Bousmekines A-E, New Alkaloids from Two Bousigonia Species: B.angustifolia and B.mekongensis
2021-04-10ZongQingHuoQianZhaoWenTaoZhuXiaoJiangHaoYuZhang
Zong-Qing Huo·Qian Zhao·Wen-Tao Zhu·Xiao-Jiang Hao·Yu Zhang
Abstract Four new monoterpenoid indole alkaloids, bousmekines A-D (1— 4), and one new pyranopyridine alkaloid, bousmekine E (5), were isolated from the twigs and leaves of Bousigonia angustifolia and Bousigonia mekongensis.Their structures including absolute confi gurations were elucidated by a combination of MS, NMR, ECD calculation, and single-crystal X-ray diffraction analysis.Compound 2 was an eburnea-type MIAs characterized by a rare chlorine atom while 5 possessed a novel pyranopyridine moiety.Their cytotoxicities against several human cancer cell lines were evaluated and compound 1 exhibited significant cytotoxicity with IC 50 values of 0.8—7.4 μM.
Keywords Bousigonia· B.angustifolia · B.mekongensis · Monoterpenoid indole alkaloids · Bousmekines A-E
1 Introduction
Monoterpenoid indole alkaloids (MIAs), constituting a large family of secondary metabolites, are mainly distributed in plants of Apocynaceae family [1— 3].MIAs exhibited intriguing biological effects, such as anticancer [4], antibacterial [5], and lysosome generating activities [6].Due to their structural complexity and biological diversity, MIAs have long been attractive objects by chemists and pharmacologists [1].The genusBousigonia(Apocynaceae family) comprises only two species,B.angustifolia and B.mekongensis, both of them are distributed in southwestern China [7].Previous chemical studies on this genus have resulted in the identification of more than 100 alkaloids, primarily consisted of aspidosperma, eburnea, and aspidosperma-eburnea type MIAs [8— 12].In order to further investigation on novel and bioactive MIAs and proffer new vision into the constitutions of the twoBousigoniaspecies, their alkaloidal extracts were investigated and four new MIAs (1— 4) and one new pyranopyridine alkaloid (5) was isolated.This paper herein describes the isolation, structural elucidation and the cytotoxicities of the isolates (Fig.1).
2 Results and Discussion
2.1 Structure Elucidation of the Compounds
Bousmekine A (1) was obtained as an optically active colorless solid,- 22 (c0.1, MeOH).Its molecular formula,C25H30N2O4, was established by HRESIMS ion atm/z423.2281 [M + H] + (calcd 423.2278), corresponding to nine degrees of unsaturation.IR absorptions implied the presence of carbonyl (1676 cm-1) function.
Detailed analysis of its NMR data (Tables 1 and 2) indicated that compound 1 had a high similarity with that of 3α-acetonyltabersonine [13], except for the presence of an additional methoxy (δH3.81;δC55.5) and a nonprotonated quaternary carbon (δC 160.0).The key HMBC correlations of OMe (δH3.81), H-9 (δH7.17, d,J= 8.4 Hz) and H-10 (δH6.44, dd,J= 8.4, 2.4 Hz) to C-11 (δC160.0) indicated that the methoxy group was located at C-11 (Fig.2), and thus established the planar structure of 1 as 11-methoxy derivative of 3α-acetonyltabersonine.2D NMR spectra (HSQC, HMBC, and 1 H- 1 H COSY) confi rmed the other parts of 1 were the same as that of 3α-acetonyltabersonine (Fig.3).The ROESY correlation of H-3/H-21, and of H-21/H-19a indicated that these protons were co-facial and arbitrarily assigned asα-oriented.Therefore, the relative confi gurations of 1 was assigned as (3S*, 7R*, 20R*, 21S*)- 1.The ECD calculation results for (3S, 7R, 20R, 21S)- 1 matched well with its experimental ECD spectrum fi nally established the absolute confi guration of 1 (Fig.4).
Bousmekine B (2) was obtained as an optically active colorless crystal,= - 98 (c0.1, MeOH).Its molecular formula was determined to be C19H21N2OCl by HRESIMS ion atm/z329.1414 [M + H]+(calcd 329.1415), suggesting 10 unsaturation degrees.The13C and DEPT spectra suggested that 2 possessed 19 carbons including one methyl, fi ve methylenes, eight methines, and fi ve nonprotonated carbons.The NMR data demonstrated that 2 was an eburneantype alkaloid similar as meloyunine [14], with exception of an additional methine (δH3.92;δC54.9).The abundance ration of 329.1414/331.1388 (3:1) in HRESIMS spectrum indicated the presence of a chlorine atom in 2 (Figure S16, Supporting Information).HMBC correlation of H-14 (δH3.92) to C-20 (δC44.3) established that the chlorine atom was located at C-14.Finally, the planar structure and relative confi guration of 2 was established by 2D NMR spectra (Fig.2), and its absolute confi guration was determined by X-ray single crystal diffraction analysis with Flack parameter = 0.104 (12) (Fig.5).
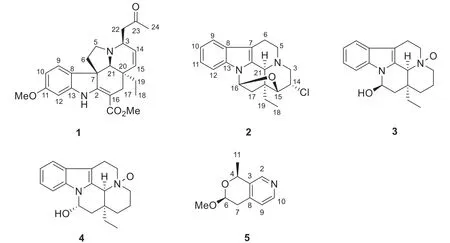
Fig.1 Structures of compounds 1— 5

Table 1 1 H NMR spectroscopic data for compounds 1-5 (δin ppm,Jin Hz)
Bousmekine C (3) had a molecular formula of C19H24N2O2, as established by HRESIMS ion atm/z313.1912 [M + H] + (calcd 313.1911), corresponding to nine degrees of unsaturation.Detailed analysis of its NMR spectra demonstrated that 3 was structurally related to eburnamine [15].The deshielded of C-3 (δC58.7, Δδ+ 13.7), C-5 (δC70.5, Δδ+ 18.9), and C-21 (δC58.7, Δδ+ 13.2) in 3 inferred that 3 was theN-4-oxide derivative of eburnamine [16].2D NMR spectra (HSQC, HMBC,1 H- 1 H COSY, and ROESY) further confi rmed the structure of 3 (Fig.2).The ROESY correlations of H-16/H-19a, H-19a/H-21 indicated these protons took the same orientation and thus established the relative confi guration of (16R*, 20S*, 21S*)- 3.Finally, the absolute confi guration of (16R, 20S, 21S)- 3 was assigned by the negative Cotton effects at 220 nm [15].
Bousmekine D (4) had the same molecular formula with 3, as established by HRESIMS analysis atm/z313.1908 [M + H]+(calcd 313.1911).Compared its NMR data with 3 indicated that both compounds had the same skeleton.The coupling constant of H-16 (br d,J= 4.8 Hz) demonstrated that H-16 tookβorientation and thus compound 4 was assigned as the C-16 epimer of 3.Further 2D NMR spectra(HSQC, HMBC, 1 H- 1 H COSY, and ROESY) and ECD data analysis confi rmed the structure of 4, as shown in Fig.1.

Table 2 13 C NMR spectroscopic data for compounds 1-5 (δin ppm)
Bousmekine E (5) was obtained as an optically active colorless solid,- 66 (c0.1, MeOH).Its molecular formula,C10H13NO2, was established by HRESIMS analysis atm/z180.1018 [M + H]+(calcd 180.1019), corresponding to fi ve degrees of unsaturation.The13C and DEPT spectra showed that 5 comprises 10 carbons, including two methyls, one methylene, fi ve methines and two nonprotonated carbons.
The1H-1H COSY spectrum gave three structural fragments: a (C-4 to C-11),b (C-6 to C-7), and c (C-9 to C-10).HMBC correlations of H-6 (δH4.78, dd,J= 8.4, 3.0 Hz) to C-4 (δC70.7), and of H-11 (δH1.59, d,J= 6.6) and H-7a (δH2.78, dd,J= 16.7, 8.4 Hz) to C-3 (δC135.9) established the linkage of a and b, which formed a pyran moiety.Meanwhile, HMBC correlations of H-2 (δH8.40, s) to C-4, C-8 (δC142.3), and C-10 (δC148.4), and of H-9 (δH7.10, d,J= 4.8 Hz) to C-3 and C-7 (δC34.7) fi nally established the connectivities of b,c, and the nitrogen atom.Thus, the planar structure of 5 was fi nally established as a novel pyranopyridine alkaloid (Fig.2).
The ROESY correlation of H-4 and H-6 indicated that both protons took the same orientation and were arbitrarily designated asα-orientation, thus its relative confi guration was determined as (4S*,6S*)- 5 (Fig.3).To allocate the absolute confi guration of 5, the optical rotation (OR) value of (4S,6S)- 5 was calculated by using density functional theory (DFT) method.The calculated [α] value for (4S,6S)- 5 was -67, which is close to its experimental value (—66), and thus the absolute confi guration of 5 was assigned as (4S,6S).
2.2 Cytotoxic Activity
Five new compounds were evaluated for their cytotoxicities against fi ve human cancer cell lines: HL-60, SW-480, A549, MCF-7, and SMMC-7721 using the MTT method with cisplatin (DDP) and paclitaxel (PXL) as positive controls [17].Among them, only compound 1 exhibited significant cytotoxicity with IC50values of 0.8—7.4 μM (Table 3).
3 Experimental
3.1 General Experimental Procedures
NMR spectra were measured via Bruker AV-500 and Avance III 600 MHz, TMS was used as an internal standard.IR spectra were surveyed on a Bio-Rad FTS-135 with KBr pellets.A JASCO P-1020 digital polarimeter was used to get optical rotations, while the ECD spectral data were measured by an Applied Photophysics Chirascan Spectrometer.HRESIMS and ESI were surveyed on Aglient 1290 UPLC/6540 Q-TOF spectrometer.Silica gel (80—100 and 100—200 mesh, Qingdao Marine Chemical Inc., China), silica gel H (10—40 μm, Qingdao Puke Chemical Inc., China), and Sephadex LH-20 (40—70 μm, Amersham Pharmacia Biotech AB), were used for column chromatography.Semi-preparative HPLC was carried out using a Shimadzu LC-20AT liquid chromatograph equipped with a YMC Triart C18 ExRS (5 μm; 10 × 250 mm) reversed-phase column.
3.2 Plant Material
The twigs and leaves ofB.angustifoliaandB.mekongensiswere collected in Xishuangbanna, Yunnan Province, People’s Republic of China, in July 2018.The samples were identified by Mr.Yu Chen, Kunming Botanical Garden.A specimen (no.ZY20180623 and no.ZY20180624) was deposited at State Key Laboratory of Phytochemistry and Plant Resource in West China, Kunming Institute of Botany, Chinese Academy of Science (CAS).
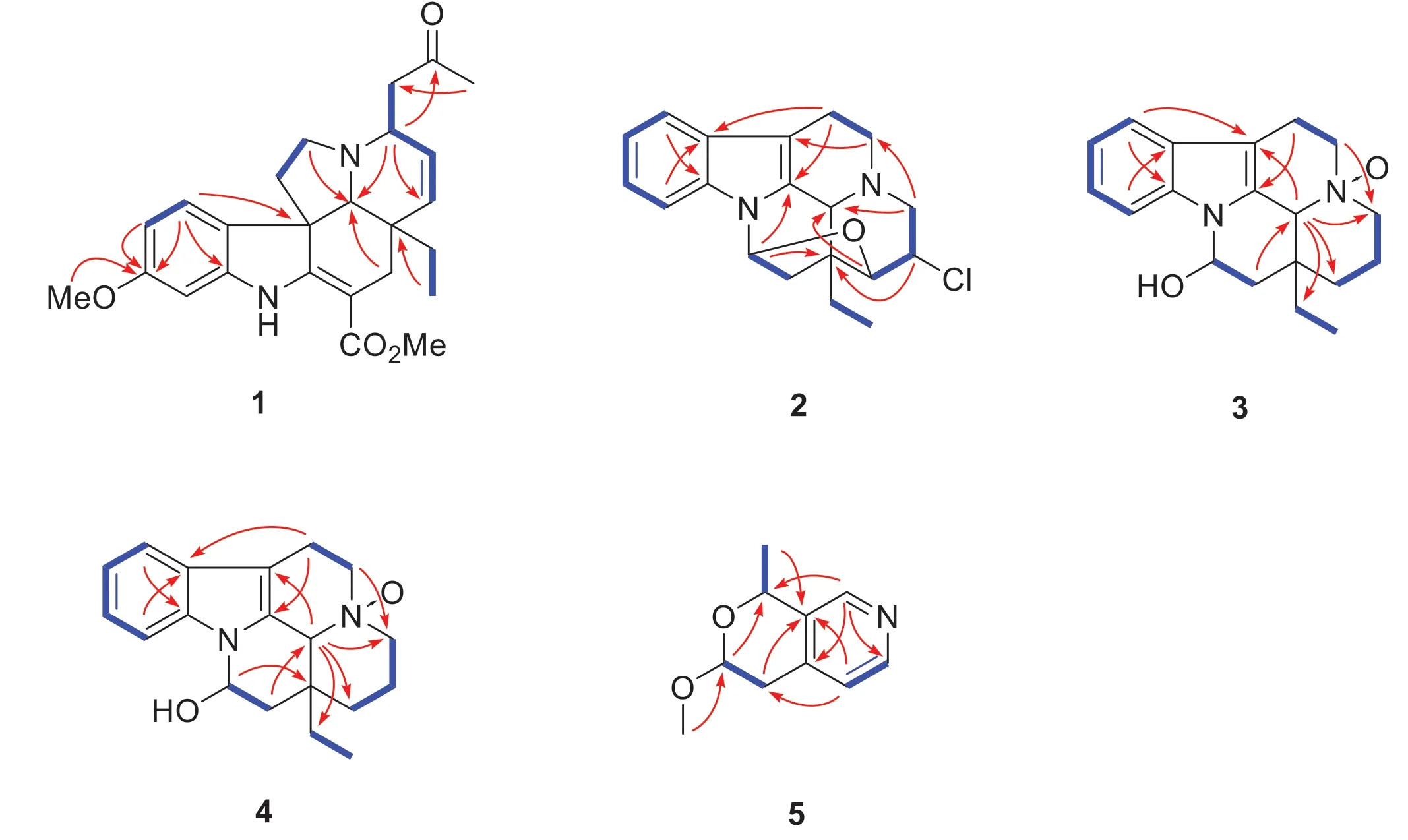
Fig.2 Key HMBC (arrow) and 1H- 1H COSY (bold) correlations of compounds 1— 5

Fig.3 Key ROESY correlations of compounds 1,3, and 5
3.3 Extraction and Isolation
The dried twigs and leaves ofB.mekongensis(30.5 kg) were powdered and extracted three times with methanol.The extract was adjusted to 2—3 with hydrochloric acid (5%) and then extracted three times with petroleum ether.The water fraction was basified to pH 9—10 with sodium hydroxide (10%), then extracted with chloroform to get the crude alkaloids.The crude alkaloids (530 g) were separated on a silica gel column (100—200 mesh), and eluted with a gradient of CHCl3 -MeOH (40:1 → 1:1) to yield fi ve fractions (A-E).Fraction D (19.1 g) was purified by a RP-18 column (MeOH/H2O, 50:50 → 100:0, v/v) to give four subfractions (DI-DIV).Subfraction DII (5.2 g) was purified by a RP-18 column (MeOH/H2O, 50:50 → 100:0, v/v) and followed by semipreparative HPLC with MeOH/H2O (75:25, 0.1% Et2NH) to give 1 (8.0 mg,tR33.0 min).Subfraction DIV (4.9 g) was purified by a RP-18 column (MeOH/H2O, 50:50 → 100:0, v/v) and followed by semipreparative HPLC with MeOH/H2O (80:20, 0.1% Et2NH) to afford 2 (12.0 mg,tR23.5 min).
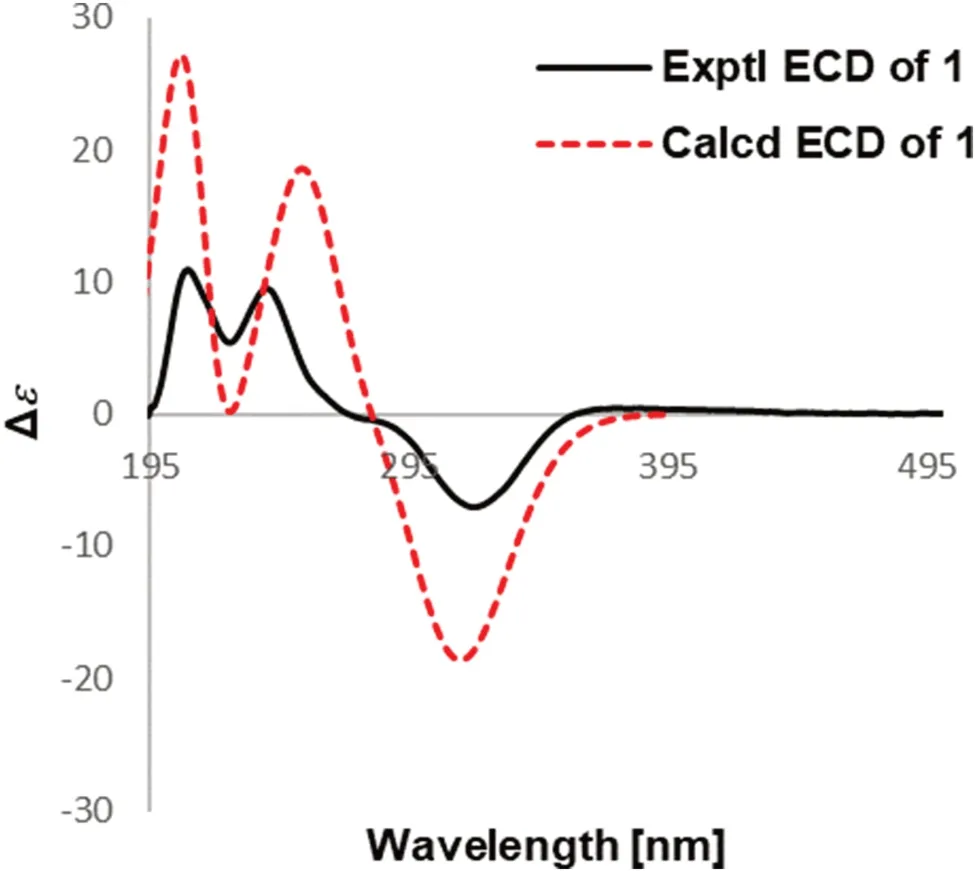
Fig.4 Experimental and calculated ECD of 1

Fig.5 X-ray crystal structure of 2

Table 3 Cytotoxicity of compound 1 (IC 50 a ,μM)
The dried twigs and leaves ofB.angustifolia(57.5 kg) were powdered and extracted three times with methanol.The crude alkaloids (730 g) were obtained according to the above method and were separated on a silica gel column (100—200 mesh) by CHCl3-MeOH (40:1 → 1:1) to yield 6 fractions (A-F).Fraction D (30.0 g) was purified by a RP-18 column (MeOH/H2O, 40:60 → 100:0, v/v) to give four subfractions (DI-DV).Subfraction DII (8.2 g) was purified by a RP-18 column (MeOH/H2O, 50:50 → 100:0, v/v) and followed by semipreparative HPLC with MeOH/H2O (50:50, 0.1% Et2NH) to give 3 (14.4 mg,tR24.0 min) and 4 (2.5 mg,tR32.0 min).Subfraction DIV (6.9 g) was purified by a RP-18 column (MeOH/H2O, 50:50 → 100:0, v/v) and followed by semipreparative HPLC with MeOH/H2O (62:38, 0.1% Et2NH) to afford 5 (2.4 mg,tR28.5 min).
3.4 Bousmekine A (1)
Bousmekine A (1): colorless solid;- 22 (c0.1, MeOH); UV (MeOH)λmax(logε): 327 (3.91) nm; ECD (0.0006 M, MeOH) λmax(Δε) 210 (+ 5.33), 226 (+ 2.64) 241 (+ 4.63), 320 (- 3.43); IR (KBr)vmax3441, 2963, 1676, 1617, 1500, 1438, 1263, 1113 cm -1 ; 1 H and 13 C NMR data (CDCl3, 600 and 150 MHz) see Tables 1 and 2; HRESIMSm/z423.2281 [M + H] + (calcd for C25H31N2O4, 423.2278).
3.5 Bousmekine B (2)
Bousmekine B (2): colorless crystal;- 98 (c0.1, MeOH); UV (MeOH)λmax(logε): 230 (4.27), 272 (3.65) nm; ECD (0.0005 M, MeOH) λmax(Δε) 207 (+ 2.60), 231 (- 17.46); IR (KBr)vmax3450, 2924, 1658, 1487, 1454, 1334, 1295, 1046 cm-1;1H and13C NMR data (CDCl3, 600 and 150 MHz) see Tables 1 and 2; HRESIMSm/z329.1414 [M + H] + (calcd for C19H21N2OCl, 329.1415).
3.6 Bousmekine C (3)
Bousmekine C (3): colorless solid;- 45 (c0.1, MeOH); UV (MeOH)λmax(logε): 223 (3.90) nm; ECD (0.0008 M, MeOH) λmax(Δε) 200 (+ 1.15), 221 (- 1.47), 234 (+ 1.24); IR (KBr)vmax3450, 2923, 1658, 1632, 1380, 1326, 1296 1011 cm -1 ; 1 H and 13 C NMR data (CD3OD, 600 and 150 MHz) see Tables 1 and 2; HRESIMSm/z313.1912 [M + H]+(calcd for C19H25N2O2, 313.1911).
3.7 Bousmekine D (4)
Bousmekine D (4): colorless solid;+ 18 (c0.1, MeOH); UV (MeOH) λmax(logε): 224 (4.33) nm; ECD (0.0004 M, MeOH) λmax(Δε) 223 (+ 9.72); IR (KBr)vmax3418, 2922, 2852, 1724, 1659, 1459, 1384, 1207, 1056 cm -1 ; 1 H and 13 C NMR data (CD3OD, 600 and 150 MHz) see Tables 1 and 2; HRESIMSm/z313.1908 [M + H] + (calcd for C19H25N2O 2 , 313.1911).
3.8 Bousmekine E (5)
Bousmekine E (5): colorless solid;- 66 (c0.1, MeOH); UV (MeOH) λmax(logε): 259 (3.08) nm; IR (KBr)vmax3442, 2918, 1736, 1647, 1542, 1467, 1384, 1261, 1048 cm-1;1H and13C NMR data (acetone—d6, 600 and 150 MHz) see Tables 1 and 2; HRESIMSm/z180.1018 [M + H]+(calcd for C10H14NO2, 180.1019).
3.9 Crystal data for Bousmekine B (2)
Bousmekine B (2): 4(C19H21ClN2O)·C2H6O,M= 1361.37,a= 11.9505(3) Å,b= 12.3727(3) Å,c= 22.7041(6) Å,α= 90°,β= 95.5750(10)°,γ= 90°,V= 3341.15(15) Å3,T= 100.(2) K, space groupP1211,Z= 2,μ(Cu Kα) = 2.091 mm -1 , 73809 reflections measured, 13008 independent refl ections (Rint= 0.1435).The fi nalR1values were 0.0675 (I> 2σ(I)).The fi nalwR(F2 ) values were 0.1701 (I> 2σ(I)).The fi nalR1values were 0.0798 (all data).
The fi nalwR(F2) values were 0.1860 (all data).The goodness of fi t onF2was 1.030.Flack parameter = 0.104(12).Crystallographic data (excluding structure factor tables) for compound 2 have been deposited with the Cambridge Crystallographic Data Center as supplementary publication (deposit number CCDC 2026043).Copies of the data can be obtained free of charge by application to CCDC, 12 Union Road, Cambridge CB 1EZ, UK [fax: Int.+ 44 (0) (1223) 336 033; e-mail: deposit@ccdc.cam.ac.uk.
3.10 Cytotoxicity Assays
Cytotoxicity evaluations were performed according to the previously described protocol [10,17].
4 Concluding Remarks
In this investigation, four new MIAs (1— 4) and one pyranopyridine alkaloid (5) were isolated from the twigs and leaves of twoBousigoniaspecies:B.angustifoliaandB.mekongensis.Among them, compound 2 was an eburnea-type MIAs and featured by a rare chlorine atom at C-14, while 5 was characterized by a novel pyranopyridine moiety.Moreover, compound 1 exhibited significant cytotoxicities against several human cancer cell lines with IC50values of 0.8—7.4 μM.The fi ndings enriched the diversity of secondary metabolites of theBousigoniagenus.
AcknowledgementsThis work was fi nancially supported by Yunnan Applied Basic Research Projects (2018FA049) and Top Young Talent of the Ten Thousand Talents Program of Yunnan (to Y.Zhang).
Compliance with Ethical Standards
Conflict of interestAuthors declare that there is no confl ict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
杂志排行
Natural Products and Bioprospecting的其它文章
- Anticancer Properties of Lobetyolin, an Essential Component of Radix Codonopsis (Dangshen)
- Plants Used as Antihypertensive
- Identification and Bioactivities of Secondary Metabolites Derived from Endophytic Fungi Isolated from Ethnomedicinal Plants of Tujia in Hubei Province: A Review
- Furan Derivatives and Polyketides from the Fungus Irpex lacteus
- Identification of Anxiolytic Potential of Niranthin: In-vivo and Computational Investigations
- Structures, Chemical Conversions, and Cytotoxicity of Tricholopardins C and D, Two Tricholoma Triterpenoids from the Wild Mushroom Tricholoma pardinum
