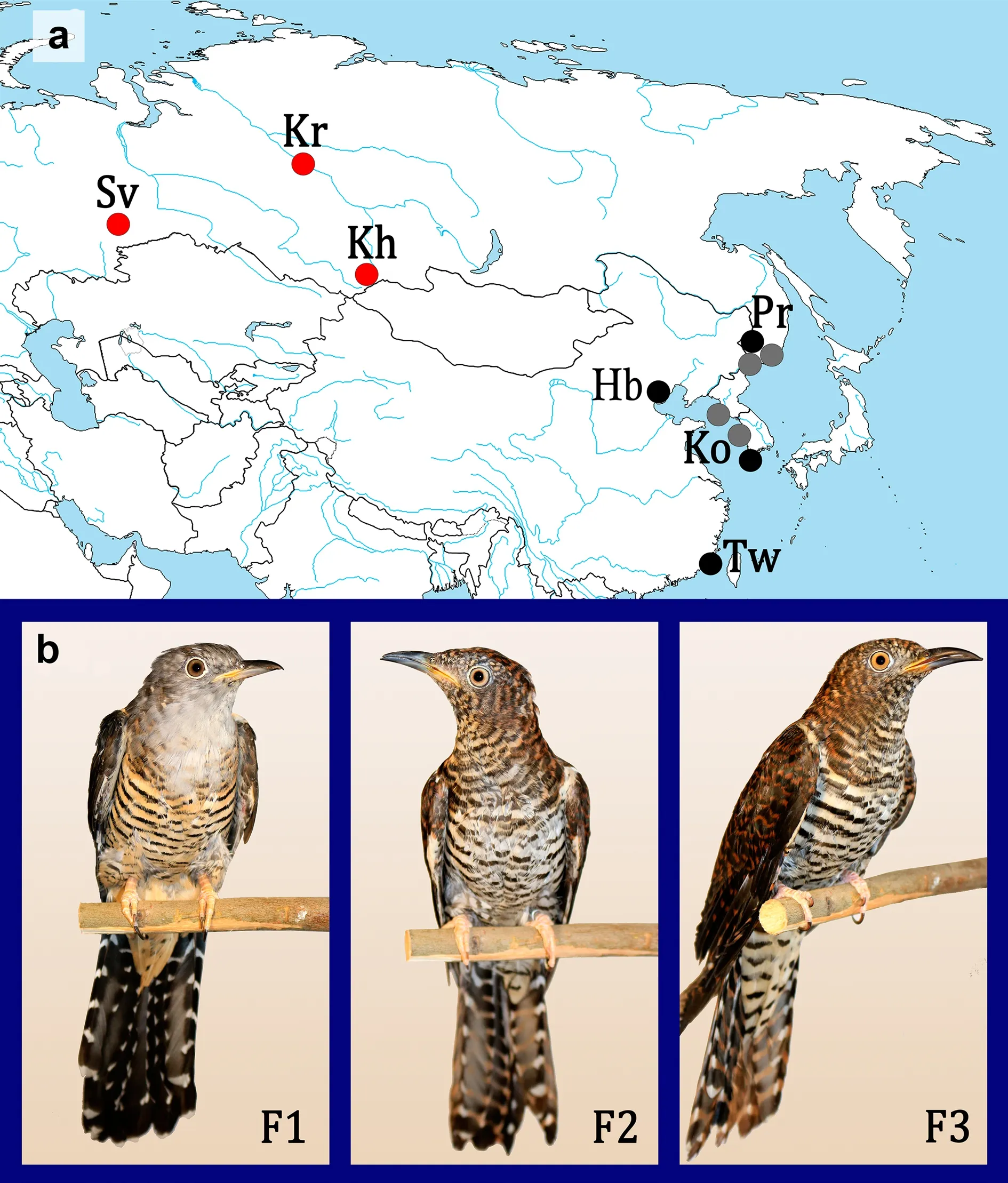
Fig. 1 Localities of origin of captive females and where analyzed recordings were obtained in the wild. a The localities of origin of captive females are shown by red circles, while other circles show localities in which the enigmatic vocalizations were either recorded (black circles) or just reporter(grey circles) in the wild. b Studied Oriental Cuckoo females in captivity (photos were taken during the molt). Abbreviations: F1, F2, F3, Oriental Cuckoo females from Khakassia (Kh), Krasnoyarsk Krai (Kr) and Ekaterinburg (Sv), respectively; Pr, recording locality of “Common × Oriental Cuckoo hybrid” in Primorski Krai; Ko, recording locality of “unknown cuckoo” in South Korea; Hb, recording locality of “atypical song of Oriental Cuckoo” in Hebei, China; Tw, recording locality of “atypical song of Oriental Cuckoo” in Taiwan, China (see Additional file 1: Table S1 for details)
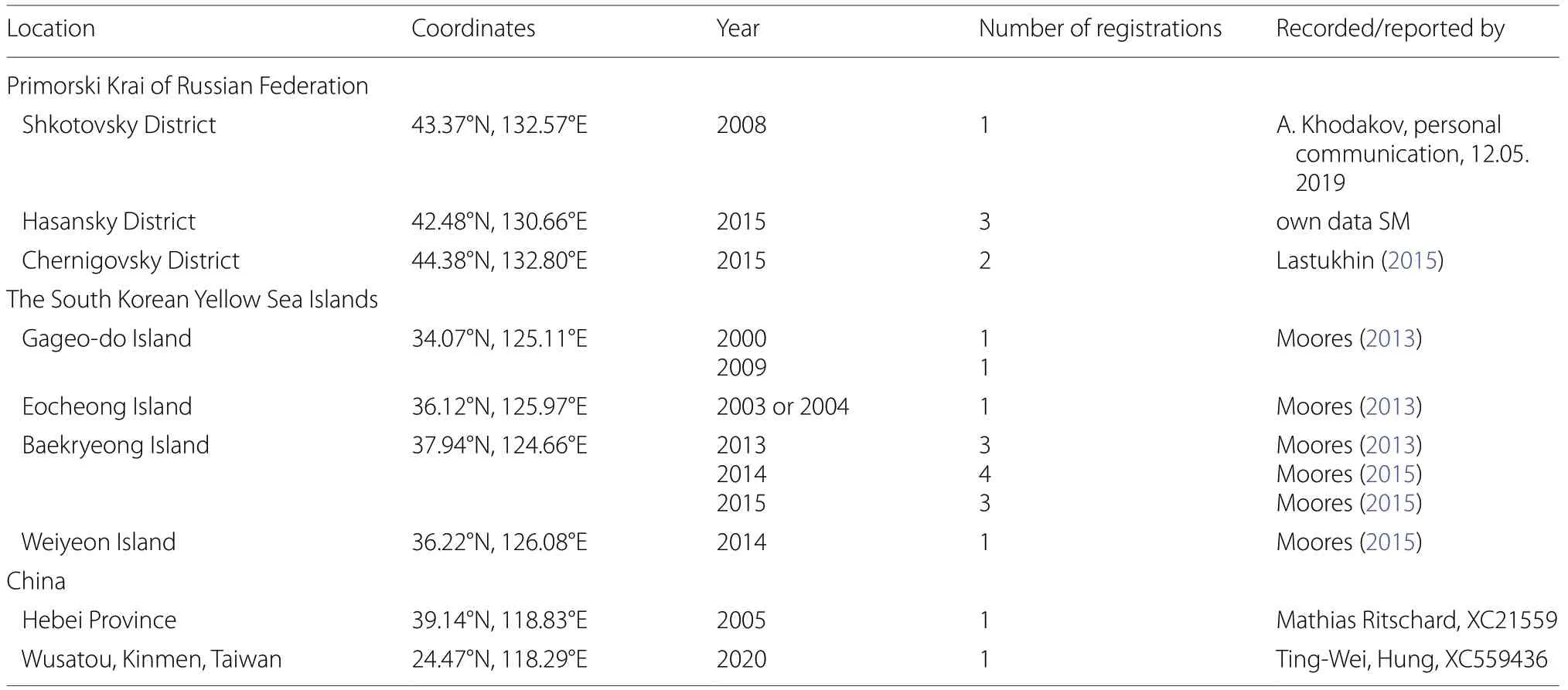
Table 1 Atypical cuckoo calls reported (recorded) from the wild
Female F1 originated from the nest of Western Greenish Warbler (Phylloscopus
(trochiloides
)viridanus
) found in Khakassia (Khakassia Nature Reserve, Maly Abakan:52.059°N, 89.597°E, Fig. 1a). This female was taken from the nest at the age of 15 days on 12 July, 2019. Female F2 was mist-netted as adult in Krasnoyarsk Krai (Enisei Ecological station ‘Mirnoe’ of Severtsov Institute of Ecology and Evolution: 62.289°N, 88.977°E, Fig. 1a) on 25 June, 2016. Female F3 was found as adult in Ekaterinburg(56.815°N, 60.537°E, Fig. 1a) on 3 June, 2017. This female was injured at that time. Female F1 was grey morph having rufous fringes of the feathers of the breast, and females F2 and F3 were rufous morph (Fig. 1b). All three females emitted bubbling calls that only female cuckoos produce. Besides, female F2 laid an egg in captivity.Collecting calls
Along with male-like vocalization of Oriental Cuckoo females (n
= 3 individuals) and enigmatic atypical cuckoo calls recorded in the wild (n
= 4 individuals), for comparison we analyzed also advertising calls of males of Oriental (n
= 10 individuals) and Common (n
= 10 individuals)Cuckoos. In total, recordings of 27 individuals were studied, and recordings of 26 of them were used in statistical analysis. For all but one individual, we analyzed 10 two-note calls per individual (only 5 atypical calls were recorded for one individual): i.e. totally 255 calls in 26 individuals.Three Oriental Cuckoo females were recorded in captivity. Among these individuals, only two were used in the statistical analysis (see below). In the analysis, we also used four enigmatic recordings of atypical cuckoo calls(Additional file 1: Table S1). Some of these recordings were previously classified as either ‘unknown cuckoo’(recorded in South Korea; Additional file 1: Table S1) or‘Common × Oriental Cuckoo hybrid’ (Lastukhin 2015;recorded in Primorski Krai of Russian Federation; Additional file 1: Table S1).
Most of the recordings of typical male advertisement call of both Common (n
= 10 males) and Oriental (n
= 10)Cuckoos were downloaded from Xeno-canto (xenocanto.org) except two recordings made by AO in Khingan State Nature Reserve (Amur Region of Russian Federation) in 2017 and 2018 (Additional file 1: Table S1). The geographic variation of advertisement call parameters had been found at least in Common Cuckoo (Wei et al.2015). Therefore, for the analysis we chose the recordings obtained in eastern Asia (Additional file 1: Table S1)to allow the correct comparison with the recordings of atypical cuckoo calls also obtained in this region.Call analysis
Following standard terminology, a ‘note’ is defined as the smallest building block visible as a continuous line on a spectrogram (Catchpole and Slater 2008). In Common and Oriental Cuckoo males, a single call (e.g. the wellknown ‘cu-coo’ of Common Cuckoo) usually but not always consists of two notes (Xia et al. 2016; Moskát et al.2017, 2018; Benedetti et al. 2018; Tryjanowski et al. 2018;Deng et al. 2019a). Only two-note calls were characteristic for three out of four analyzed recordings of enigmatic atypical vocalization (Fig. 2d-f), while one individual began to vocalize with three-note calls but switched then to two-note calls (Fig. 2g). For Oriental Cuckoo females, however, it was typical to alternate single-note,two-note and three-note male-like calls in a sequence(Fig. 2a, b). In the statistical analysis, we used two-note female calls only in order to compare them with other recordings. Only two out of three females were included in this analysis because the recordings of the third individual consisted mostly from single-note calls, and only three two-note calls were recorded from this female.Nevertheless, females’ single-note and three-note calls considered below briefly.
Besides, we described briefly the syntax of male-like and atypical call sequences. To do so, we analyzed 39 uninterrupted call sequences of females F1-F3, and six atypical call sequences recorded in the wild (four audioand two video-recordings; the latter were not included in the call analysis mentioned above).
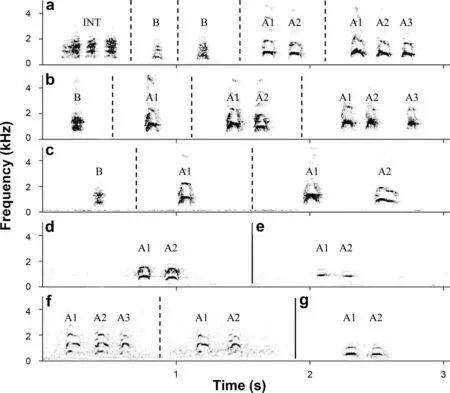
Fig. 2 Spectrograms of male-like a-c and atypical d-g vocalizations. The figure shows vocalization of a female F1 Oriental Cuckoo, b female F2 Oriental Cuckoo, c female F3 Oriental Cuckoo, d “unknown cuckoo” from South Korea, e “Common × Oriental Cuckoo hybrid” from Primorski Krai(Russia), as well as f two atypical calls recorded in Taiwan (China) and g atypical call recorded in Hebei Province (China). For A-notes (i.e. notes of both male-like and atypical calls), the numbers highlight position of note in the call (e.g. A2 means the second A-note). Variants of male-like and atypical calls: A1, single-note calls, A1-A2, two-note calls, and A1-A2-A3, three-note call. B, Kuk-call; INT, introductory call. Calls separated by dotted lines are from the same individual; all others are from different individuals
Sonograms were produced using Syrinx PC v. 2.6(John Burt, www.syrin xpc.com) with an FFT size = 512,and a window type = Hanning. First of all, we allocated each note of each call in a single wav file. The subsequent analysis was done in R software (R Core Team).We used ‘autodetect’ function in warbleR package in R(Araya-Salas and Smith-Vidaurre 2017) to detect signal (i.e. note) within each wav file across the frequency range of 0-5 kHz using threshold of 5%. We then used‘specan’ function (frequency range 0-5 kHz, threshold 5%) in warbleR package in R to automatically measure 21 acoustic parameters (listed in Additional file 2: Table S2)of each note of two-note call across all analyzed wav files(i.e. 42 parameters for each two-note call; Additional file 3: Table S3).
Analysis of video-recordings
To get insights into the mechanisms of call production,we video-recorded female F1 Oriental Cuckoo during emitting of either male-like vocalization (eight recordings) or bubbling call (one recording) using action camera Sony FDR-AS3000 with remote control. Female was recorded with a speed of 60 frame/s, from 8 to 20 February, 2020. Totally, we analyzed nine recordings ofX
± SE = 0.9 ± 0.5 min each (8.1 min in total), and these recordings were not used in the acoustic analysis described above. Brief inspection of the recordings revealed that female emitted the male-like call with the closed beak and inflated neck. It is well known that such features are characteristic for so-called ‘closed-mouth vocalization’, that is, the vocal behavior with the closed beak and simultaneous inflation of the esophagus or tracheal pouches; closed-mouth vocal behavior generally favors low-frequency sounds (Fletcher et al. 2004;Riede et al. 2004, 2016). Therefore, while analyzing videorecordings of female F1, we looked for the compliance between the bird posture and the produced sound by comparing the screenshots of a video-recording with the corresponding time points in the spectrogram. Totally,we analyzed in such a way seven notes from three different calls of female F1. We aimed to estimate the amount of the extension of the neck region. To do so, we selected six manually placed points in each successive video frame of each note. We then estimated the area of polygon between these points (in pixels) using Adobe Photoshop 21.1. The minimal value of this measurement of note, that was measured either immediately before or just after that note, was considered as 100% (separately for each note).Then, the area of the neck region in each video frame of note was estimated relative to this minimal value. We then compared the median relative volume of the neck region during the first and second parts of each note. To do so, we compared three screenshots that corresponded to the first half of a note with three screenshots from the second half.The data on female F1 Oriental Cuckoo were then compared with the video-recordings of calling males of the four Palearctic cuckoo species: Oriental Cuckoo (recordings ofn
= 21 of individuals), Common Cuckoo (n
= 24),Indian Cuckoo (C. micropterus
;n
= 13), and Lesser Cuckoo (C. poliocephalus
;n
= 22). The video-recordings of these individuals were downloaded from YouTube(Additional file 4: Table S4).Statistical analysis
In order to assess and visualize difference/similarity in two-note calls of the analyzed individuals in the multivariate acoustic space, we ran a principal component analysis (PCA) using factorextra package in R (Kassambara and Mundt 2017). In the analysis, we used measurements of each parameter of both first and second notes of a two-note call (i.e. 21 × 2 = 42 parameters per each call). Before the PCA was ran, we used REdaS package in R (Hatzinger et al. 2014; Maier 2015) to evaluate whether our data were suitable for this analysis. We found our data suitable, because Bartlett’s test of sphericity showed that the correlation matrix was significantly different from an identity matrix (Chi-square test: χ= 41,365.2,P
< 0.0001), and the Kaiser-Meyer-Olkin measure of sampling adequacy was 0.82 (above the minimum acceptable value of 0.5; Kaiser 1974) indicating that a large proportion of variance in the variables can be explained by the components.Two-sample Studentt
-tests were used to test differences between four groups: (1) Common Cuckoo males,(2) Oriental Cuckoo males, (3) two captive females, and(4) four individuals that produced atypical calls in the wild. Additionally, in the analysis of video-recordings we used dependent two-group Wilcoxon Signed Rank test in R software.Results
When and on what circumstances was male-like vocalization reported in captivity and in the wild
In captivity, we recorded male-like vocalization of females at the time of spring and autumn migratory activity (Fig. 3). This activity lasted ca. 2-2.5 months and displayed the cyclic pattern: a 5-10-day period of nightly activity alternated with a 1-7-day period of nightly inactivity. The exact dates of migratory activity and its duration differed among the three females. By contrast,bubbling call produced almost year-round several (up to three) times per week, but the most often during the breeding season (i.e. in May-July: up to several times per day; Additional file 5: Movie S1).
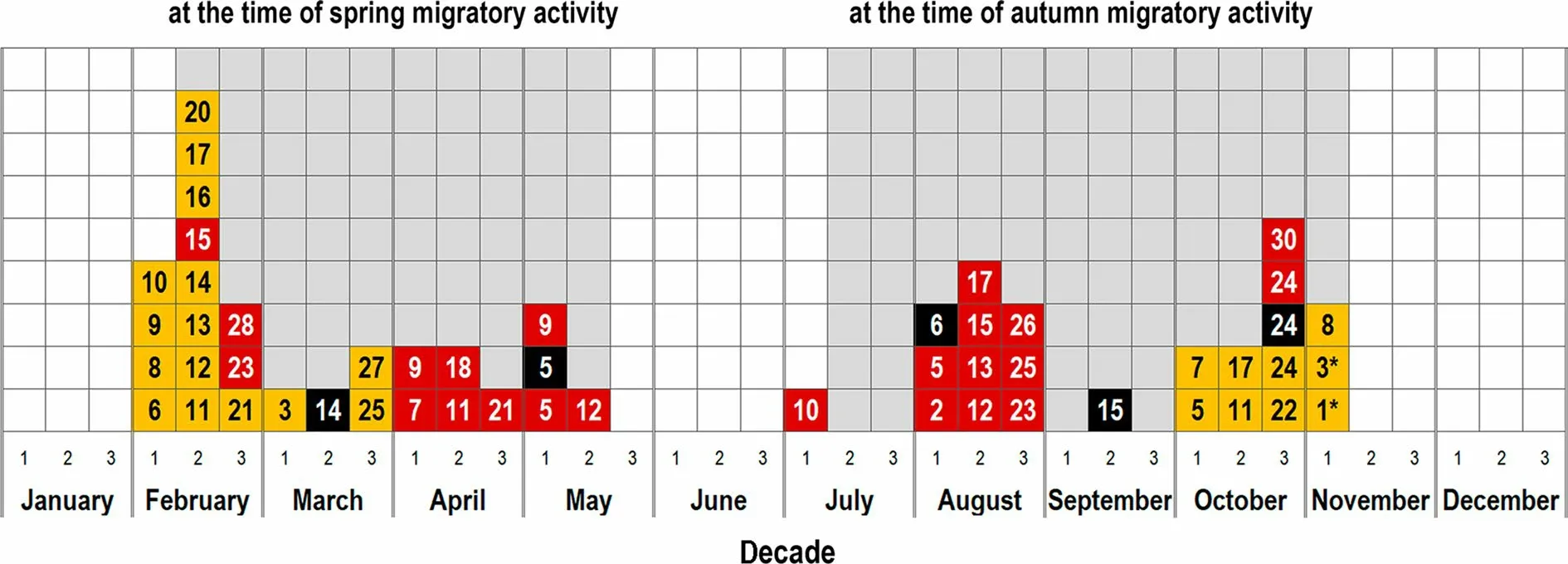
Fig. 3 Annual dynamic of male-like vocalization of females Oriental Cuckoo in captivity. Yellow color corresponds to female F1, red to F2, and black to F3. The numbers inside squares indicate a date when an individual called (dates when a given individual called in different years are marked with asterisks)

Fig. 4 Daily dynamic of calls of Oriental Cuckoo females in captivity. a Male-like calls, b Babbling calls. Yellow color corresponds to female F1, red to F2, and black to F3
At nights, females emitted bubbling call only (Fig. 4b).By contrast, male-like vocalization (Fig. 4a) produced in either morning (6:00 a.m.-01:00 p.m.) or evening(03:20-09:50 p.m.) hours, and much rarely in the night(10:00 p.m.-01:00 a.m.). While emitting male-like vocalization, females used the only perch in 60-70% of observations. This perch was lit by rising or setting sun, and the sky was readily seen from the perch (Additional file 6:Movie S2). Females other than calling one did not show any changes in behavior while the male-like vocalization was produced.
In the wild, the enigmatic atypical cuckoo calls have been mostly reported in May, that is, during spring migration. In Taiwan, China, calls were recorded on 18 May (Kinmen 2020). In South Korean Islands, the earliest date was 9 May (Gageo-do Island, 2000), and the latest dates were 25 May (Gageo-do Island, 2009)and 26-28 May (Baekryeong Island, 2013 and 2014)(Moores 2013, 2015). The dates for Primorski Krai of Russian Federation were: 17 May and 19 May, 2015 in Chernigovsky District (Lastukhin 2015), 30 May and 1-2 June, 2015 in Hasansky District (own data of SM),and 8 July, 2008 (recorded by A. Khodakov). In the wild,the atypical cuckoo calls were reported between 7:30 and 10:40 a.m. (n
= 3).Structure and organization of female male-like call sequences
Females emitted three variants of male-like call having either one, two or three notes (variants A1-A3; Fig. 2a-c;Additional file 7: Audio S1). It was typical to alternate these call variants in a single sequence, and most long call sequences contained both three-, two- and singlenote call variants. In females F1, F2 and F3, the length of recorded male-like call sequences varied from 8 to 93 s(mean ± SD = 56.1 ± 22.4 s,n
= 39), and the number of calls in sequence was from 4 to 79 (mean ± SD = 45 ± 19,n
= 39).Two main syntactic features were characteristic for male-like call sequences of females (Additional file 8: Figure S1). Firstly, females usually called with eventual variety, where every call variant occurred in a bout consisting of several repetitions of the same variant. Secondly, typically there were more notes in calls produced in the beginning of a sequence rather than in calls emitted at the end of that sequence.
Two other call types can be rarely found in bouts of male-like vocalization. The first was Kuk-call (after Cramp 1985; call type B in Fig. 2a-c) that appeared to be more typical for among-individual communication during feeding or resting, when another female flew closely to the calling one (own observation in captivity). The second was the introductory call consisted of 4-7 broadband notes (Fig. 2a). In female F1 sequences began often with an introductory call (Additional file 8: Figure S1).Besides, four additional short acoustic sequences (these sequences not shown in Additional file 8: Figure S1) consisted from either the only introductory call or the introductory call followed by 1-2 male-like calls.
The length of atypical cuckoo call sequences recorded in the wild varied from 5 to 60 s (mean ± SD = 31.3 ± 23.0 s,n
= 6), and the number of calls in sequence was from 5 to 43 (mean ± SD = 23 ± 17,n
= 6). It seemed possible, that at least some of these recordings were not made from the very beginning of call sequence. All but one of these sequences consisted from two-note calls only. In one recording, cuckoo began calling with three-note calls,and then switched to two-notes calls.Acoustic characteristics of calls
In this section, we considered two-notes calls only. Using PCA, we extracted the first two PCs (eigenvalues 1.7 and 3.9, respectively). PC1 explained 81.6% of the total variation, and PC2 explained a cumulative 99.0%. Peakedness of the spectrum of the first note, as well as peakedness of the spectrum and asymmetry of the spectrum of the second note showed the highest absolute correlation with PC1. The following parameter showed the strongest correlation with PC2: peakedness of the spectrum and asymmetry of the spectrum of both notes A1 and A2, and modulation index of note A2.
The PCA clearly separated the analyzed individuals into three groups. The first two groups were males of either Common or Oriental Cuckoos. The third group included both two captive females and four individuals that produced atypical call in the wild (Fig. 5). We thus hypothesized that atypical cuckoo calls might have been produced by females (presumably Oriental Cuckoo females because the male-like call is unknown in well studied Common Cuckoo).
These three groups appeared for us to differ clearly aurally. Two frequency characteristics of both female male-like call and atypical call seemed to best distinguish them from advertising call of males of both Common and Oriental Cuckoos. First, they located in higher frequency ranges: e.g. both notes had the highest values of third quartile and maximum dominant frequencies (Additional file 3: Table S3). Secondly, the fundamental frequency of these calls covered the wider range that was expressed in e.g. the highest values of interquartile frequency range and modulation index of the first note (Additional file 3:Table S3).
Besides, in males of both Common and Oriental Cuckoos, the majority of energy was in fundamental frequency and there was little detectable energy in higher harmonics. By contrasts, both female and atypical calls have prominent harmonics along with fundamental frequency(Fig. 2).
Motor patterns of calling in Oriental Cuckoo female
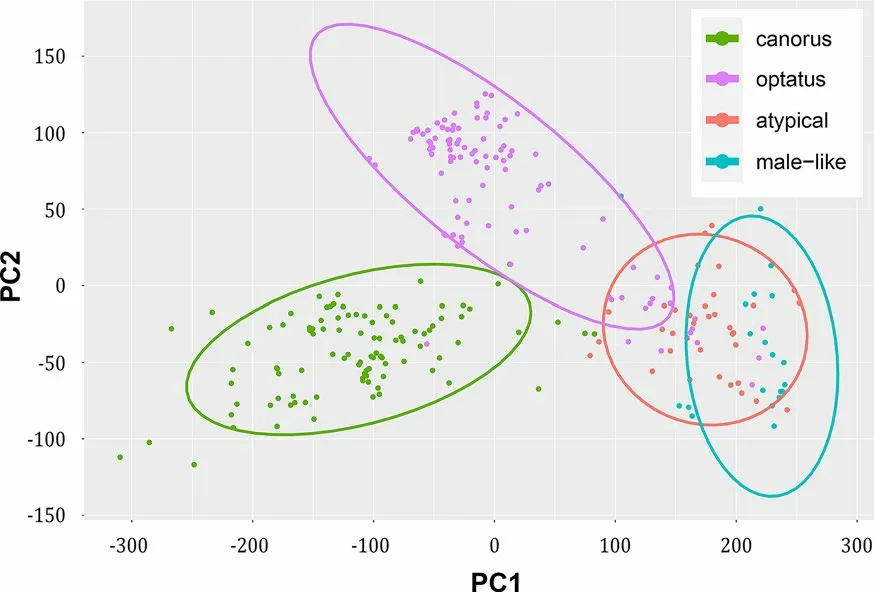
Fig. 5 PCA of 42 acoustic parameters of two-note calls of four groups of individuals
While producing male-like call, female Oriental Cuckoo adopted a striking posture slightly leaning forward and stretching the neck, beak closed and the ventral and dorsal neck regions considerably inflated (Additional file 8:Figure S2). The enlargement of the neck regions was obviously produced by airflow through the syrinx in the expiratory direction while the beak was closed. It was unexpected, however, that the dorsal neck region inflated more than the ventral region (Additional file 8: Figure S2b). In other respects, the posture of calling female did not have any specific features: as usually, female sat on a branch in a vertical position, wing tips laying on top of the tail, head slightly raised, and neck extended. Besides,at each note, the wings threw up pressing against the body.
The analysis of video-recordings revealed that the inflation of the neck region, assessed by its relative area,began to increase some 1-3 ms before the note are started to produce. The median of maximum increase was 118% (range 110-126%,n
= 7 notes) relative to minimal volume (100%) of the neck region between calls. The neck region did not completely collapse between the successive notes of a call (3 in Fig. 6b).We compared the median relative volume of the neck region during the first and second parts of a note. We found that the volume during the first half of a note exceeded that of the second half (dependent two-group Wilcoxon Signed Rank test:V
= 28,n
= 7,P
= 0.022). The median difference between median relative volume of the first and second halves of a note was 3%, range 1-6% (the median volume of the neck region during the first half of a note exceeded that of the second half in all analyzed notes). Therefore, at the beginning of each note the neck region attained the maximum volume, and then generally decreased towards the end of the note (the middle note in Fig. 6b, c is a bright example).
Fig. 6 Changes in the amount of neck region inflation while emitting male-like call. The figure illustrated a sequence of three notes of three-note male-like call of female F1. The amount of inflation was estimated by measuring the area of the neck region as indicate by red dotted lines a; the enlargement of the neck region was estimated as the percentages relative to minimal inflation, i.e. before and/or after calling b. Three notes are shown spectrographically in the bottom panel c
While producing bubbling and introductory calls,female did not adopt any specific posture. She did not inflate the neck region, and widely open the beak during calling (data not shown; Additional file 5: Movie S1).
Motor patterns of calling in males of the four Palearctic cuckoo species
Males of all species lower their wing tips below the tail level while producing advertising call (Additional file 8:Figure S3). This is the only feature of the calling postures of both Indian and Lesser Cuckoos, the later sometimes also leaning forward. It should be noted that Lesser Cuckoo produces calling not only from a perch, but often also in flight (Panov 1973; own observations in Primorski Krai of Russia, and in Hunan Province of China). While calling, both Indian and Lesser Cuckoos widely open the beak, while only slightly inflating the throat.
The most peculiar posture is characteristic for Common Cuckoo male. Male of this species lifts the tail up,spreads the tail feathers, and swings tail from side to side.While sitting on a steady perch (e.g. on a thick branch or a stone), male can prominently lean forward until the body took almost horizontal orientation (Additional file 8: Figure S3). Each call of a male consists of two different notes (Fig. 7g), and the motor pattern differs between the first and second note of a call. The first, more highpitched note coincides with the slight opening of the beak, and the neck stretching; the ventral and dorsal neck regions (‘throat sac’) inflates moderately (Fig. 7e). When the second note produces, the beak closed, the ‘throat sac’ inflated prominently, the head slightly leaned down,and the neck shortened (Fig. 7f).
The large ‘throat sac’ (i.e. both ventral and dorsal neck regions) extension is characteristic for the production of both (apparently identical) notes of Oriental Cuckoo call. The beak closes during call production (Fig. 7b) and opens slightly only immediately before calling (Fig. 7a).While calling, male bended the back becoming ‘roundshouldered’, shortened the neck, and sometimes also slightly raised the tail showing the undertail-coverts.Before emitting a note, male slightly raised his head, and then lowered it down simultaneously with calling.
Discussion
The origin of enigmatic male-like cuckoo calls: from whom and when?
Recently, cuckoo calls exhibited a combination of vocal traits of both Oriental Cuckoo and Common Cuckoo that were reported from East Asia (Moores 2013, 2015;Lastukhin 2015; own data SM). The calling individual was not identified exactly in any report because either the birds keep shy or automated audio recorders were used for call recording. The author who took photos of three calling individuals was not able to identify the species exactly (Moores 2015).
Here, we found that the enigmatic male-like calls recorded in the wild might have been produced by females (most probably—Oriental Cuckoo females). We concluded this based on the PCA analysis of 42 automatically measured acoustic parameters of cuckoo calls. The analysis showed that PC1 and PC2 coordinates clearly placed the two groups of individuals among each other’s:the first group consisted of individuals that produced enigmatic atypical calls in the wild, and the second group consisted of captive Oriental Cuckoo females that produced male-like calls. Both atypical and male-like calls have detectable energy in higher harmonics. By contrast,aberrant cuckoo calls (that were not considered in this paper) apparently lack higher harmonics in both Common (see Fig. 1 in Moskát et al. 2021) and Oriental (see recording of SM at Xeno-canto.org: XC609612) Cuckoos.
In captivity, Oriental Cuckoo females produced singleand three-note males-like calls along with two-note calls.In the wild, two- and three-note atypical male-like calls were recorded. In two- and three-note calls, either the first note (in two-notes call) or both the first and second notes (in three-note call) usually were more high-pitched(Additional file 3: Table S3) and louder. The same pattern have been found in ‘cu-coo’ and ‘cu-cu-coo’ calls of Common Cuckoo male (Payne 2005; Erritzøe et al. 2012). In this species, two- and three-note calls have been found to use in different context, and the ‘cu-cu-coo’ calls are associated with females emitting their bubbling call (Xia et al.2019).
In the wild, male-like vocalization have been only rarely reported, and only three out of six captive females produced this type of vocalization. Therefore, probably not all females emitted this call. However, the rarity of reports of the male-like call in the wild could be also due to the overall shyness of comparatively badly studied Oriental Cuckoo as compared to Common Cuckoo (Cramp 1985; Payne 2005; Erritzøe et al. 2012).
Our data suggested that one more reason could explain the rarity of female male-like vocalization. We supposed that females produce male-like call during migration only. The vocal behavior of parasitic cuckoos is almost completely unknown in this time (Cramp 1985; Payne 2005; Erritzøe et al. 2012). Several facts were in favor of our point of view. Firstly, we reported vocalization of captive females during their migratory activity only, starting from the age of several months,that is, from the age of the first migration. Secondly, the locations from which enigmatic calls were reported in the wild are located in a strip stretching from south to north along the coast of eastern Asia (Fig. 1a), that is,on the typical migration route of cuckoos. Thirdly, the calls were reported from the wild mostly in May, i.e. at the time of spring migration (in East China and Russian Far East, the Oriental Cuckoo migration occurs from middle April until late May (La Touche 1931-1934;Gluschenko et al. 2016). Fourthly, Oriental Cuckoo is a regular migrant but probably does not breed on South Korean Island Baekryeong from which enigmatic calls were reported (Moores 2007, 2013). Fifthly, the obviously migrating bird was observed calling in Hasansky District by the authors. Within three days, this individual stayed in a broken oak forest where leaf-warblers(host species) do not breed.
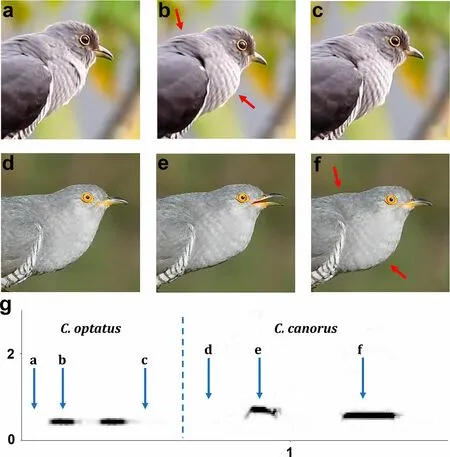
Fig. 7 The screenshots of calling postures of males of Oriental a-c and Common d-f Cuckoos. The spectrograms of calls are shown in the bottom panel (g, x-axis = time in s; y-axis = frequency in kHz), and the lowercase letters over blue arrows indicate the time position of corresponding still-frame images a-f. Common Cuckoo male produces the first note of a call with the open beak e, and the second note is produced while the beak is closed f. By contrast, Oriental Cuckoo male produces both notes with the closed beak b, and the beak is opening slightly only just before calling a. The enlargement of throat is characteristic for calling in both species (red arrows), and is the most apparent when the beak is closed. The video-recordings used for the analysis were downloaded from https://www.youtube.com/watch?v=4rq4J8i9QNo (Oriental Cuckoo) and https://www.youtube.com/watch?time_continue=11&v=wejl6 ukN8y c&featu re=emb_logo (Common Cuckoo)
We do not know yet, however, can females of otherCuculus
species produce male-like call? If the answer will be ‘yes’, then do the calls of different species differ?For instance, female bubbling call differs among species in frequency and number of notes (Kim et al. 2017b),although overall structures are similar with each other(Payne 2005; Erritzøe et al. 2012).Towards the mechanisms of call production in male and female cuckoos
During calling, Oriental Cuckoo male closes the beak and prominently inflates the throat. Common cuckoo male does the same while emitting the second note of its‘cu-coo’ call, but the first note produces with the slightly opened beak and only moderately inflated throat. Probably, esophagus inflation is the most likely mechanism that causes the throat (‘throat sac’) extension during calling in both cuckoos, as it was suggested or proved for Pectoral Sandpiper (Calidris melanotos
) (Riede et al. 2015),Ring Dove (Streptopelia risorii
) (Riede et al. 2004), and White-browed Coucal (Centropus superciliosus
) (Brumm and Goymann 2017). However, other mechanisms, e.g.the inflation of the tracheal or gular vocal sacs (reviewed in Dantzker 2015) are possible. The closed beak during calling allows for the inflation of the esophagus (and/or associated vocal sacs), and means that the bird vocalizes into the closed, inflated cavity with the sound radiating through the skin of the neck.The low fundamental frequency and absence of higher harmonics are characteristic for the advertising call of males of both Common and Oriental Cuckoos. Moreover, the frequency seemed to depend on the motor pattern. For example, both notes of Oriental Cuckoo call have the lowest peak frequency (mean 0.46 kHz; Additional file 3: Table S3), and males of this species appeared to inflate the throat the most. While emitting the second note of a call (mean peak frequency = 0.66 kHz; Additional file 3: Table S3), Common Cuckoo males also prominently inflate the throat (cf. Fig. 7b, f). At last, the first note of a call of Common Cuckoo that produces with the slightly open beak had the highest peak frequency(mean 0.81 kHz; Additional file 3: Table S3).
It is well known that all the aforementioned features(except that of the first note of Common Cuckoo call) are the most important characteristics of the closed-mouth vocalization: (1) low fundamental frequency, (2) absence of higher harmonics, (3) inflation of the ‘throat sac’, and(4) closed beak (Fletcher et al. 2004; Riede et al. 2004,2016). We thus conclude that both the advertisement call of Oriental Cuckoo and the second note of Common Cuckoo call appear to be true closed-mouth vocalizations. By contrast, advertising vocalization of both Indian and Lesser Cuckoos is open-mouth as the beak widely opens while calling.
At the first glance, male-like call of Oriental Cuckoo female was apparently the closed-mouth vocalization as well. Indeed, this sound was prominently lower than the much more typical for female bubbling call. In Common Cuckoo, bubbling call had frequency between 1 and 2.5 kHz (Deng et al. 2019b; Moskát and Hauber 2019),and in Oriental Cuckoo the corresponding values were 1.2 and 2.3 kHz (Kim et al. 2017b). By contrast, male-like call had the peak frequency below 1 kHz (means of the first and second notes were 0.82 and 0.74 kHz, respectively; Additional file 3: Table S3). The peculiar calling posture of Oriental Cuckoo female with inflated neck and closed beak that we describe in this study could play a role in lowering the voice of female, as it was found for closed-mouth vocalizations in other species.
Two features of female male-like call, however, contradicted treating of this call as the true closed-mouth vocalization. Firstly, male-like call had the prominent spectrum as the higher harmonics were readily seen on the spectrograms (Figs. 2a-g, 6c). By contrast, the typical closed-mouth vocalization had only one major resonance frequency, that is, the fundamental frequency of the signal. The absence of higher harmonics may result from the low-pass filter characteristic of the esophagus wall and overlying skin (Fletcher et al. 2004).
Secondly, as birds typically (but not exclusively) vocalize during expiration (Wild et al. 1998), in closed-mouth vocalization maximum inflation occurs at the end of the sound because the enlargement of the ‘throat sac’ is produced by airflow through the syrinx in the expiratory direction while the beak and nares are closed (Riede et al.2004). By contrast, in female Oriental Cuckoo maximum neck extension was observed at the beginning of a note,and the amount of neck inflation generally decreased towards the end of the note (Fig. 6b). This pattern is characteristic for open-mouth vocal behavior. For example, in Radde’s Warbler (Phylloscopus schwarzi
, an open-mouth singer) at the beginning of each song syllable the ‘throat sac’ attains the maximum volume, while at the end of each song syllable the ‘throat sac’ volume reduces to minimal value (Opaev and Shishkina 2020).Therefore, unlike male advertising call, male-like call of female may not be typical closed-mouth vocalization in Oriental Cuckoo. It might be that the latter call produces without complete rerouting of the air into an inflatable structure (e.g. with a narrow beak gape and/or open nares). The vocalizations with an almost closed beak are well known in birds (Reichard and Welkin 2015),but should be treated as open-mouth vocalization (Riede et al. 2016). Nevertheless, the Oriental Cuckoo female vocalization appeared to be rather unusual among birds as it combined features of both closed-mouth (almost or completely closed beak, inflated neck region, and low frequency of the sound) and open-mouth (prominent harmonic spectrum and the pattern of dynamic of the amount of neck extension during calling) vocalizations.
It should be noted that during calling the dorsal neck region of female inflated more than the ventral neck region (Additional file 8: Figure S2b). Similarly, in males of both Common and Oriental Cuckoos the dorsal neck region inflated noticeably (Fig. 7b, f). It seems unusual,because in birds the dorsal neck region typically does not extend during vocalization. We do not know yet what particular structure has inflated in the dorsal part of the neck of Oriental Cuckoo female.
Functional significance of cuckoo calls
The possible functional significance of female male-like vocalization is puzzling. To date, only two cuckoo calls have been studied in details: male advertising call and female bubbling call. Both call types are used during the breeding season and have multiple functions. We consider these functions below in order to hypothesize what the possible function of female male-like call might be.
First of all, male advertising call and female bubbling call are used in spacing behavior. In the breeding grounds, both males and females have home ranges of roughly similar sizes that are defended to some extent from same-sex rivals (Nakamura and Miyazawa 1997;Honza et al. 2002; Vogl et al. 2004; Nakamura et al. 2005;Payne 2005; Erritzøe et al. 2012; Williams et al. 2016;Moskát and Hauber 2019; Moskát et al. 2019; Yun et al.2019). To repel same-sex rivals, males use advertising call (Moskát et al. 2017, 2018; Tryjanowski et al. 2018)while females emit bubbling call (Davies 2015; Moskát and Hauber 2019). In females, aggression towards other females appear to be important as they compete strongly for the nests of host-species (Nakamura and Miyazawa 1997; but see Vogl et al. 2004). We suppose that malelike call does not play a role in spacing behavior as it did not use during breeding season when home ranges are monopolized most.
Cuckoos might also use their calls to communicate individuality. Indeed, advertising calls of males have been found to differ among individuals (Jung et al. 2014; Li et al. 2017; Zsebök et al. 2017). However, within-season consistency of individually distinctive vocal characteristics appeared to be small (Deng et al. 2019a), and this call is thus unlikely to reveal certain individual. Bubbling calls also vary greatly in each individual (Deng et al. 2019b).Similarly, it seems unlikely that females use male-like calls for individual recognition. The latter vocalization was very rare, found in only a fraction of individuals, and did not use in the breeding season when individual recognition appeared to be the most important.
It is generally assumed that male cuckoos use advertising call for mate attraction (Payne 2005). However, recently it was found that females did not exhibit response to playback of both typical and aberrant male calls (Xia et al. 2019; Moskát et al. 2021). Similarly, malelike call did not elicit female response in captivity (own data of SM). By contrast, the strong response to female bubbling call has been found both in males (Kim et al.2017b; Lee et al. 2019; Moskát and Hauber 2019; Xia et al. 2019) and females (Lee et al. 2019; Moskát and Hauber 2019), including captive females (own data of SM).Because males are usually attracted to advertising calls of unfamiliar individuals (Moskát et al. 2017, 2018), one can assume that they would respond to female male-like vocalization as well.
Probably, the vocalization could facilitate either prebreeding intersexual interactions or resting group gathering at stopover sites. Although cuckoos usually encounter alone on migration, it is known that they can migrate in loose groups, and sometimes also forage in groups in wintering grounds and at stopover sites (Cramp 1985;Payne 2005; Erritzøe et al. 2012).
Recently, it was found that both male and female cuckoo calls influence both current host species (York and Davies 2017) and rare suitable and unsuitable hosts(e.g. swallows, starlings, thrushes and some corvids;Lyon and Gilbert 2013; Tryjanowski et al. 2018). A group mobbing is normally observed in the presence of calling cuckoo. It should be noted that some host species are able to distinguish between Common Cuckoo and a predator (e.g. Sparrowhawk Accipiter nisus) because their acoustic responses and behaviors differ depending on the presence of either species (Welbergen and Davies 2008;Yu et al. 2017a, b, 2019, 2020). The mobbing, in turn,can be useful for cuckoos, making it easier to find host nests (Marton et al. 2019). We do not know yet whether host species react to cuckoo outside the breeding season. However, it seems unlikely that the mobbing has any functional significance for cuckoos in this time.
Conclusions
Our results might be interesting from the evolutionary point of view as well. We found that Oriental Cuckoo females can produce male-like calls outside the reproductive season. It might be that male-like vocal behavior has not any function. It is possible that the male-like calling could be just a seasonal manifestation of the female singing lost in the evolution from non-parasitic to parasitic cuckoos. Female singing and duetting are indeed found in several non-parasitic cuckoo species (Tobias et al. 2016; Brumm and Goymann 2017).
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1186/s40657-021-00246-9.
Additional file 1: Table S1.
Recordings of analyzed individuals, including source of recordings and origin of females recorded in captivity.Additional file 2: Table S2.
List of acoustic parameters (21) measured by specan function of warbleR package and used to compare call structure.Additional file 3: Table S3.
Univariate statistics for measurements of twonotes calls of Oriental Cuckoo (OC) and Common Cuckoo (CC).Additional file 4: Table S4.
YouTube video-recordings of males of fourCuculus
species used in the analysis.Additional file 5: Movie S1.
Female F1 emitting bubbling call during migratory activity (Video-recording 13 July 2020, 11:17 p.m.).Additional file 6: Movie S2.
Female F1 emitting male-like calls (Videorecording 20 February 2020, 08:57 a.m.)Additional file 7: Audio S1.
Male-like calls of Female F2 (Audio-recording 15 February 2020, 00:21 p.m.)Additional file 8: Figure S1.
Schematic representation of 39 uninterrupted male-like call sequences of three Oriental Cuckoo females recoded in captivity, and of 6 atypical call sequences (all produced by different individuals) recorded in the wild.Figure S2.
Calling females Oriental Cuckoo.Figure S3.
The screenshots of calling postures of cuckoo males.Acknowledgements
The authors thank A.A. Meshcheryagin for his help in recording of captive females; Y.N. Gluschenko, S.G. Surmach and G.N. Bachurin for their development of interest to enigmatic cuckoo vocalization and for informing the authors about reports of such vocalization in Russian Far East; O.V. Bourski and M.S. Galisheva for providing cuckoos to be held in captivity; A.A. Lastukhin for providing cuckoo recording; authors who uploaded their audio- and videorecordings of cuckoos to Internet collections and made them freely available.
Authors’ contributions
SM recorded cuckoo calls in captivity and collected information about atypical cuckoo vocalization in the wild, AO carried out the analysis, SM and AO wrote the manuscript. Both authors have read and approved the final manuscript.
Funding
This study was performed within the frameworks of state contract with the Institute of Plant and Animal Ecology, Ural Branch, Russian Academy of Sciences (project number 18-9-4-22), and as a part of Program of the Russian Academy of Sciences 2013-2020, No. AAAA-A18-118042690110-1 [0109-2019-0003] ‘Ecological and evolutionary aspects of animal behavior and communication’. Call analysis was supported by the Russian Science Foundation (grant number 20-14-00058).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files. Examples of male-like vocalization and bubbling call of Oriental Cuckoo females are available at Xeno-canto (F1: XC624308,XC624310, XC624312, XC624316, XC624322; F2: XC624326, XC624329,XC624365; F3: XC624337, XC624350, XC624361). Other audio/video recordings used in the study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
All applicable international, national, and institutional guidelines for the care and use of animals were followed. This is an observational study. All work complied in accordance with the Federal law of Russian Federation No. 498-Ф3 ‘On responsible treatment of animals’. The Research Bioethics Committee of the Institute of Plant and Animal Ecology has confirmed that no ethical approval is required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author details
Institute of Plant and Animal Ecology, Ural Branch of the Russian Academy of Sciences, Ekaterinburg, Russia.A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow, Russia.
Received: 24 September 2020 Accepted: 24 February 2021
杂志排行
Avian Research的其它文章
- Vineyards, but not cities, are associated with lower presence of a generalist bird,the Common Blackbird (Turdus merula),in Western France
- Breeding biology of a relictual Maghreb Magpie (Pica mauritanica) population in Tunisia
- Habitat use and space preferences of Eurasian Bullfinches (Pyrrhula pyrrhula)in northwestern Iberia throughout the year
- High levels of genetic diversity and an absence of genetic structure among breeding populations of the endangered Rufous-backed Bunting in Inner Mongolia, China: implications for conservation
- Nest site selection and reproductive parameters of the threatened Atlantic Royal Flycatcher (Onychorhynchus swainsoni) and their significance for conservation
- The Grey-backed Shrike parents adopt brood survival strategy in both the egg and nestling phases
