Functional maturation of immature β cells: A roadblock for stem cell therapy for type 1 diabetes
2021-04-08ZiYiSunTingYanYuFangXuJiangWeiWang
Zi-Yi Sun, Ting-Yan Yu, Fang-Xu Jiang, Wei Wang
Zi-Yi Sun, Ting-Yan Yu, Fang-Xu Jiang, Wei Wang, Department of Endocrinology, Xiang’an Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen 361100, Fujian Province, China
Fang-Xu Jiang, School of Biomedical Science, University of Western Australia, Nedlands 6009, Australia
Fang-Xu Jiang, School of Health and Medical Sciences, Edith Cowan University, Perth 6000, Australia
Abstract Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease caused by the specific destruction of pancreatic islet β cells and is characterized as the absolute insufficiency of insulin secretion.Current insulin replacement therapy supplies insulin in a non-physiological way and is associated with devastating complications.Experimental islet transplantation therapy has been proven to restore glucose homeostasis in people with severe T1DM.However, it is restricted by many factors such as severe shortage of donor sources, progressive loss of donor cells, high cost, etc. As pluripotent stem cells have the potential to give rise to all cells including islet β cells in the body, stem cell therapy for diabetes has attracted great attention in the academic community and the general public.Transplantation of islet β-like cells differentiated from human pluripotent stem cells (hPSCs) has the potential to be an excellent alternative to islet transplantation.In stem cell therapy, obtaining β cells with complete insulin secretion in vitro is crucial.However, after much research, it has been found that the β-like cells obtained by in vitro differentiation still have many defects, including lack of adult-type glucose stimulated insulin secretion, and multihormonal secretion, suggesting that in vitro culture does not allows for obtaining fully mature β-like cells for transplantation.A large number of studies have found that many transcription factors play important roles in the process of transforming immature to mature human islet β cells.Furthermore, PDX1, NKX6.1, SOX9, NGN3, PAX4, etc., are important in inducing hPSC differentiation in vitro.The absent or deficient expression of any of these key factors may lead to the islet development defect in vivo and the failure of stem cells to differentiate into genuine functional β-like cells in vitro.This article reviews β cell maturation in vivo and in vitro and the vital roles of key molecules in this process, in order to explore the current problems in stem cell therapy for diabetes.
Key Words: Stem cell therapy; Type 1 diabetes mellitus; β cell; Maturation
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by the absolute deficiency of β-cell function.Disorders of the immune system cause destruction of β-cells, resulting in the absolute lack of insulin secretion and the inability to properly regulate blood glucose homeostasis[1,2].This process is mediated by autoimmunity, with the participation of both innate and adaptive immunity[3,4].Due to insufficient insulin secretion, blood glucose rises rapidly in the short term.This can cause life-threatening conditions such as hypoglycemia unawareness, diabetic ketoacidosis, or diabetic hyperosmolar coma.Long-term hyperglycemia will damage the cardiovascular and cerebrovascular systems and microcirculation in varying degrees[5], resulting in complications including eye disease, nephropathy, peripheral neuropathy, and coronary atherosclerotic heart disease.Significantly, T1DM is also associated with some other chronic autoimmune diseases, such as celiac disease[6,7].Finally, as most patients with T1DM have had the condition since childhood, longterm insulin use is not only an inconvenience to daily life, but also an economic burden on society.Therefore, research on new treatment methods for T1DM is crucial.
Since several decades ago, T1DM has been experimentally treated by whole pancreas and then islet transplantation[8,9].However, due to its unfeasibly high costs and insufficient donor sources for the increasing number of T1DM patients, this treatment cannot be widely implemented in practice.To resolve this problem, the ultimate goal is to develop a stem cell therapy for diabetes, namely, differentiate islet β-like cells from human pluripotent stem cells (hPSCs) capable of glucose stimulated insulin secretion (GSIS) similar to mature β cells, and effectively regulating blood glucose homeostasis in the body after transplantation.Great efforts have thus been concentrated on discovering technologies in how to effectively differentiate hPSCs into genuine β-like cells that could maintain the long-term survival and functional stability if transplanted.This review article summarizes the latest progresses on the β-cell development and functional maturityin vivoand on the differentiation of insulinsecreting β-like cellsin vitrofrom human pluripotent or multiple stem cells.
BRIEF SUMMARY OF AUTOIMMUNITY IN T1DM
The etiology and pathogenesis of T1DM are not fully understood, but they are generally believed to be related to genetic and environmental factors.Although most patients do not have a family history of T1DM, genetic susceptibility is an important factor.A combination of epigenetics such as DNA methylation and histone modification, altered microRNA profiles, and other pathological mechanisms may also be related to the development of T1DM and affects the immune reaction on islet β cells.Studies have shown that the main genetic risk factors for T1DM are located in the major histocompatibility complex class II human leukocyte antigen (HLA) region, and the genetic polymorphism in this region largely determines the genetic risk of T1DM[10-12].
The autoimmunity in T1DM patients is manifested by the presence of circulating islet autoantibodies and autoreactive T cells.The human body temporally establishes an immune balance after birth and the pancreatic islet self-reactive T cells are regulated and suppressed from becoming active[13,14].CD4+ helper cells and CD8+ cytotoxic T lymphocytes play an important role in the pathogenesis of T1DM by producing autoantibodies and recognizing β-cell proteins as autoantigens[15].HLA molecules induce the proliferation of pathogenic T cells by presenting self-antigens to naive T cells, and producing self-reactive CD4+ T cells.These activated CD4+ T cells then produce cytokines, which in turn activate β cell-specific cytotoxic CD8+ T cells.Subsequently, these activated T cells are recruited to pancreatic islets and stimulate macrophages and other T cells.This leads to the destruction of pancreatic β cells[16,17].
DEFECTS OF CURRENT T1DM TREATMENT
Patients with T1DM need lifelong insulin replacement therapy.Exogenous insulin supplementation is not only a cumbersome process but is also associated with acute hypoglycemia unawareness episodes.It can lead to chronic devastating complications such as heart and kidney failures, blindness, foot necrosis, and cancers.Although islet transplantation can replace destroyed β cells and exert insulin secretion function in the human body, this method has many limitations, such as a shortage of donors, high costs, strong immune system rejection after transplantation, and long-term use of antirejection drugs[18,19].Immunotherapy includes non-self-antigen-specific and self-antigen specific therapies.The former involves regulatory T cell replacement therapy that aims to self-reactivate T cells, B cells, and inflammatory cytokines, while the latter mainly targets the regulation and inactivation of self-antigen.Unfortunately, a T1DM immunotherapy that can totally replace the standard insulin replacement therapy has not yet been developed[20].Researchers have also tested the possibility of mysenchymal stem cells (MSCs) as an innovative treatment for autoimmune diseases.MSCs are a class of multipotent stem cells with the ability to self-replicate.Their inherent selfrenewal potential and immune regulation ability are considered to be an exciting starting point for the treatment of autoimmune diseases[21].For example, MSCs may have the ability to prevent the autoimmune destruction of β cells in T1DM animal models and generate functional β cells to maintain blood glucose homeostasis[22,23].
At present, there are several issues in the clinical application of stem cell therapy, including selection of appropriate encapsulation materials and transplantation site, and the need for further research on improving immune regulation and new blood vessel formation methods.However, the most pressing issue is how to obtain fully functional and mature β cells throughin vitroculture.In order to solve these problems, it is critical that the maturation process of islet β cellsin vivoandin vitro, and major functioning transcription factors and other critical molecules are better understood.
MATURATION PROCESS OF β CELLS IN VIVO
The pancreas consists largely of exocrine glands and in a smaller proportion, endocrine glands[24].The exocrine glands are composed of pancreatic acinar tissues and pancreatic ducts, and the endocrine glands are composed of cell clusters of different sizes, known as the islets of Langerhans[25].The islet is an endocrine micro-organ, consisting of at least five types of endocrine cells: α cells (15%-20%), β cells (60%-80%), δ cells (5%-10%), ε cells (< 1%), and pancreatic polypeptide-secreting (PP) cells (2%)[26].Observed by optical projection tomography, there are about 1000 islets in the pancreas of 8-wk-old mice, with each islet containing an average of 800 β cells.There are about 1000000 islets in the human pancreas, each containing about 400-600 β cells.The β cells as polygonal cells have a diameter of 13-18 μm, and each contains about 100000 insulin secretory vesicles[27,28].Insulin is stored in a crystallized form in these vesicles that are ultimately released through exocytosis[29].The exocytosis of insulin granules is controlled by the ATP-sensitive K (KATP) channel and requires calcium ions to flow into the cells through the cell membrane calcium channel.When the blood glucose concentration rises, the glucose uptake and metabolism in β cells also increase, which leads to an increase in ATP production.These changes in adenine nucleotide concentrations cause the KATPchannel to close, triggering calcium influx and insulin secretion[30].
Embryonic pancreatic development begins with ventral and dorsal pancreatic buds.In mice, the dorsal bud appears on day 9.0 of the embryo development (E9) and the ventral bud appears on E9.5 along the dorsal and ventral surfaces of the posterior foregut endoderm (Figure 1).The first transformation of mouse pancreatic morphology begins from E9.5 to E12.5, during which time pancreatic progenitor cells rapidly proliferate to form the pancreatic endoderm.At E12 to E13, the ventral and dorsal buds contact and fuse together.At E13, pancreatic endodermal cells proliferate, and pancreatic progenitor cells give rise to neurogenin 3 (NGN3) positive progenitor cells, which then form mature endocrine cells[31-33].By E14.5, the developing islets consist of many insulin-producing β and glucagon-producing α cells, and δ cells that secrete somatostatin appear for the first time.PP cells begin to appear before birth.At birth, β cells in mice do not have adult-type insulin secretion function, but gradually mature within 2-3 wk after birth[34].
During human embryonic development, dorsal pancreatic buds appear around the fourth week of gestation, followed by abdominal buds.In contrast to the early presence of glucagon-expressing cells in mouse pancreatic buds, human endocrineexpressing cells are not detected until G7.5–8w after the dorsal buds grow for 3 wk in early embryonic pancreas.These endocrine cells are derived from NGN3+ endocrine progenitor cells, and among them, the first to appear are insulin-producing β cells.The transcription factors PAX6, PAX4, NKX2.2, NKX6.1, HLXB9,etc.are involved in the process of differentiation from endocrine progenitor to insulin-producing β cells[35].βcell replication is easily detectable at G9w and peaks around G14-16w[36-38].At this time, embryonic β cells are multi-hormonal cells that produce insulin, glucagon, and growth hormone, as they are still in an immature state.Immature β cells have strong proliferative ability, but they do not have the functions of mature β cells.
The hallmark feature of functional β cells is mature GSIS, which means when postprandial blood glucose increases, pancreatic β cells secrete a sufficient amount of insulin to prevent hyperglycemia, and inhibit insulin secretion under fasting conditions to prevent hypoglycemia.This is also known as the biphasic model that is established after β-cell maturation.Human studies have shown that neonatal β cells do not have this biphasic secretion function, because these β cells are not fully mature at this stage.After birth, pancreatic islet cells gradually lose their proliferative capacity and develop highly sensitive and powerful GSIS capacity under the control of transcription factors such as MafA[39].There is no definite conclusion about the time point at which β cells fully mature in humans, but it is closely aligned to the time when a newborn begins to take food supplements.According to the experiment of Otonkoskiet al[40], human islet β cells obtain mature insulin secretion function at about 26-44 wk of age.However, further studies are required to confirm the stage of β-cell maturation.
KEY MARKERS IN THE PROCESS OF β CELL MATURATION
At present, due to the limitations on human studies, most of the understanding of βcell maturation comes from rodent studies.In mice, the β cells in the fetal stage are immature and highly proliferative.At this time, the β cells can already generate insulin granules and show high basal insulin levels, but the regulated mechanism of insulin secretion remains to be established.After birth, the β cells have not yet obtained a mature phenotype to respond to the stimulation of changing glucose concentration to properly secrete insulin[41,42].The first mature wave in mice appears 2 wk after birth.At this time, β cells are still proliferative, but this characteristic is gradually lost since β cells follow the biphasic maturation model and need to adapt to the dietary changes of the newborn.The second maturation wave occurs in the third week after birth, which coincides with the weaning period.This is also true for the human newborn[43-45].During this period, the proliferative property of β cells gradually disappears, and is replaced by an adult GSIS feature.The GSIS contains a variety of cellular processes, in which β cells sense changes in glucose concentration through specific glucose transporters (GLUT1 & GLUT2)[46,47].Subsequently, glucose stimulation causes the mitochondria to actively participate in the control and enhancement of insulin secretion in the mature GSIS process.Finally, the insulin granules fuse with the cytoplasmic membrane and secrete insulinviathe exocytosis[48].
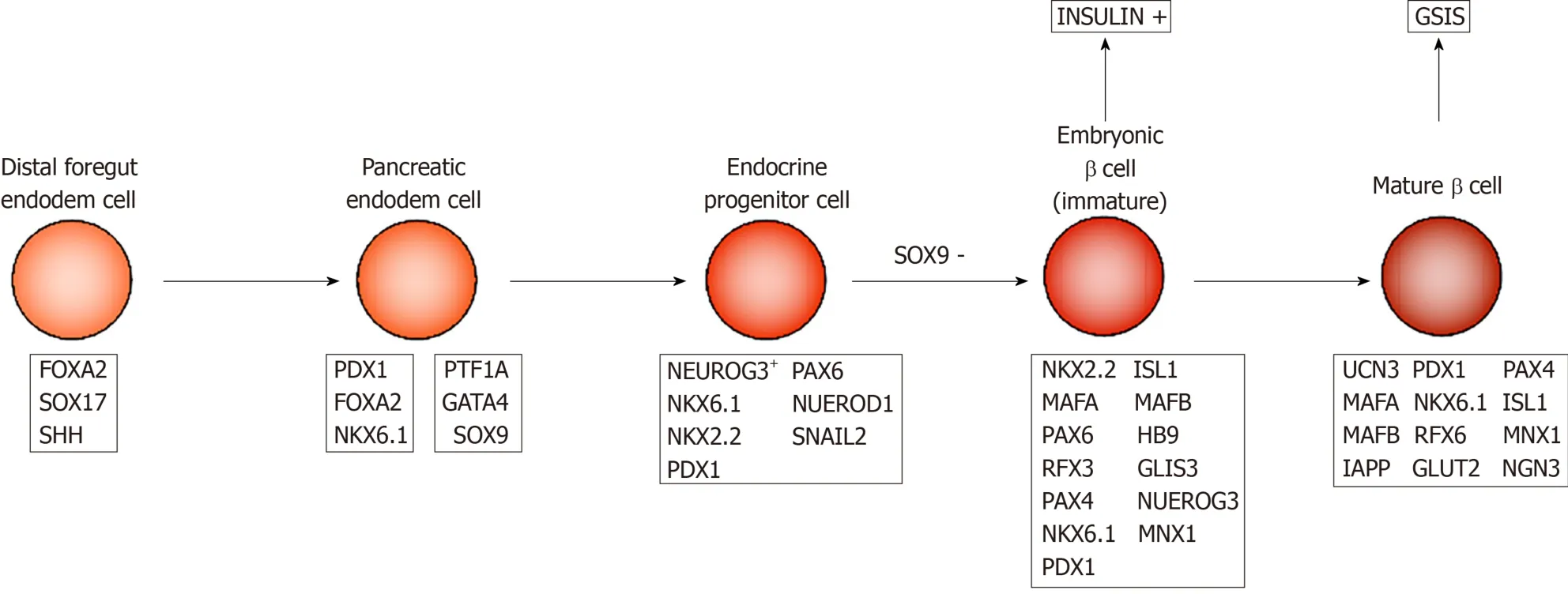
Figure 1 β-cell development and maturation in vivo.
There are many key transcription factors involved in the process of β cell maturation.For example, the transcription factors MAFA and MAFB play important roles in the development and maturation of β cells, respectively.MAFB is expressed earlier than MAFA, and appears in mouse pancreatic epithelial cells at E10.5[49], while MAFA is originally expressed in insulin+ cells at E13.5[50].At E15.5, 50% and 90% of cells with insulin secretion ability express MAFA and MAFB, respectively.However, in adult mice, MAFA is only expressed in β cells while MAFB is in α cells[51], suggesting the transformation from MAFB to MAFA signals β cell maturation.Critical for the development of immature insulin+ cells, MAFB is expressed in most insulin+ cells at E15.5 and E18.5.However, it is only expressed in a small amount of P14 mouse β cells, and by P28, MAFB expression is completely absent[52].In contrast, the level of MAFA in islet β cells in immature P2 mice is only 7% of that in adult mice, in which the GSIS properties of these β cells are also poor.MAFA overexpression in P2 β cells can substantially enhance the GSIS ability[53].Other experiments indicate that the expression level of MAFA in mature β cells is significantly higher than that in immature cells and is positively related to insulin secretion capacity[54].Taken together, the increased expression of MAFA and the disappearance of MAFB signal the maturation of β cell function and are important links for β cells to obtain adult GSIS.
Many other transcription factors are not direct markers of β-cell transformation from immaturity to maturity, but play important roles in the process of β-cells achieving functional maturity.During endocrine cell formation, NGN3 regulate the early differentiation of islet cells and formation of endocrine cells during development[55].All pancreatic endocrine cells are derived from NGN3 expressing endocrine progenitor cells.Individuals lacking NGN3 will not be able to produce any functional pancreatic endocrine cells and will subsequently die from diabetes[56,57].Individuals lacking NGN3 expression can still produce embryonic β cells and produce insulin, however, they cannot respond to glucose stimulation and eventually obtain functionally mature β cells[58], suggesting that NGN3 expression is essential for β-cell maturation.The transcription factor PDX1 is activated in the mouse foregut endoderm at E8.5 and expressed in multipotent pancreatic progenitor cells of early pancreatic buds[59].In 5-wk-old male mice, the lack of PDX1 expression results in changes in the expression of genes that control β-cell function and proliferation status (such asMAFAandGLUT2), leading to decreased insulin secretion levels[60].The β cell glucose tolerance of PDX1-deficient mice is impaired, plasma insulin levels are reduced, and the adult-type GSIS is impaired[61].
In addition to the above, the transcription factors NEUROD, MNX1, PAX4, NKX6.1,etc.also play important roles in the process of β-cells becoming functionally mature[62-65].
UCN3 can be used as a marker of β cell maturity[66].UCN3 is a member of the CRF (corticoltropin release-factor) family[67,68].As an endogenous ligand of the CRF receptor 2, it is closely related to the regulation of energy balance and/or glucose metabolism in the body[69].It is also expressed as a secreted protein in local areas of the brain and the pancreas.In mouse islets, UCN3 first appears in β cells at E17.5 and is expressed as a characteristic marker of β cells from P7 to the entirety of the adult period.Given that in P14, UCN3 and insulin expression completely overlap, Blumet al[70]used Western blot and immunohistochemistry to analyze the protein expression levels and found that UCN3 expression in mature β cells is 7 times higher than in immature cells.Immunofluorescence staining indicates that UCN3 expression is high in all adult β cells; however, this was not detected in embryonic islets at E18.5[70].
The functional maturation of β cells involves the switching of cell signals from mTORC1 to AMPK (5' adenosine monophosphate activated protein kinase)[71-73](Figure 2).mTOR is a nutrition-sensitive kinase and essential for regulating the proliferation and growth of postnatal pancreatic β cells[74,75].Studies have shown that mTORC1 promotes β-cell proliferation in embryonic and neonatal stages by regulating cyclins D2 and D3 and CDK4.The specific loss of mTORC1 in mouse β-cells can lead to severe glucose intolerance, which is related to an insufficient number of β-cells[76,77].AMPK is an effective inhibitor of mTORC1, and its kinase activity is regulated by the intracellular ratio of ATP to AMP/ADP[78].Loss of LKB1 (AMPK upstream activator) can increase β-cell proliferation and mass by inducing mTORC1, resulting in increased insulin output[79,80].Helmanet al[81]found that the function of β-cells after birth is closely related to changes in the nutritional environment, which is mainly due to amino acid-stimulated insulin secretion and GSIS and through the mTORC1 signaling pathway.These researchers found that under two nutritional conditions, there was no difference in the expression of PDX1, NKX6.1, UCN3, MAFA, and other transcription factors in β cells, indicating that the switch to adult-type GSIS is not affected by the expression of these markers.Instead, changes in glucose reactivity are related to the activation of mTORC1 after changes in nutritional conditions, and there is a positive correlation between insulin secretion and mTORC1 activation.Disrupting the nutritional sensitivity of mTORC1 in mature β cells will cause their insulin secretion to return to a functional immature state[82].
Synaptotagmin 4 (Syt4) may play an important role in the maturation of β cells[83].As a non-Ca2+binding paralog of the β cell Ca2+sensor Syt7, it increases approximately 8-fold during β-cell maturation, and the absence of Syt4 will increase the secretion of basal insulin in newborn mice.The role of this protein is to reduce the sensitivity of immature β cells to calcium ions that directly regulate the exocytosis of insulin granules and influence the normal secretory process of insulin[84].
Recently, a Wnt/Plane cell polarity effector protein Flattop (Fltp) was found to distinguish immature (Fltp-) and terminally mature (Fltp+) β cells[85].Fltp+ cells have higher expression levels of β-cell functional genes (i.e.,SLC2A2,NKX6.1,UCN3,MAFA,etc.), and it can be observed that the number of mature secreted granules is significantly increased, the mitochondrial physiological function is enhanced, and the static GSIS is higher[86].
Other studies showed that the microenvironment is also important for obtaining mature β cells[87,88].Freshly isolated β cells in suspension culture release much less insulin than scattered β cells that re-aggregate into islets, suggesting that the composition of pancreatic islets, cell polarity, contact between homotype cells, contact between heterotype cells, and interaction with the surrounding tissues and environment can all lead to differences in glucose reactivity and insulin secretion.
Among them, paracrine regulation plays an important role in β cell function (Figure 3).Even if the islets are dispersed to the cellular level, most β cells still retain the link with α cells[89,90], which suggests the co-evolution of the two types of endocrine cells is necessary for the pancreatic islet development, and may be of great significance to the pancreatic islet maturation.Islet paracrine signals from one cell type can regulate others in the same pancreatic islet by spreading through the gap or circulating through intra-islet blood vessels[91].For example, glucagon secreted from α cells inhibits insulin secretion from β cells (Figure 3B).Insulin receptors are found on both α and β cell membranes, which further confirms the existence of the paracrine effect of islet cells[92].Insulin secreted from β cells activates the GABAA receptor on the α cell membrane, leading to a large influx of Cl- and inhibiting the secretion of glucagon[93].β cells can electrically couple to surrounding α and δ cells through the gap, to secrete synchronously, thereby generating insulin secretion pulse[94].Moreover, secretory molecules of pancreatic islet pericytes and local macrophages have nutritional effects on β cells[95,96].
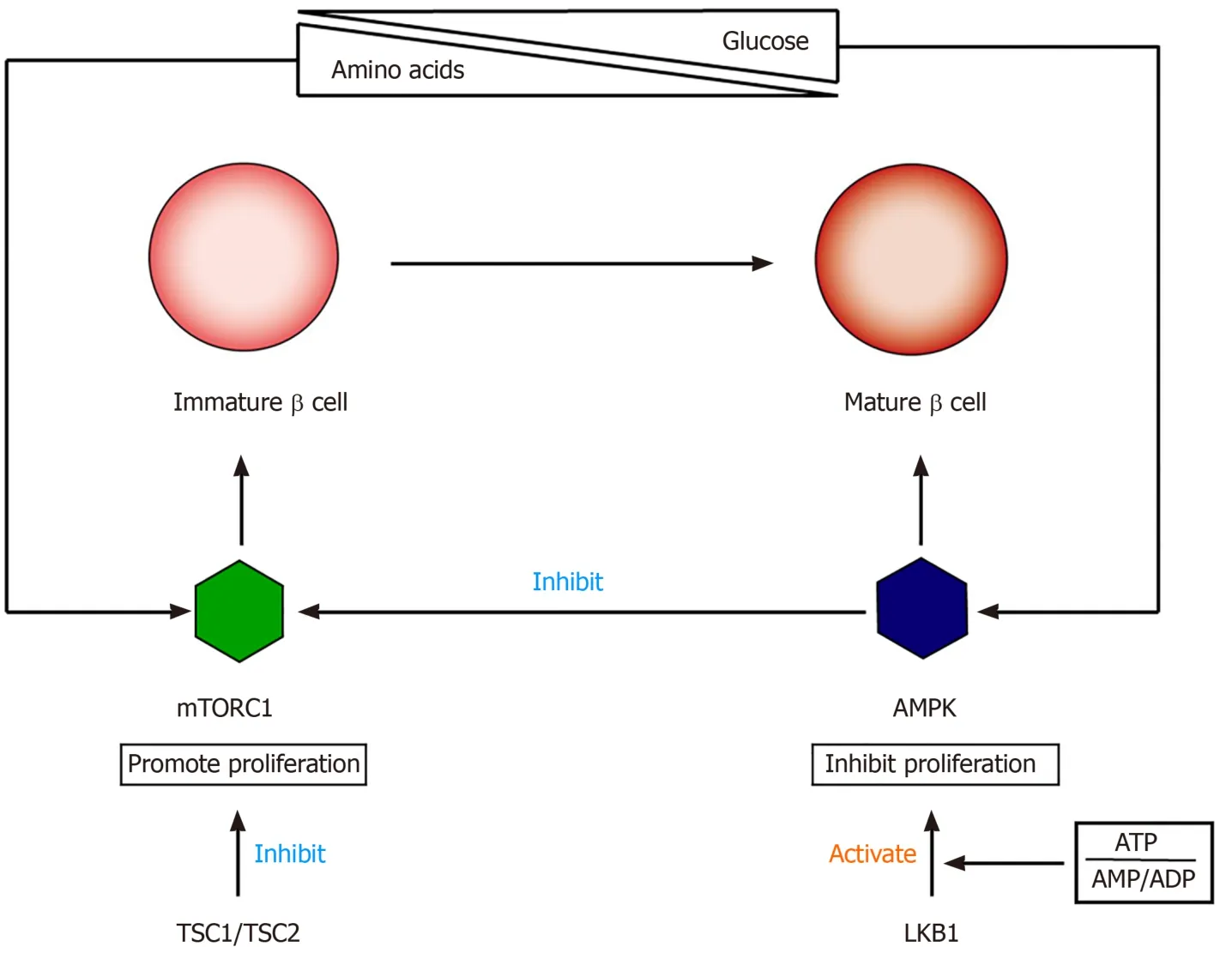
Figure 2 Relationship between mTORC1 and AMPK in the process of β cell transformation from immature to mature.
At low glucose concentrations, the islet α cells secrete glucagon that activates the glucagon receptor on the β cell membrane in a paracrine manner and inhibits insulin secretion[97].Additionally, ghrelin locally released by ε cells in pancreatic islets can inhibit GSIS.This process may involve the activation of GHSR-coupled Gi, the opening of voltage-dependent K+channels, and the inhibition of Ca2+influx[98].Finally, enteroendocrine hormones released after food ingesting participate in the GSIS.The most important hormones are glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)[99].Studies have confirmed the expression of GLP-1 and GIP receptors in pancreatic β cells.After activation, these two hormones bind to the receptor coupled to the heterotrimeric Gs protein, thereby activating adenylyl cyclase, increasing intracellular cAMP, and enhancing GSIS[97].
INDUCTION OF β CELLS IN VITRO AND CURRENT PROBLEMS
Due to the increasing demand forin vitrodifferentiation of hPSCs into β cells to treat T1DM, researchers have actively explored ways to obtain functionally β cellsin vitro.hPSCs include human embryonic stem cells (ESCs) and induced pluripotent stem cells (hiPSCs).These cells are considered a reliable source for β-cell replacement due to their ability to self-renew and differentiate into all major somatic lineages[100,101].After experimenting many methods, researchers found that the induction of genuine β cellsin vitromust follow the same differentiation processin vivo.Using Matrigel or lowdensity mouse embryonic fibroblasts as the culture platform, hPSCs can finally differentiate to β-like cells following the sequence of definitive endoderm, primitive intestinal canal pancreatic progenitor cells, endocrine progenitor cells, and hormoneexpressing endocrine cells, when regulated by specific doses and sequences of growth factors and signaling molecules (such as retinoic acid, BMP pathway inhibitors, FGF10, and FGF7)[102].However, β-like cells producedin vitroby this method are mainly insulin positive multi-hormonal cells.They will only exhibit limited GSISin vitrodue to their lack of expression of key transcription factors of β-cells.Once transplanted into mice, they lose the ability to respond to glucose concentration stimuli[103].
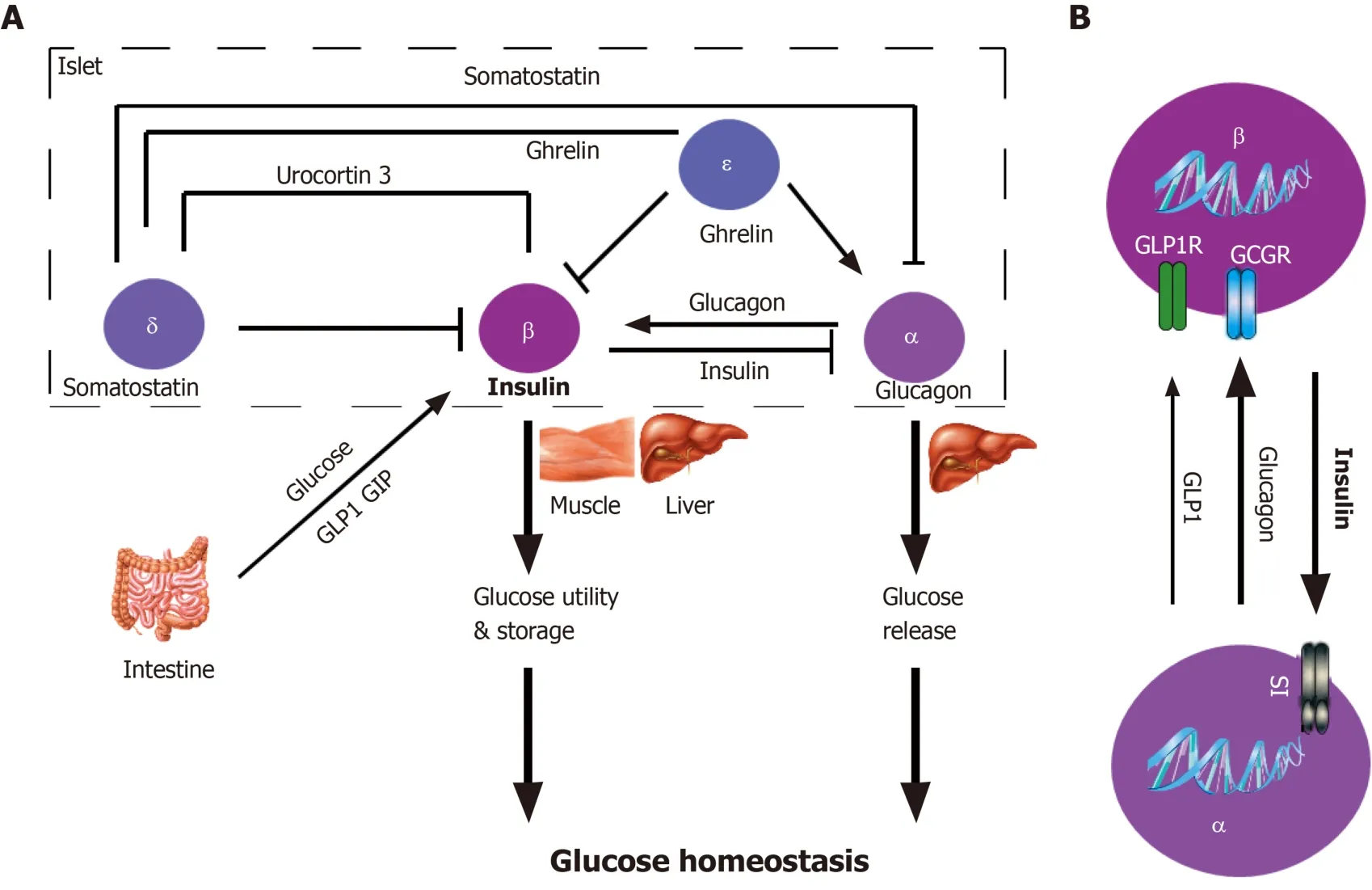
Figure 3 Paracrine regulations within the pancreatic islets.
Pagliucaet al[104]used a scalable suspension-based culture system to generate and cultivate hPSCs, and cell clusters (about 100-200 mm in diameter, each cluster contains hundreds of cells) from hESC line (HUES8) or hiPSC line (hiPSC-1 and hiPSC-2) were induced to transform into definitive endoderm (> 95% SOX17+ cells, DE), and subsequently differentiated into early pancreatic progenitor cells (> 85% PDX1+ cells, PP1).Culturing PP1 for 5 d under KGF or FGF7, retinoic acid, and SANT1 (sonic hedgehog signaling pathway antagonists) conditions can lead to forming pancreatic progenitor cells (PP2) expressing PDX1+/NKX6.1+, and producing functional β cells 3-4 mo after transplantation into mice.After testing the insulin secretion of these β-like cells, researchers found that their function is similar to that of adult β cells.
These stem cell (SC)-derived β-like cells are known as SC-β cells[104]that are arguably the most advanced β-like cells inducedin vitrofrom hPSCs.The increased level of UCN3 expression was found to coincide with the functional maturation of the SC-β cells[105,106].In SC-β cells, the nutritional regulation of mTORC1 activity is mainly determined by the amount of amino acids, not glucose, which is very similar to fetal β cells in the body[107].This is consistent with the fact that SC-β cells are not mature at this time.After reducing the amount of amino acids in the culture medium, the SC-β cells can be induced to be more mature[108].In adults, the supply of nutrients is periodic, so the activity of mTORC is also dynamic, which requires the participation of AMPK, TSC1, TSC2 (the upstream inhibitors of mTOR),etc.
Daviset al[109]found that SC-β cells have lower GSIS levels than cadaver islet β cells.They found that glucose metabolism is a restricting factor that inhibits the formation of mature GSIS in SC-β cells.Abnormal mitochondrial metabolism is also associated with the immature GSIS in SC-β cells that can be used to explore the metabolic processes and quantify their ability to transport glucose.Furthermore SC-β cells can sense and respond to changes in metabolic flux, but the metabolism of glyceraldehyde 3-phosphate is the key "defect" or "bottleneck".The activities of the enzymes GAPDH and PGK1 are significantly lower in SC-β cells than in cadaver islets, and these two enzymes can catalyze the enzymatic conversion of 3-phosphoglyceraldehyde to 3-phosphoglycerate.When the enzyme concentrations are reduced, the slow glycolysis flux in SC-β cells inhibits the production of phosphoenolpyruvate in mitochondria, resulting in restrictions on GSIS promoted by downstream mitochondrial phosphoenolpyruvate carboxykinase.Therefore, bypassing the above-mentioned defect in the glycolysis process that is unique to thein vitroculture process can drastically increase the intracellular PEP and make the cells have mature islet-like insulin secretion characteristics[110,111].The researchers proposed that treating differentiated SC-β cells with metabolized, cell-permeable intermediates that skip these enzymatic steps in glycolysis can result in islet-like insulin secretion and acts through the same mechanisms that underlie glucose sensing in functional islets.Taken together, these data suggest that thein vitrofunctional maturation of SC-β cells can be achieved by improving the nutritional conditions in the culture medium.However, further exploration is required as to how this bottleneck is formed in the differentiation processin vitro.
In the process of differentiation of β cellsin vitro, it is necessary to ensure the generation of other islet cells.Indeed, other cells may also be differentiated in addition to SC-β cells.Vereset al[112]showed that in SC-islets derived from hPSCs, there are also α-like cells expressing GCG, ARX, IRX2, and INS and enterochromaffin cells that express CHGA, TPH1, LMX1A, and SLC18A1.These cells are multi-hormonal cells and when transplanted, they can improve the function of β cells through local interactions or autocrine signaling in SC islets.Furthermore, CD49a was found as a surface marker of SC-β cells, and it was showed that pure SC-β cell clusters can be obtained by magnetic separation.
Protein transduction technology for delivering targeted transcription factors is also used to obtain insulin-producing cells from stem cellsin vitro[113].Protein transduction domains (PTD) or cell penetrating peptides can be directly internalized into cells when the protein is synthesized as a recombinant fusion molecule or covalently crosslinked to the PTDs, the mechanism of PTD-mediated protein transduction through endocytosis as a vesicle into the cytoplasm[114].Thus, PTD may provide a new strategy of generating insulin-secreting cells from stem/progenitor cells without transferring foreign transcription factor genes such asPDX-1,B2/NEUROD,NGN3, andISL-1[115,116].
In addition to hPSCs, multipotent stem cells including hematopoietic stem cells, mesenchymal stromal cells/MSCs, and adipose-derived stem cells are also possible sources for generation of insulin-producing cells[117].MSCs from various tissues and organs and the umbilical cord blood can be differentiated into islet-like cells or insulinproducing cells (IPC) that express key transcription factors such as PAX6 and ISL1[118].Besides IPC differentiation, MSCs may also secrete various cytokines and growth factors to help regenerate endogenous islet β cells[119].Siet al[120]found that injection of MSCs into diabetic rats can lead to significant endogenous β-cell regeneration.Meanwhile, Ianuset al[121]found that approximately 1.7% to 3% of regenerated islet β cells originated from transplanted MSCs.
CONCLUSION
T1DM is an autoimmune disease, which is generally early onset and is characterized by an absolute lack of insulin secretion.Therefore, the current treatment is to supplement the required insulin from an external source.However, there are many problems with this treatment method, including cumbersome procedures and associated devastating complications.If patients forget to take their medicine, acute complications are immediately developed, such as diabetic ketoacidosis.Therefore, in recent years, researchers have proposed other treatments for T1DM, such as islet transplantation, immunotherapy, and stem cell therapy.Among them, stem cell therapy is the most promising treatment method, but it still faces many obstacles such as generation of matured β-like cells in achieving the clinical application.First, the changes in the nutritional conditions, the surrounding microenvironment, and the related molecular mechanisms may all play a part in the maturation of β-like cells.Second, abnormal mitochondrial function in glucose metabolism is closely related to immature GSIS of β-like cells.Developing normal mitochondrial function in SC-β cells must be achieved in the next few years to generate mature β-like cells.Third, the microenvironment forin vitrodifferentiation also appears to be crucial for the functional maturation of β cells as isolated β cells cannot have adult-type GSIS, which is related to the paracrine effects among pancreatic islet cells as described above.This suggests that the reconstruction of islet-like structures, for example 3D bioprinting within vitrodifferentiated SC-β and other endocrine cells, is necessary for generating mature SC-β cells.Finally, critical molecules/compounds to mature the reconstructed islet-like structures need to be discovered.It is hoped that in the near future, stem cell therapy can ultimately become a viable curative treatment for most T1DM patients.
