Identification and Bioactivities of Secondary Metabolites Derived from Endophytic Fungi Isolated from Ethnomedicinal Plants of Tujia in Hubei Province: A Review
2021-04-10KeYeHongLianAiJiKaiLiu
Ke Ye ·Hong-Lian Ai ·Ji-Kai Liu
Abstract Tujia is a national minority, inhabiting in the mountainous Wuling area in China.Since 1978, Tujia medicine has been studied, summarized and developed, leading to numerous achievements by Chinese researchers, such as the publishing of approximately 30 monographs of Tujia medicine.These publications are focused on summarizing and improving the theory of Tujia medicine and developing clinical therapies from this system of medicine.The shortage of natural medicinal plants used in Tujia medicine has created the need to discover new resources to replace them and protect endangered natural plant species.Endophytic fungi are one of the conservation options, are considered a source of new bioactive natural products, and are a renewable and inexhaustible source of new drugs and agrochemicals.This review summarizes 260 compounds from endophytic fungi that have been previously isolated from the medicinal plants of Tujia.These compounds include steroids, terpenoids, meroterpenoids, polyketides, alkaloids, peptides, aliphatic compounds, aromatic compounds, and heterocyclic compounds.
Keywords Tujia medicine · Endophytic fungi · Secondary metabolites · Bioactivities
1 Introduction
Endophytic fungi are microorganisms that inhabit in the inner healthy tissues of host plants.They typically do not induce any apparent symptoms of disease in the host [1].Since anticancer agent paclitaxel (Taxol) was discovered inTaxomyces andreanae, an endophytic fungal strain isolated fromTaxus brevifolia[2], interest in bioactive natural products derived from endophytic fungi has increased.During the past two decades, a considerable number of natural products with novel structures and interesting bioactivities have been reported, and endophytic fungi have been identified as to be a source of new bioactive natural products [3— 5].
Interestingly, endophytic fungi can produce the same or similar bioactive metabolites as their host plants [6].Thus, they can be used to develop a substitutable approach to producing valuable bioactive compounds to protect plant and conserve resources and the natural environment [7].Tujia medicine is a type of Chinese medicine that has unique advantages and potential in curing different diseases, but some Tujia medicinal plants are endangered.Therefore, endophytic fungi isolated from Tujia medicinal plants of the Tujia could be a novel source of natural products, thereby protecting endangered plants.
This review summarizes metabolites, including steroids, terpenoids, meroterpenoids, polyketides, alkaloids, peptides, aliphatic compounds, aromatic compounds, heterocyclic compounds and others as well as their bioactivities of endophytic fungi isolated from the antirheumatic and anti-traumatic medicinal plant of the Tujia in Hubei province.In addition, different medicinal plant classes are described.
2 Cephalotaxus Fortunei Hook
Cephalotaxus fortuneiis a perennial, coniferous shrub or small tree belonging to the family Cephalotaxaceae.It is mainly distributed in the subtropical regions up to the northernmost Qinling Mountains and the Huai River in central China.C.fortuneicontains the anticancer alkaloid harringtonine, which has made it important for medicinal use in treating leucocythemia [8].
Trichodermanin A (1), a novel diterpenoid with skeletal carbons arranged compactly in a fused 6/5/6/6 ring system, was isolated from the subculture of endophytic fungusTrichoderma atroviride, which was obtained from the bark ofC.fortunei [9].
Two sesquiterpenes, named trichoderiols A (2) and B (3), were also isolated from cultures of the same endophytic fungusT.atroviride[11].In bioactivity studies, these three compounds showed good antifungal effects againstCandida albicans,Cryptococcus neoformans, andTrichophyton rubrumas well as some antitumor activity [10].Furthermore, compounds 2 and 3 were evaluated for their anti-infl ammatory activity against nitric oxide (NO) production and showed significant NO scavenging effects, with half-maximal inhibitory concentrations (IC50) values of 15.3 and 9.1 μM, respectively.The results of a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay indicated that none of the concentrations used in the experiment were cytotoxic [11].
In addition to terpenoids, other types of compounds have been isolated from this endophytic fungus, including 3-oxo-1-cyclopentene-1-propanoic acid (4), 7-methoxy-4,6-dimethyl-1(3H)-isobenzofuranone (5), 4-(hydroxymethyl)-7-methoxy-6-methyl-1(3H)-isobenzofuranon (6), tetrahydro-4-hydroxy-6-phenyl-2-pyran-2-one (7), vanillic acid (8),P-hydroxyethyl phenol (9), 2,4-dihydroxy-3,5,6-trimethylbenzoate (10), gallic acid (11), kaermferol (12), n-docosanoic acid (13), caffeic acid hexaeosanyl ester (14), andβ-sitosterol (15) [10] (Fig.1).
(-)-Trichodermadione (16a) and (+)-trichodermadione (16b), two novelN-furanone amide enantiomers, were isolated from the solid culture ofT.atroviridein the bark ofC.fortunei.Moreover, trichodermadiones B (17) and C (18), a cyclohexanone sesquiterpenoid and a diterpenoid, respectively, were obtained, along with the following twelve compounds: 1,9,R-mevalonolactone (19), anhydrome valonolactone (20), 5-methoxymethyl-1H-pyrrole-2-cabaaldehyde (21), 3-(1-aminoethylidene)-6-methyl-2H-pyran-2,4(3H)-dione (22), mollisilactone (23), 4-(2-formyl-5-(methoxymethyl)-1H-pyrrol-1-yl)butanoic acid (24), 5-hydroxy-2,3-dimethyl-7-methoxychromone (25), lignoren (26), ascotrichic acid (27) and catenioblin C (28).However, in bioactivity studies, compounds 16a,16b,17 and 18 did not show anti-infl ammatory activity [12,13] (Fig.2).
Ma et al.[14] reported a furan derivative, 5-acetoxymethylfuran-3-carboxylic acid (29), which was isolated from the endophytic fungusAspergillus flavusinC.fortunei.This compound exhibited potent antibacterial activity againstStaphylococcus Aureus, moderate antioxidant activity, and has the potential to be an antibacterial drug.
Six compounds isolated fromA.fl avusinC.fortuneiwere identified as compound 15, 5-hydroxymethyl furan-3-carboxylic acid (30), ergosterol (31), stigmasta-7,22-diene-3β,5α,6α-triol (32), gliotoxin (33) and succinic acid (34).Among these compounds,30 and 33 showed good antibacterial activity and 30 exhibited a good inhibitory effect againstEscherichia coli, with a minimum inhibitory concentration (MIC) of 15.6 μg/mL [15].Bioassay-guided fractionation of the ethyl acetate (EtOAc) extract of the endophytic fungusAspergillus piperisin the stem of theC.fortuneiled to the isolation and identification of several compounds, including 31 and 33, emodin-8-O-methylethe (35), monomehtylsulochrin (36), 1,5-dimethyl citrate (37), D-mannitol (38), and ergosterol peroxide (39), which showed antibacterial activity [16].An endophytic fungus of thePenicilliumsp.was collected and identified fromC.fortune, and the following fi ve compounds were identified as compounds 31,38, and 39, 3-isopropenyl-Z-butenedioic acid monomethyl ester (40), and methyl-O-β-D-glucopyranosid (41) [10] (Fig.3).
3 Huperzia serrata (Thunb.ex Murray) Trev
Huperzia serratais used in the traditional Chinese medicine preparation.Qian Ceng Ta grows at an altitude of 300—2700 m in damp forests and rock crevices in China.This plant produces the alkaloid huperzine A (42), which is marketed in China as a new drug for Alzheimer’s disease (AD) treatment and used in the USA as a supplement to prevent further memory degeneration [17— 20].Endophytic fungi often have the ability to produce the same or similar bioactive metabolites as their host plants.Five endophytic fungi isolated fromH.serrataproduced metabolites that were similar or identical to those of huperzine A.These endophytic fungi were characterized and identified asAlternariasp.,Shiraiasp.,Fusarium oxysporum, and two different strains ofColletorichum gloeosporides.Moreover,Alternariasp.produced 6-methoxy-7,4′-dihydroxyisofl avone (43) and arbutin (44) [21— 25] (Fig.4).
A strain of thePenicilliumsp.collected from the stem ofH.serratawas found to produce alkaloids in preliminary experiments.Systematic experiments isolating alkaloids from the fungus led to the identification of four diketopiperazine alkaloids, tryhistatin (45), 16-hydroxyroquefortine C (46), roquefortine C (47), and cyclo(dehydrohistidyl-L-tryptophyl) (48).They are an important class of fungal metabolites and this class of alkaloids has shown great potential in the development of therapeutic drugs [26] (Fig.5).
Ceriponols L (49) and M (50 ), two tremulane sesquiterpenoids, were obtained from an endophytic fungusCeriporia lacerateisolated from the stems ofH.serrata[27].Meroterpenoids are synthesized via a common intermediate produced by the hybridization of a polyketide intermediate and the terpenoid precursor farnesyl diphosphate.Qi et al.[28] reported fi fteen 3,5-dimethylorsellinic acid derived meroterpenoids identified as chrysogenolides A—H (51— 58), berkeleyacetals A—C (59— 61), purpurogenolide C (62), 22-epoxyberkeleydione (63), berkelrydione (64), and berkeleyone B (65).These compounds were produced byPenicillium chrysogenum, an endophytic fungus collected fromH.serrata.Among these compounds, chrysogenolides C (53), D (54), and F (56); berkeleyacetal C (61); and purpurogennolide C (62) showed inhibition of NO production in lipopolysaccharide (LPS)—activated RAW 264.7 macrophages with IC50values in the range of 4.3—78.2 μM (Fig.6).
Fungal polyketides are one of the largest and most structurally diverse classes of naturally occurring compounds [29].Four new dimeric spiro-azaplilone derivatives cochliodones E—H (66— 69) were obtained from an endophytic fungus of theChaetomiumsp., which was obtained fromH.serrata.Furthermore, four compounds assayed and all exhibited antibacterial activity.In particular, compound 68 inhibitedE.coligrowth to levels almost the same as cefotaxime did [30].In addition, Yu et al.[31] isolated eight compounds from an endophytic fungus of theChaetomiumsp., which was collected fromH.serrata, consisting of seven polyketides and one fungal toxin.They were identified as chaetoviridine F (70), chaetoviridine E (71), (7R,4′S,5′S,11S)-chaetoviridin A (72), (7R,4′S,5′R,11S)-chaetoviridin A (73), xanthoquinodin A1 (74), xanthoquinodin A2 (75), xanthoquinodin B (76) and chetomin (77) (Fig.7).
Chemical investigation of the metabolites of an endophytic fungus of thePenicilliumsp.obtained from the stems ofH.serrata, led to the isolation of compounds 31 and 39, sorbicillin (78), 2,3′-dihydrosorbicillin (79), 2-chloro-N-phenylpropranamide (80),N-(2-hydroxyphenyl)-acetamide (81), thymine (82) and a chromone derivative (2S)-2,3-dihydro-7-hydroxy-6,8-dimethyl-2-[(E)-prop-1-enyl]-chroman-4-one (83).Moreover, compounds 78,79 and 83 were subjected to an in vitro cytotoxicity assay.Compound 78 exhibited potent cytotoxicity against HeLa cells and weak activity against HepG2 cells with IC50values of 1.6 and 27.2 μM, respectively.Compound 79 showed moderate activity against HeLa cells and weak activity against HepG2 cells with IC50values of 7.4 and 44.4 μM [32] (Fig.8)
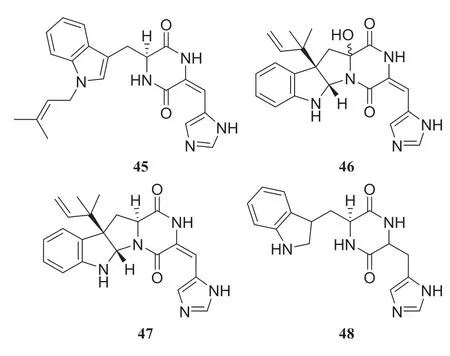
Fig.5 Structures of compounds 45— 48
Systematic investigation of metabolites from the endophytic fungusCercospora lagenariaeisolated fromH.serrateled to the isolation of nine polyketides identified as cerecolagenlic acid A (84), alternariol (85), alternariol-9-methyl ether (86), (+)-nigrosporal A (87), alternarienonic acid B (88), 2-methyl-5-carboxymethyl-7-hydroxylchromone (89 ), 2,5-dimethyl-7-hydroxychromone (90), 1-deoxyrubrelactone (91) and (-)-alternarlactam (92).Among these compounds,90 exhibited some inhibitory effects on NO production in LPS-activated RAW 264.7 macrophage cells, with an IC50of 57.5 ± 1.2 μM [33].Zhan et al.[34] reported the isolation and structural elucidation of six furanone derivatives, huaspenone A (93), huaspenone B (94), aspertetronin A (95), aspertetronin B (96), gregatin E (97) and penicilliol A (98).These compounds were isolated from the cultures of an endophytic fungus of theAspergillussp.obtained from the stems ofH.serrata.Eight metabolites isolated from an endophytic fungus of thePeyronellaeasp.fromH.serratawere identified as compounds 19 and 20, glycerol 2-acetyl-3,5-dihydroxyphenylacetate (99), curvulinic acid (100),O-methylcurvulinic acid (101), methyl curvulinate (102), andrastin A (103) and fuscoatramide (104) [35] (Fig.9)
Eight diphenyl ether derivatives obtained from an endophytic fungusP.chrysogenumisolated fromH.serratewere identified as penicichrysogenillide A (105), penicichrysogenillide (106), talaromyone A (107), isopencillide (108), penicillide (109), hydroxypenicillide (110 ), purpactin A (111) and penicichrysogenillide (112).Furthermore, in additional studies, compounds 105 and 106 showed inhibitory activity against NO production in LPS-stimulated RAW264.7 macrophage cells with IC 50 values of 76.2 and 41.2 μM, respectively [36].
Several compounds were isolated from three different endophytic fungi collected fromH.serrate.The fi rst strain,Shiraiasp.produced eburicol (113), 2,3-dihydroxypropyl-9Z,12Zoctadecadienoate (114),(R)-2,3-hydroxypropyl stearate (115), 2,3-dihydroxypropyl-hexadecanoate (116), linoleic acid (117), hypocrellins A (118), hypocrellins B (119), elsinochromes B (120), and elsinochromes C (121).In bioactivity studies, compounds 114— 116 and 118 showed antibacterial activity.The second strainAspergillus fumigatusproduced compound 31, dioctyl phthalate (122), 1′,9,12-linoleic acid-2′,3′-dihydroxypropyl ester (123), microsphaerone C (124), and helvolic acid (125).Moreover, compounds 123— 125 exhibited inhibitory activity against acetylcholinesterase (AChE) with rates of 73.5%, 84.1% and 77.6% and IC50values of 0.057, 0.038, and 0.05 mg/mL, respectively.The third strain of theNeofusicoccumsp.produced compounds 31,39, cerebroside C (126), fusaproliferin (127), adenosine (128), 1-(furan-2-yl)-hyroxyethanone (129), versicolactone A (130), and versicolactone B (131) [37— 39] (Fig.10)
4 Pinus massoniana Lamb
Pinus massonianais widely grown in Asia and is an important source of timber and oleoresin in southern China [40].An endophytic fungus of thePhomopsissp.was isolated fromPinus massonianaand nine compounds were isolated, which consisting of four nonenolides, three alternariol derivatives, and two phthalide derivatives.The systematic names of the four nonenolides are (5S,8S,9R,10R,E)-5,8,9-trihydroxy-10-pentyl-3,4,5,8,9,10,-hexahydro-2H-oxecin-2-one (132), (5S,8S,9R,10R,E)-5,8,9-trihydroxy-10-nonyl-3,4,5,8,9,10,hexahydro-2H-oxecin-2-one (133), (5S,6S,9S,10R,E)-5,6,9-trihydroxy-10-pentyl-3,4,5,6,9,10-hexahydro-2H-oxecin-2-one (134), and (5R,8S,9R,10S,E)-5,8,9-trihydroxy-10-([R]-4-hydroxyoctyl)-3,4,5,8,9,10-hexahydro-2H-oxecin-2-one (135).
The two phthalide derivatives were 3,5-dihydroxy-7-mehtoxy-4-(methoxymethyl)-6-methyl-isobenzofuran-1(3H)-one (136) and 5-hyroxy-3,7-dimethoxy-4-(methoxymethyl)-6-methyl-isobenzo-furan-1(3H)-one (137).Three alternariol derivatives are compounds 85,86, and alternariol 4,10-dimethyl ether (138).All compounds were evaluated for antitumor and antibacterial activities, but none of them showed obvious activity [41].Another endophytic fungus of theGlomerellasp.was obtained fromP.massoniana, and two lanostane-type triterpenoids and four steroid derivatives were isolated.
The systematic names of the two lanostane-type triterpenoids are 3-carboxyl-4,12β,28-trihydroxyl-3,4-seco-5α-27-ketone-lanostane (139), and 3-carboxymethyl-4,12β,28-trihydroxyl-3,4-seco-5α-27-ketone-lanostane (140).Four sterol derivatives were identified as compounds 31,39,3β,5,8-trihydroxy-D-homo-ergosterol (141) and (20S,22E,24R)-ergosta-7,22-dien-3β,5α,6β-triol (142).Furthermore, the evaluation of all compounds for antitumor and antibacterial activities showed that only compound 139 showed weak cytotoxicity, with an inhibition rate of 21.1% at 20 μg/mL, whereas others showed no apparent activity [41] (Figures 11,12,13).
5 Dysosma versipellis (Hance) M.Cheng ex Yang

Fig.6 Structures of compounds 49— 65
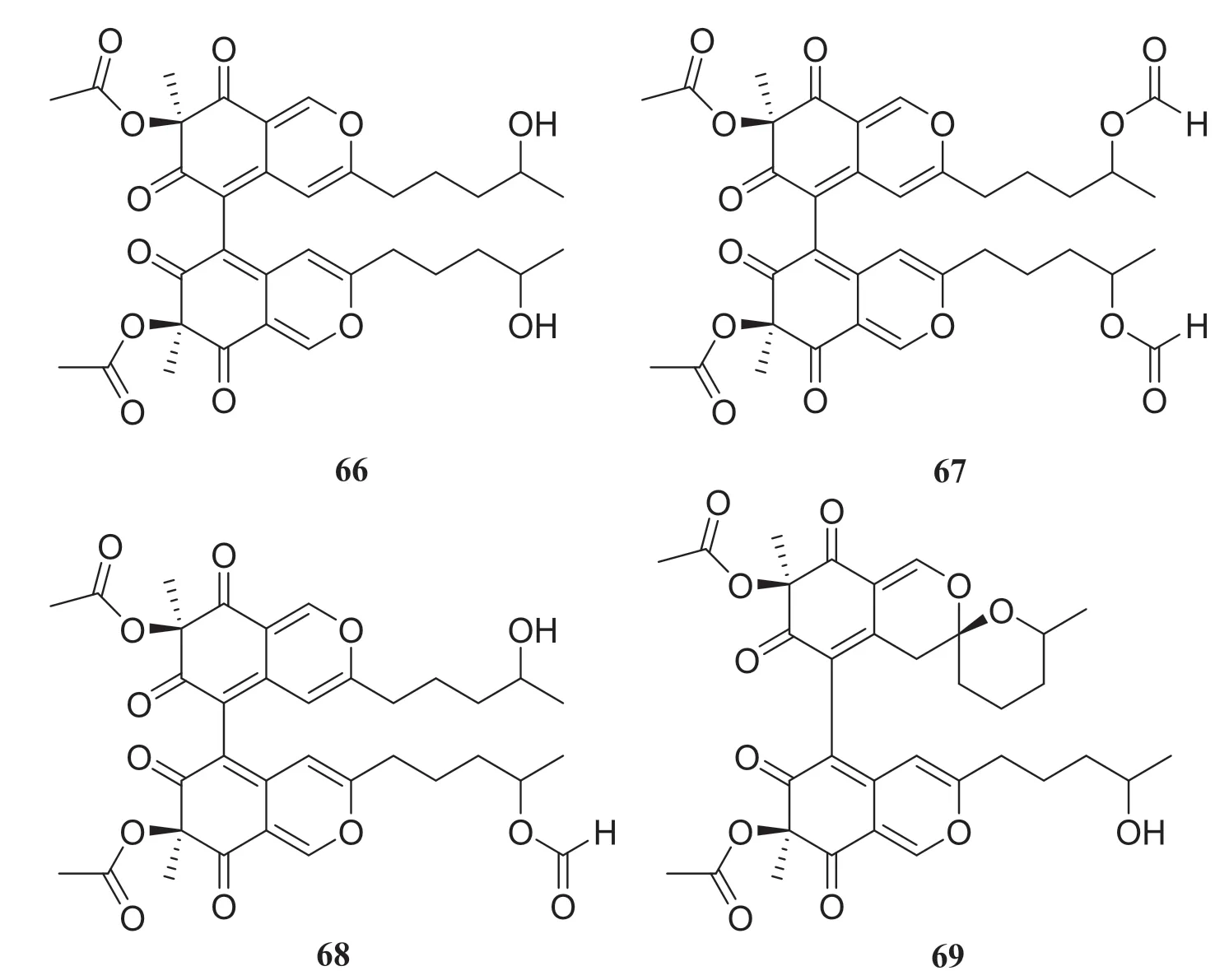
Fig.7 Structures of compounds 66— 69
Dysosma versipellisis an herbaceous perennial species that grows in the understory of mixed evergreen and deciduous forests in China.As an important endangered medicinal plant species,D.versipellisis restricted to eastern and southern China [42].A strain of thePenicillium sp.was isolated and cultured from the fresh leaf ofD.versipellis.Studies on the metabolites of the crude extract led to the isolation of the following twelve compounds: seven meroterpenoids, 11β-acetoxyisoaustinone (143), austin (144), austinolide (145), dehydroaustinol (146), dehydroaustin (147), chrodrimanin A (148) and chrodrimin B (149), a butyrolactone, isoberkedienolactone (150), and four other types of compounds,O-methylmellein (151), 3-(propan-2-ylidene)-pyrrolidine-2,5-dione (152), (E)-3-[2,5-dioxo-3-(propan-2-ylidene)-pyrrolidin-1-yl]acrylic acid (153) andN-(4-hydroxy-2-methoxyphenyl)-acetamide (154).All the compounds were evaluated for cytotoxicity in vitro using the MTT method but they only showed weak cytotoxicity [43].
Sixteen compounds isolated from an endophytic fungusPaecilomyces Bainerisolated from the roots ofD.versipelliswere as follows: nine sterols,15,39, cholesterol, 5,8-epidioxy-5α,8α-ergosta-6,9,22E-tien-3β-ol (155), ergosta-4,6,8(14),22-tetraene-3-one (156), ganodemaside B (157), 5α,6α-epoxy-3β-hydroxy-(22E)-ergosta-8(14), 22-dien-7-one (158), stigmasterol (159) and (Z)-stigmasta-5,24(28)-dien-3β-ol (160), two fatty acids, hexadecenoic acid (161) and oleic acid (162), two glycerides glycerol monooleate (163) and one nucleotide (128).In MTT assay, these compounds showed some cytotoxicity, and compound 157 showed strong cytotoxicity [44] (Fig.14).
6 Celastrus anglatus Maxim
Celastrus anglatus, which is heavily distributed in the mountains of southwest China, has been exploited as a natural insecticide resource and is a popular ingredient in folk medicine because of its active ingredients [45].The following three compounds were isolated from the endophytic fungusOospora Wallr, which was isolated fromC.anglatus: 31, cytochalasin D (164), and ducitol (165).All compounds were evaluated for their inhibitory activity against plant pathogens on spore germination at a concentration of 100 μg/mL.The inhibitory activity of compound 164 was strong, with a half-maximal effective concentration (EC50) of 35.01 μg/mL againstAlternaria longipes[46].Bioassay-guided fractionation led to the isolation of fi ve antibacterial compounds from the fermentation broth of unknown endophytic fungus isolated fromC.anglatus.These compounds were identified as 3′-chlorotrypacidin (166), asterric acid (167), methylasterrate (168), methyl-4′,6′-dichloroasterrate (169), and methyl-4′-chloroasterrate (170).The inhibition rates of compounds 165 and 166 (at 500 μM) againstCurvularia lunatawere 100% and 67.6%, respectively, and they showed strong inhibitory activity againstBacillus subtilis[47].An endophytic fungus isolated from the phloem ofC.anglatuswas identified asFusarium proliferatumand three cyclopeptides isolated from this strain were named enniatin A1 (171), enniatin B1 (172) and enniatin B (173) [48] (Fig.15)
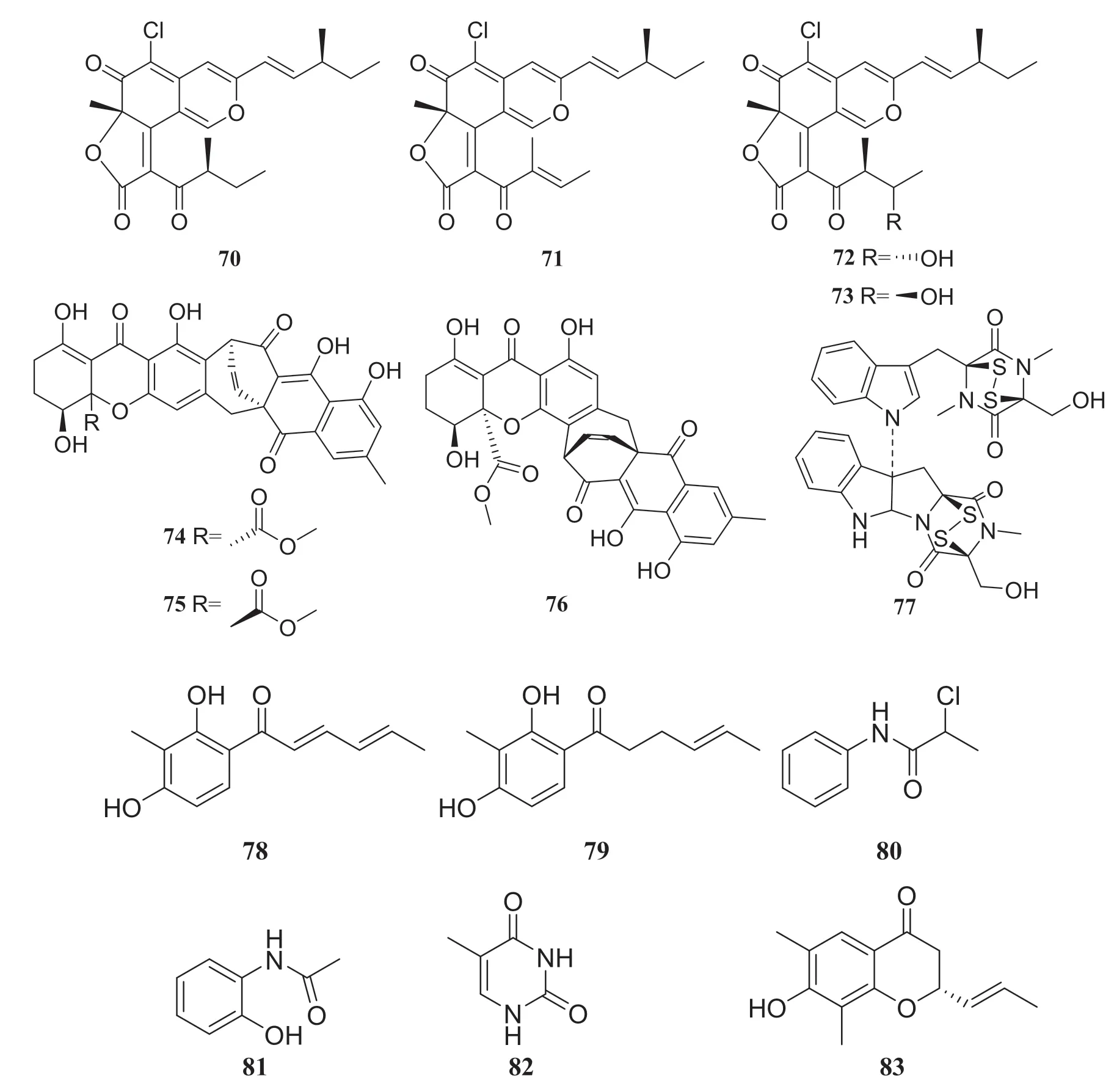
Fig.8 Structures of compounds 70— 83
7 Camptotheca acuminata Decne
Camptotheca acuminatais a tree species indigenous to southern China, which is referred to as “xi shu” and is of particular interest because of its importance of secondary metabolite, camptothecin and its analogs.Camptothecin (174) is known for remarkable inhibitory activity against tumor cells and the human immunodefi ciency virus (HIV) [49].Endophytic fungi can produce the same or similar metabolites as the host plant and, therefore,174 and its derivatives, 9-methoxycampothecin (175) and 10-hydroxycampothecin (176), have been obtained from several endophytic fungi isolated fromC.acuminata.(Table 1) [50— 55] (Fig.16)
Chemical investigation of the secondary metabolites of a fermented endophytic fungus of theAspergillussp.isolated several complex alkaloids.These compounds were identified as pseurotin A (177), FD-838 (178), fumitremorgin C (179), cyclotryprostatin B (180), 12,13-dihydroxyfumitremorgin C (181), fuminquinazoline C (182), fumiquinazoline J (183), spirotryprostatin A (184) and tryprostatin B (185).This fungus was isolated from the inner bark ofC.acuminata[56] (Fig.17).

Fig.9 Structures of compounds 84— 92
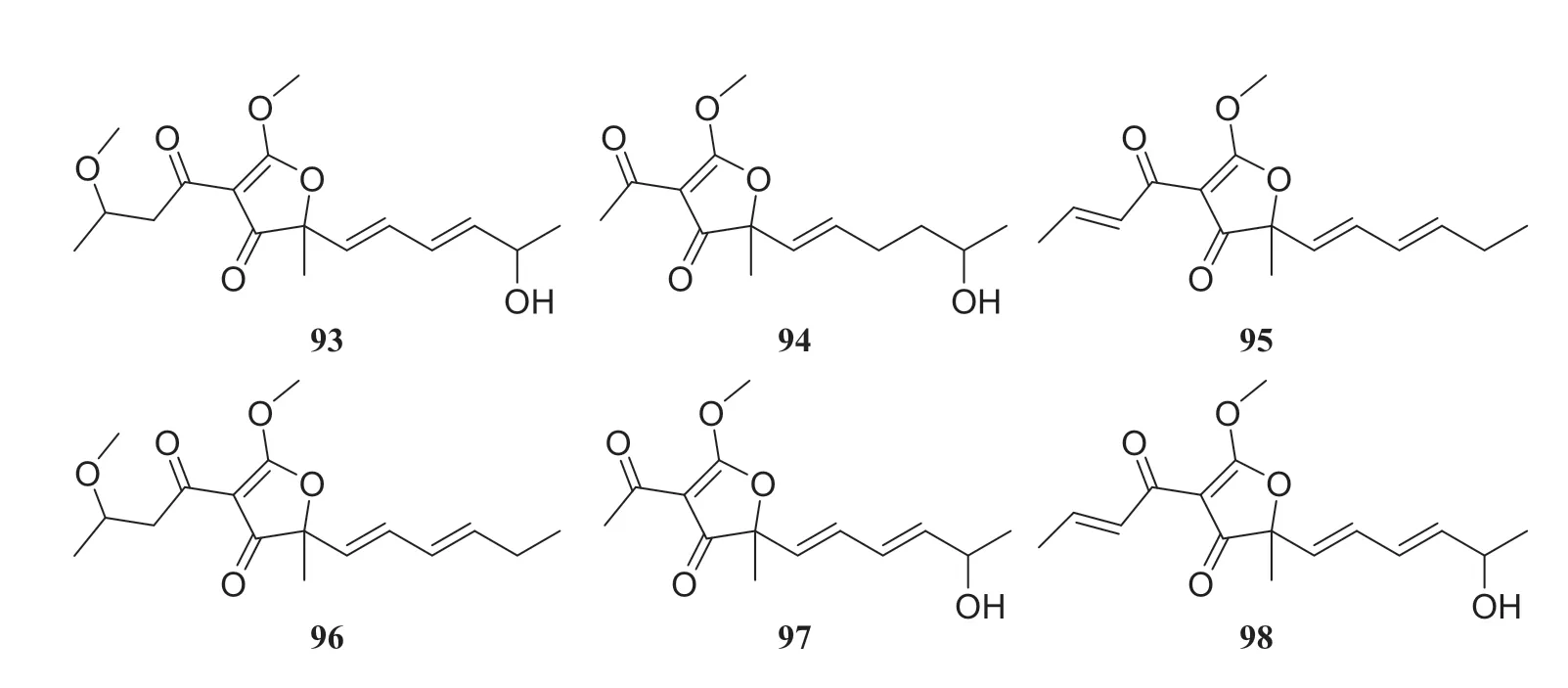
Fig.10 Structures of compounds 93— 98

Fig.11 Structures of compounds 99— 104

Fig.12 Structures of compounds 105— 112
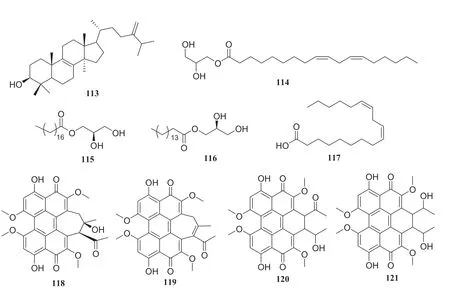
Fig.13 Structures of compounds 113— 121
Tan et al.[57] reported the isolation of fi ve 10-membered macrolides and an unsaturated fatty acid and its methyl ester from the fermentation products of the endophytic fungal strainPhomopsissp.isolated fromC.acuminata.The seven compounds were identified as 8-O-acetylmultiplolide A (186), multiplolide A (187), 8-O-acetyl-5,6-dihydro-5,6-epoxymultiplolide A (188), 5,6-dihydro-5,6-epoxymultiplolide A (189), 3,4-deoxy-didehydromultiplolide A (190), (4E)-6,7,9-trihydroxydec-4-enoic acid (191), and (4E)-6,7,9-trihydroxydec-4-enoate (192).Furthermore, fi ve 10-membered macrolides,186— 190, exhibited no obvious antifungal activity againstC.albicansat 200 μg/mL.The cytotoxic activities of compounds 186-189 against humantumorRajicells were tested using the MTT assay, but none exhibited cytotoxicity.Only compound 186 exhibited significant inhibitory activity against AChE, with an IC50of 1.19 μg/mL.
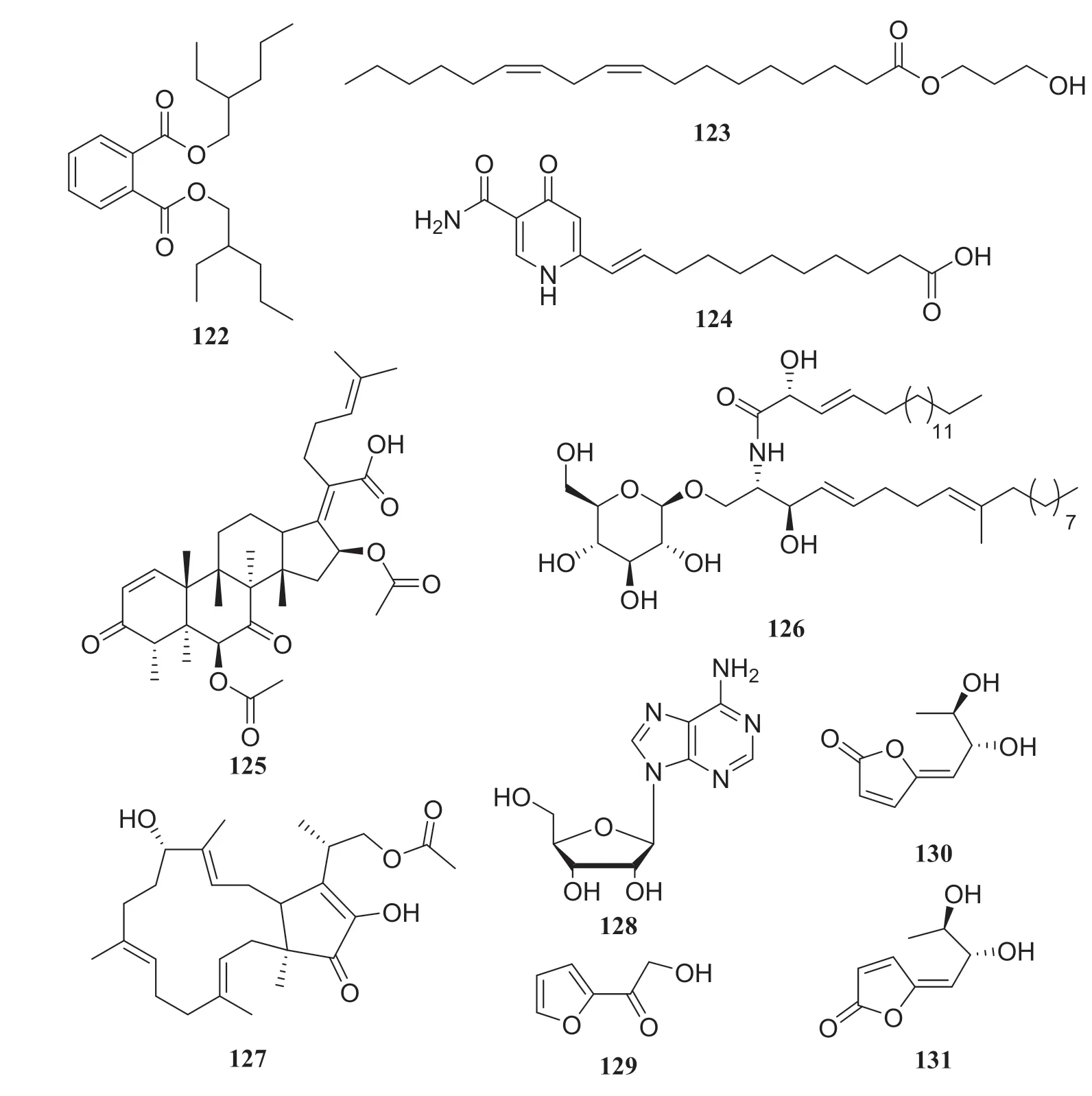
Fig.14 Structures of compounds 122— 131
Studies on the secondary metabolites from an endophytic fungus of theDiaporthesp.isolated from surface-sterilized twig tissues ofC.acuminata, led to the discovery of four new polyketides,rel-(2R,3S,4R,5R)-4-ethyltetrahydro-3-methyl-5-propylfuran-2,3-diol (193), methyl 5-[(1R)1-hydroxyethyl]-g-oxofuran-2-butanoate (194), butyl 5-[(1R)-1-hydroxyethyl]-γ-oxofuran-2-butanoate (195), 5-[(1R)-1-hydroxyethyl]-γ-oxofuran-2-butanoic acid (196), 3,4-dihydro-5′-[(1R)-1-hydroxyethyl][2,2′-bifuran]-5(2H)-one (197), phomopsolide B (198), and (2S,3S)-3,6-dihydro-6-oxo-2-{(1E)-2-[(4S,5S)-2,2,5-trimethyl-1,3-dioxolan-4-yl]ethenyl}-2H-pyran-3-yl (2E)-2-methylbut-2-enoate (199).In biostudies, compound 198 showed strong cytotoxicity against human-tumor HeLa cells, with an IC50of 0.019 μm/mL.Compounds 196,198 and 199 were tested for their antibacterial and antifungal activities and at a concentration of 50 μg/disk, compound 198 showed an inhibitory zone with diameter of 1.4 cm againstShigella dysenteriae[58] (Fig.18).
Research on the secondary metabolites from an endophytic fungal strain of thePhomopsissp., which was also isolated from the surface-sterilized twig tissues ofC.acuminata, led to the isolation of 15 compounds: eleven naphthalene-type fungal polyketides, oblongolides B, C, N, O, P, Q, R, S, T, U, V (200-210); two new linear furanopolyketides named 5-{5-[(R)-1-hydroxyethyl]furan-2-yl}dihydrofuran-2(3H)-one (211) and 5-{5-[(R)-1-methoxyethyl]furan-2-yl}dihydrofuran2(3H)-one (212); one meroterpene named dihydroxysabinae (213); and one sesterterpene terpestacin (214).Furthermore, all compounds except 206,208, and 210 were tested for their antimicrobial activities, but none showed a substantial effect [59] (Fig.19).
From an endophytic fungus of thePhomopsissp., isolated fromC.acuminata, the following 12 compounds were isolated: oblongolide compounds 200,201,203,204,209, oblongolides C1 (215), D (216), P1 (217) and X1 (218), a phomodiol 6-hydroxyphomodiol (219), (3R,4R,5S,6R)-6-hydroxy-5-methylramulosin (220), and (3R)-5-methylmellein (221).Some oblongolides previously obtained from this endophytic fungus were isolated from another fungus of thePhomopsissp.All the compounds were evaluated for their cytotoxicities.Compounds 215,217,218, and 219 exhibited modest selective activities against the HepG2 cancer cell lines, and compound 201 showed minor selective activity against A549 cells[60].Compounds 85,86,204,210,211,212,216, and oblongolide H (222) were isolated from a different endophytic fungus of theDiaporthesp.[61] (Fig.20)
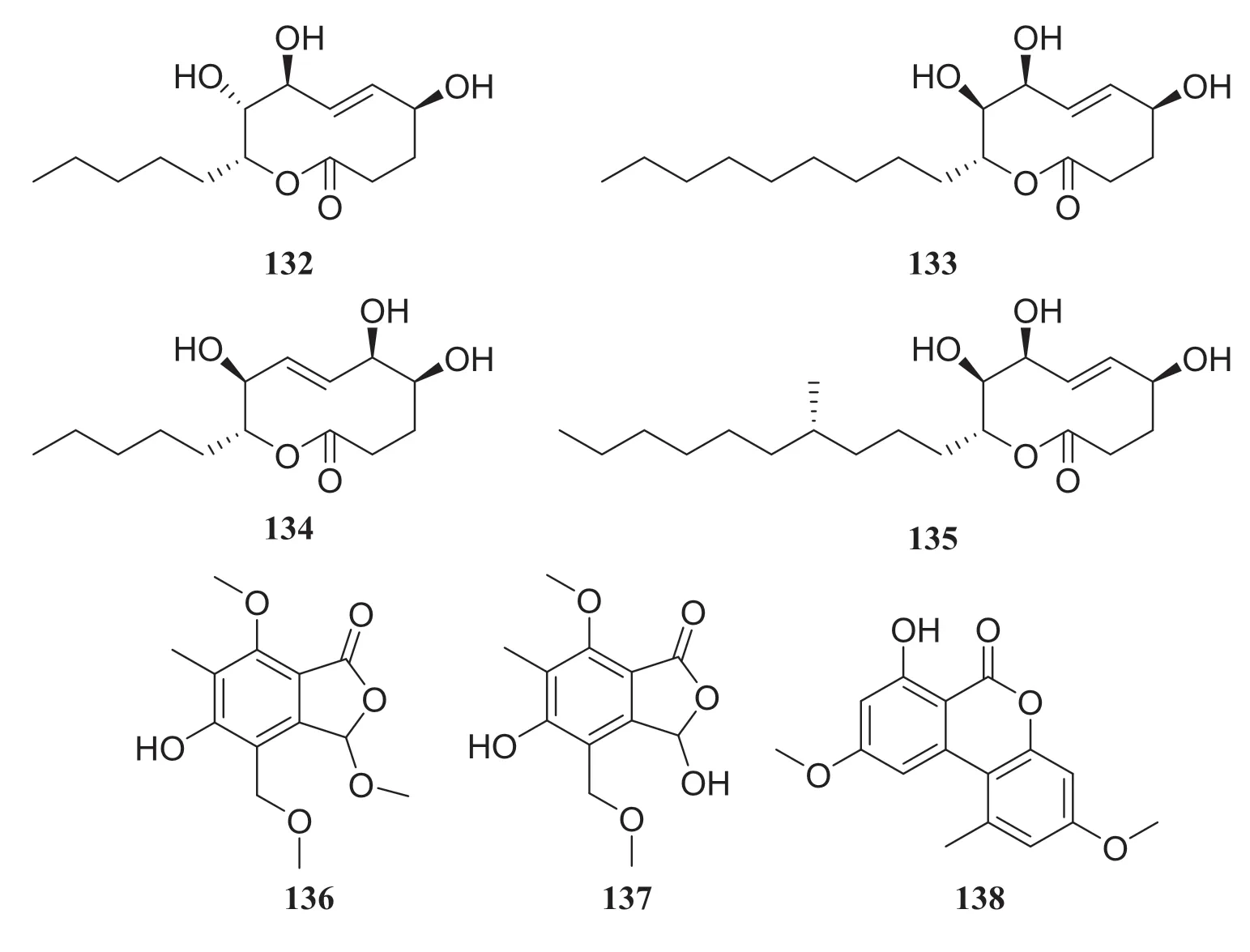
Fig.15 Structures of compounds 132— 138
Primary chemical profi ling ofC.acuminata-derived endophytic fungusPenicillium polonicumisolated nine compounds.Based on the nuclear magnetic resonance (NMR) and MS data, they were identified as polonicin A (223), polonicin B (224), 1233A (225), fusarubin (226), 3-methy ether-fusarubin (227),5,8-dihydroxy-3-methoxy-7-methyl-6-(2-oxopropyl-1,4-naphthoquinone (228), anhydrofusarubin (229), 2-isopropanol-3-methyl-7-methoxy-naphthazarin (230) and 5-hydroxydihydrofusarubin D (231).All compounds were evaluated against the HepG2 hepatocellular carcinoma cell lines and compounds 228— 231 showed cytotoxicity against the HepG2 cells.Additionally, all compounds were evaluated for their antidiabetic activity against L6 cells at a concentration of 30 μg/mL and compounds 223,224, and 225 increased the rate of glucose uptake by 1.8, 1.5 and 1.25 times, respectively.Moreover, incubation of L6 cells with compound 223 stably increased the fluorescence intensity on the membranes by 2.1fold [62] (Fig.21).

Table 1 Camptothecin and its derivatives from endophytic fungi
Two lactone derivatives were isolated from an endophytic fungus of theDiaporthesp.cultivated onC.acuminata, and identified as 5-([E]-1,4,5-trihydroxyhex-2-enyl)furan-2(5H)-one (232) and (5Z)-5-(2,3,4,5-tetrahydroxyhexyidene)furan-2(5H)-one (233).An MTT assay of compound 232 showed its antitumor activity against human cervical carcinoma cells Hela, and compound 233 exhibited strong inhibitory effects against MCF-7 breast cancer cells, human SH-SY5Y neuroblastoma and Lewis 3LL lung carcinoma cells [63] (Fig.22).
8 Paris polyphylla Smith
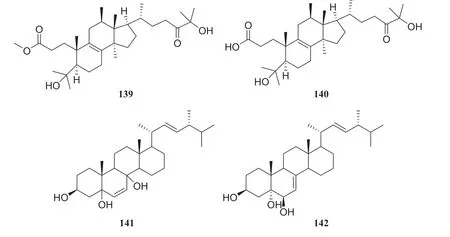
Fig.16 Structures of compounds 139— 142

Fig.17 Structures of compounds 143— 154
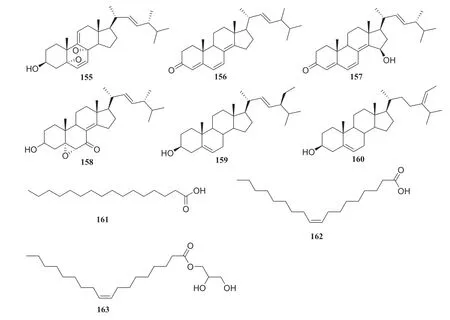
Fig.18 Structures of compounds 155— 162
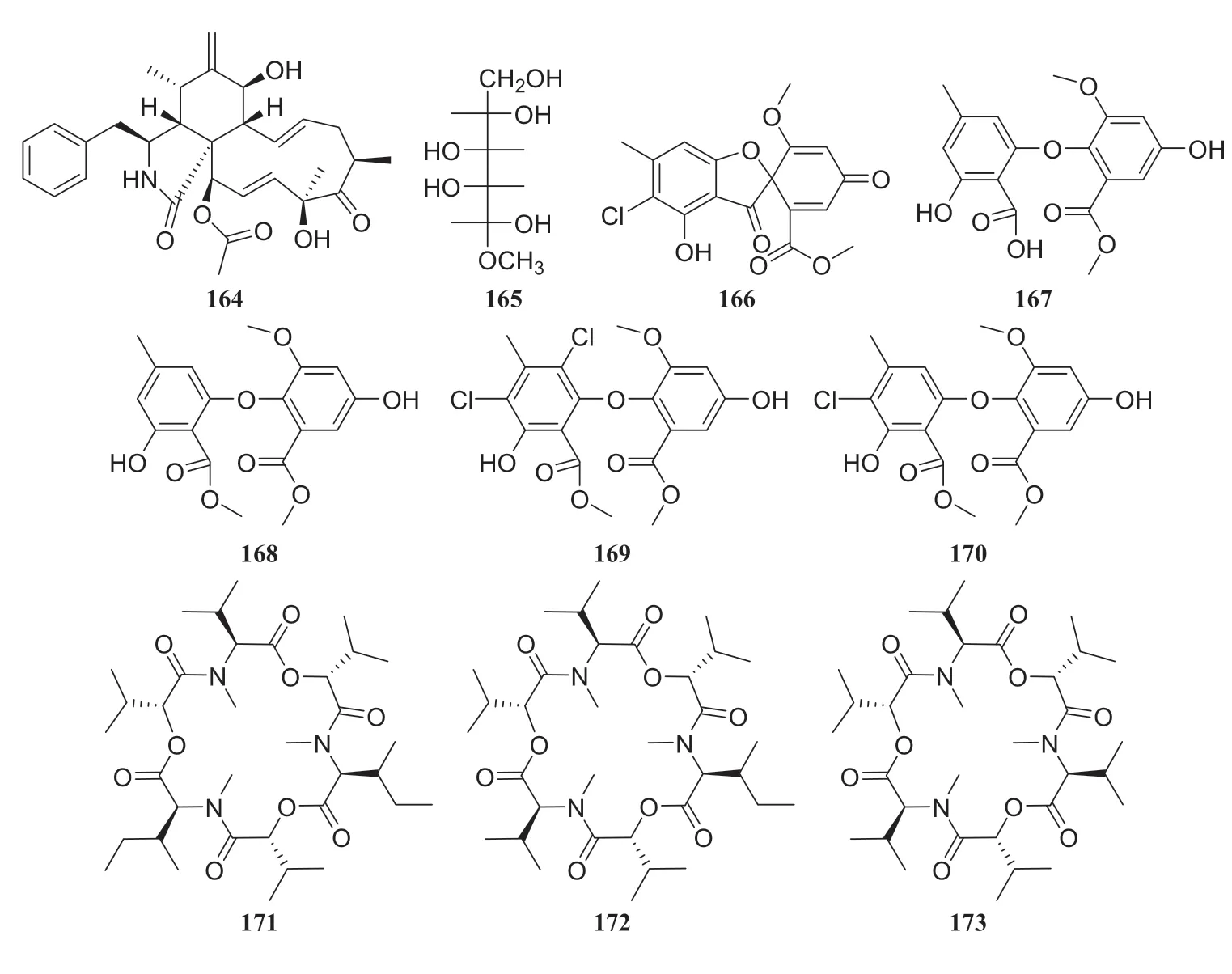
Fig.19 Structures of compounds 164— 173
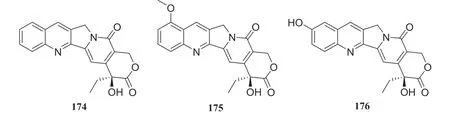
Fig.20 Structures of compounds 174— 176
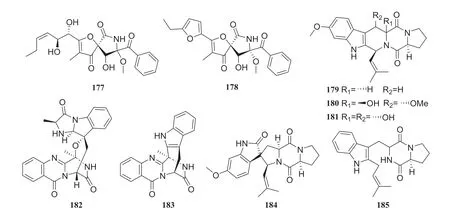
Fig.21 Structures of compounds 177— 185

Fig.22 Structures of compounds 186— 192
Paris polyphyllais a perennial medicinal plant that has been used to treat traumatic injuries such as bleeding and snake bites as well as conditions such as carbuncles, furuncles, scrofula, and chronic bronchitis [64].Bioassayguided fractionation of the EtOAc extract of an endophytic fungus of thePenicilliumsp.led to the isolation of eight polyketides: 90, 1,3,4-trimethoxyl-6-methyl-9,10-anthraquinone (234), bostrycin (235), isorhodoptilometrin (236 ), physcioin (237), emodin (238), aloesol (239), and coniochaetone B (240).This endophytic fungus was isolated from the leaves of theParis polyphylla.Furthermore, eight compounds were evaluated for antitumor activity against the HepG2 cell line, and only compounds 234— 236 showed inhibitory activity, with IC 50 of 15.6, 6.5, and 13.2 μg/mL, respectively [65].Five sterols with unusual bicyclo [4.4.1] skeletons isolated from the same strain of aPenicilliumsp.were identified as 22-acetyisocyclocitrinol A (241), neocylcocitrinols A (242), B (243), C (244), and D (245) [66] (Fig.23).

Fig.23 Structures of compounds 193— 199
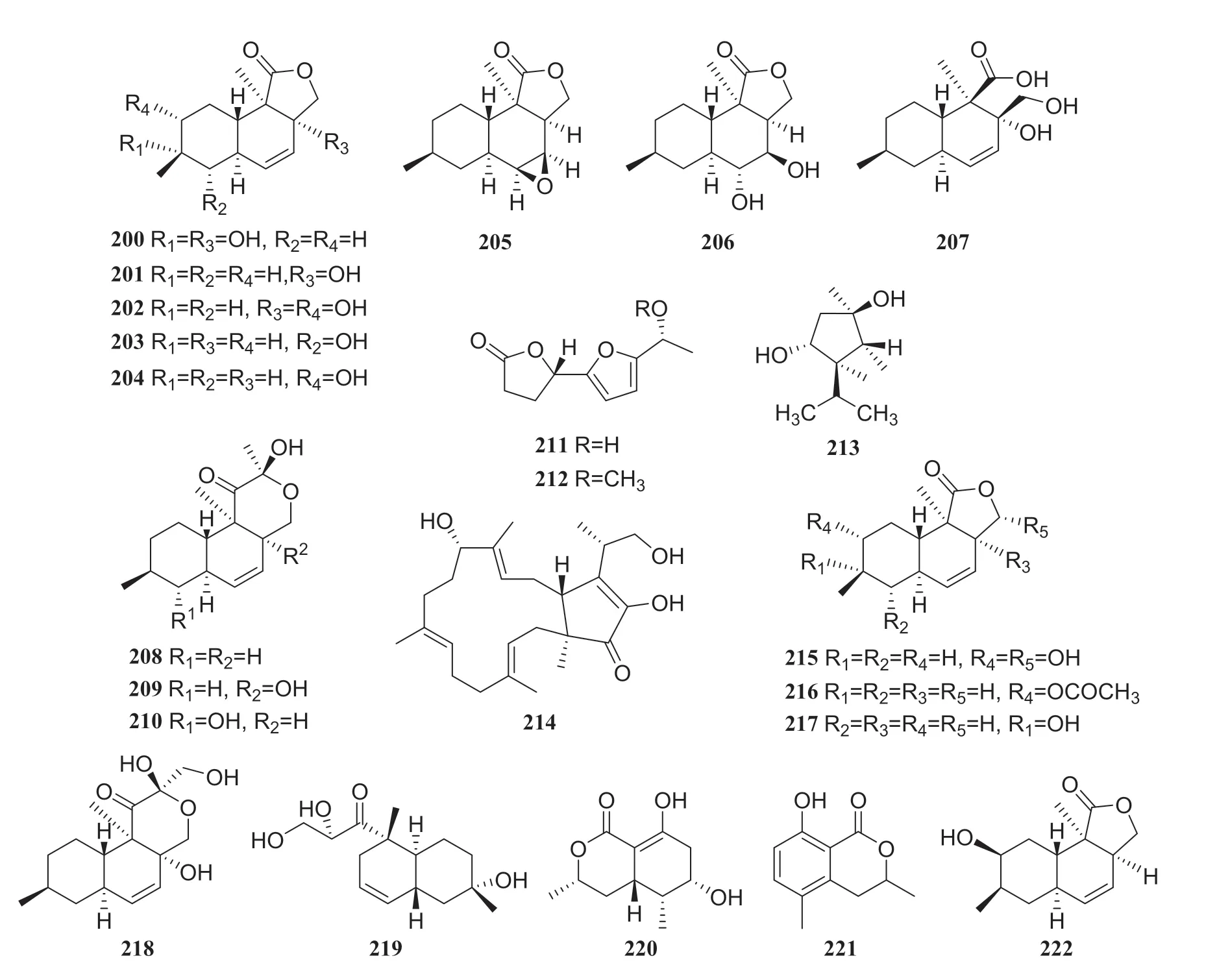
Fig.24 Structures of compounds 200— 222
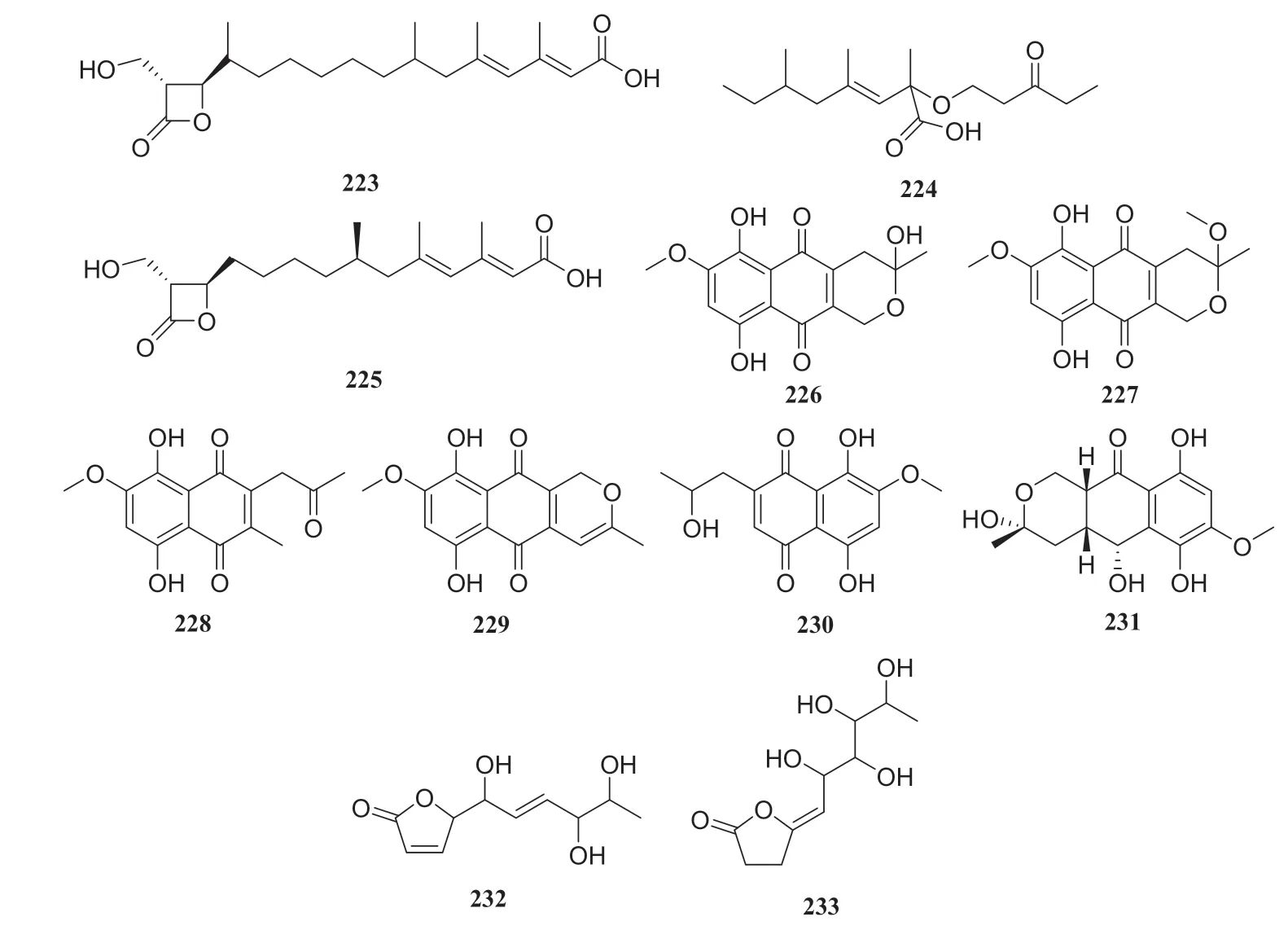
Fig.25 Structures of compounds 223— 233
Eight compounds isolated from another strain of aPenicilliumsp.isolated fromParis polyphylla, were identified as 109, citrinin H1 (246), dehydroisopenicillide (247), 7-en-nonadecanoic acid monoglyceride (248), 7,9-diennonadecanoic acid monoglyceride (249), silbaticol (250), 5-hydroxy-2-pyridinemethanol (251), and 2,4,6-octatrienoic acid (252).In an MTT assay evaluating eight compounds in HepG2 cell lines,109,246,247, and 251 showed inhibitory activity with IC 50 values of 8.5, 12.5, 15.0, and 18.2 μg/mL, respectively [67] (Fig.24)
9 Miscellaneous
Two known metabolites isolated from the endophytic fungusTrichoderma ovalisporumobtained fromCaesalpinia decapetalwere identified as 163 and (Z)-9-heptadecenoic (253) [68].Five known alkaloids were isolated from the endophytic fungusFusarium oxysporumobtained from the roots ofIris tectorum.The compounds were identified as beauvericin (254), 4-oxopentanoic acid (255),N-(4-oxopentyl)-acetamide (256), 5-butyl-2-pyridinecarboxylic acid (257) and 5-butylene-2-pyridinecarboxylic acid (258).In biostudies, only beauvericin showed strong antibacterial activity againstS.aureusandE.coli.All compounds were evaluated for their cytotoxic activity against HepG2, HepG3 and LO2 cells using an MTT assay.Among evaluated compounds, beauvericin, 5-butyl-2-pyridinecarboxylic acid and 5-butylene-2-pyridinecarboxylic acid exhibited weak antitumor activity, with IC50of 65.3—120.5 μg/mL, as well as weak cytotoxic activity against LO2 cells [69].Bioassay-guided fractionation of an EtOAc extract of the endophytic fungusBionectria ochroleucaled to the isolation of a known compound glutaric acid methyl ester (259), along with seven unattuned compounds.This endophytic fungus was isolated fromVitex negundo[70].Several endophytic fungi, includingFusarium oxysporum,Trichoderma hamatum, andFusariumsp., isolated from theParis polyphyllaSm.var chinensis (Franch.) can produce diosgenin (260) [71] (Fig.25).
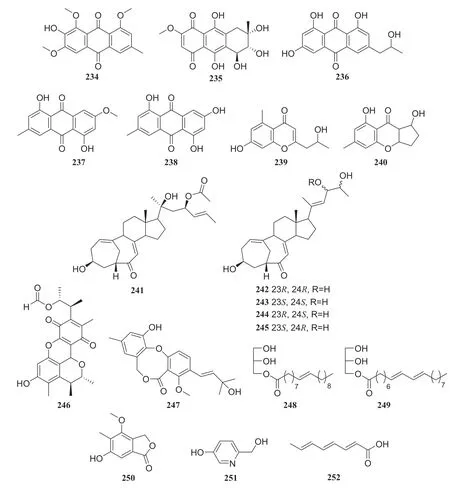
Fig.26 Structure of compounds 234— 252
10 Conclusion
It is important to point out that endophytic fungi produce highly diverse secondary metabolites and, therefore, could be used to as sources to discover novel natural products with important bioactivities.Considering the vulnerability and limitation of productivity of plants, endophytic fungi are a potential renewable and inexhaustible source of novel drugs and agrochemicals (Fig.26).
Interactions between endophytic fungi and host plants are established through complex chemical and biological networks.Endophytic fungi inhibiting plants can colonize their internal tissues without causing disease symptoms.The plant hosts in mutualistic symbioses provide favorable conditions for endophyte development.The microorganisms can produce the same compounds found in the medicinal plants, probably because an exchange of genetic material occurs between the endophyte and plant.Studying and understanding these interactions is essential to achieving the sustainable production of natural products with significant bioactivities from endophytic fungi [72] (Fig.27).
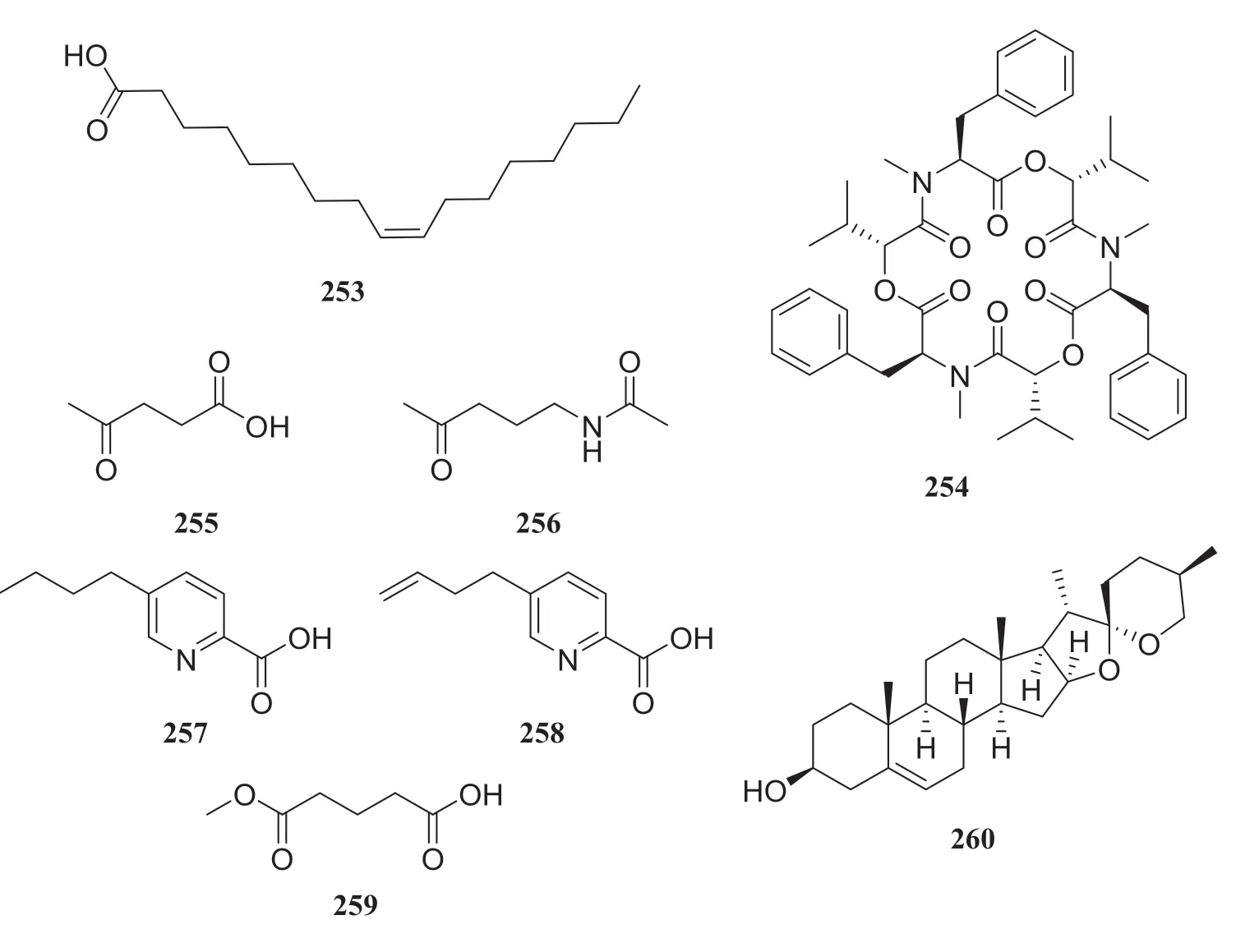
Fig.27 Structures of compounds 253— 260
In Hubei province, over 2000 natural medicinal plants are used in Tujia medicine [73 ].Because their overdevelopment and overuse, many medicinal plants are becoming scare, and some are facing extinction.Thus, the use of medicinal plants for the isolation of endophytic fungi is one conservation options.Moreover, endophytic fungi may significantly reduce the use of agrochemicals (fertilizers, fungicides, insecticides, and herbicides) in the cultivation of medicinal plants.The loss of endophytic microbes from medicinal plants during cultivation could be mediated by the transfer of endophytes from wild relatives of medicinal plants to cultivated species.Furthermore, endophytes from medicinal plants used in Tujia medicine have been poorly investigated and should not be neglected because of their natural origin.Finally, the study of endophytic fungi as a renewable source is in its infancy, and should be further explored in future research.
AcknowledgementsThis work was financially supported by the National Natural Science Foundation of China (Grants No.32000011 and 21961142008).
Compliance with Ethical Standards
Conflicts of interestThe authors declare no confl ict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
杂志排行
Natural Products and Bioprospecting的其它文章
- Anticancer Properties of Lobetyolin, an Essential Component of Radix Codonopsis (Dangshen)
- Plants Used as Antihypertensive
- Bousmekines A-E, New Alkaloids from Two Bousigonia Species: B.angustifolia and B.mekongensis
- Furan Derivatives and Polyketides from the Fungus Irpex lacteus
- Identification of Anxiolytic Potential of Niranthin: In-vivo and Computational Investigations
- Structures, Chemical Conversions, and Cytotoxicity of Tricholopardins C and D, Two Tricholoma Triterpenoids from the Wild Mushroom Tricholoma pardinum
