多胺螯合纳米纤维高效去除Pb(Ⅱ)的特性与机制
2021-04-07范佩邱金丽于伟华李杰刘福强
范佩 邱金丽 于伟华 李杰 刘福强


摘 要:优选二乙烯三胺(DETA)作为胺化试剂对聚丙烯腈(PAN)纳米纤维进行改性,制备多胺纳米纤维吸附剂D-PAN,探究对Pb(Ⅱ)的吸附特性与机制。对D-PAN进行SEM、BET、FTIR和XPS表征,并通过试验研究溶液初始pH值、接触时间、温度、无机盐等因素对D-PAN吸附过程的影响。结果表明:多胺基团被成功引入D-PAN的三维网络中,多孔道结构有利于改善D-PAN对Pb(Ⅱ)的吸附性能。pH值为5.0时,D-PAN对Pb(Ⅱ)的静态吸附性能最优,由Langmuir模型拟合获得的最大吸附容量高达1.73 mmol/g,准一级动力学速率常数高达0.06 min-1。含盐体系中D-PAN对Pb(Ⅱ)的吸附量可提高近1倍,“盐促”效应显著。结合XPS和DFT结果分析,多胺基团中N原子可与Pb(Ⅱ)形成双齿和三齿螯合物。多次再生利用性能显示,D-PAN结构性能具有优良的稳定性。D-PAN具有吸附快、容量大、易再生等优点,具有广阔应用前景。
关键词:铅离子;多胺;螯合;纳米纤维;盐促
中图分类号:TU375.4 文献标志码:A 文章编号:2096-6717(2021)02-0182-08
Abstract: In order to explore the adsorption characteristics of Pb (Ⅱ) and mechanism, polyacrylonitrile was selected as the matrix to be chemically modified with diethylenetriamine (DETA),preferred as an amination reagent,and a kind of polyamine chelating nanofiber (D-PAN) has been successfully prepared. The physical and chemical structure analysis by SEM, BET,FTIR and XPS was performed, and the effects of initial pH value, contact time, temperature, inorganic salt and other factors on the adsorption process of D-PAN were studied.The results show that: amine groups was successfully introduced into three-dimensional network of D-PAN, and multi channel structure is conducive to improve the adsorption properties of D-PAN for Pb (Ⅱ). It had the best static adsorption property at pH 5.0. The maximum adsorption capacity was 1.73 mmol/g obtained by fitting with Langmuir model, and the quasi-first-order kinetic rate constant is up to 0.06 min-1. Moreover, D-PAN had a significant “salt-promoting” effect, and the adsorption amount of Pb (Ⅱ) in the salt-containing system could be nearly doubled.Combining the results of XPS and DFT, it was found that Pb(Ⅱ) could be removed by chelating with N atoms in polyamine groups to form bidentate and tridentate complexes.Furthermore, D-PAN exhibited excellent structural stability after multiple regeneration. In summary, D-PAN has the advantages of fast adsorption, large capacity and easy regeneration, and has broad application prospects.
Keywords:lead ions; polyamine; chelation; nanofiber; “salt promoting” effect
矿冶、铅酸蓄电池、电镀等行业生产是水中重金属铅的主要来源[1]。近年来,陕西凤翔县[2]、湖南衡东县[3]、广东紫金县[4]等多地爆发铅污染事件,严重威胁水生态安全以及人类健康[5-6]。中国将强毒性铅列为第一类污染物,《地表水环境质量标准》(GB 3838—2002)中Ⅲ类水的限值为0.05 mg/L,而且相关行业铅的排放限值也十分严格[7-8]。含铅废水常用治理技术包括化学沉淀法、电解法、膜分离法和吸附法等[9]。吸附法因操作简便、选择性好并可循环使用,成为水体铅污染的深度治理主流技术之一[10]。
多种吸附剂及其改性材料已被广泛用于Pb(Ⅱ)的吸附去除。例如,Niu等[11]、张雪彦等[12]、蘭舫等[13]分别利用复合生物炭、改性乙酸木质素和交联羧甲基罗望子胶吸附Pb(Ⅱ),但最大吸附量仅分别为0.58、0.39、0.64 mmol/g。Ma等[14]利用层状双金属氢氧化物纳米颗粒吸附Pb(Ⅱ),可在1 h内达到吸附平衡,且最大吸附量高达1.40 mmol/g,然而,因难以回收再利用限制了其实际应用。因此,亟需研发容量大、速率快、易回收的高效除Pb(Ⅱ)吸附剂。
纳米纤维具有质轻、高长径比、多孔道三维网络结构等特点[15]。由其制备的块体材料不仅具有纳米材料的快速吸附优势,而且易于回收[16]。其中,聚丙烯腈(PAN)纳米纤维已被广泛用于去除重金属离子,例如:Deng等[17]发现聚乙烯亚胺(PEI)修饰的碳管改性PAN纳米纤维对Pb(Ⅱ)的最大吸附量可达1.12 mmol/g。Wang等[18]制备了盐酸羟胺改性的PAN多孔层状吸附剂,对Pb(Ⅱ)的最大吸附量可达1.17 mmol/g,且共存NaCl可将其平衡吸附量提升约45%,表现出“盐促”效应[19]。因此,利用多胺化合物修飾PAN纤维类吸附剂,可综合发挥氮原子配位能力以及PAN纳米纤维基体易回收的结构优势,实现Pb(Ⅱ)的高效去除及吸附剂的再生利用[20]。笔者优选二乙烯三胺(DETA)对PAN纳米纤维进行化学改性,制备高效吸附剂D-PAN用于Pb(Ⅱ)的去除,观察溶液初始pH值、接触时间、温度等因素对吸附过程的影响,并结合X射线光电子能谱(XPS)和密度泛函理论计算(DFT)[21],系统分析D-PAN对水中Pb(Ⅱ)的吸附特性与机制。
1 试验
1.1 仪器
油浴锅(AL404),上海森信实验仪器有限公司;冷冻干燥箱(Scientz-12N),上海上登实验设备有限公司;分析天平(AL104)和pH计(FE20K),梅特勒托利多仪器(上海)有限公司;全温培养振荡器(HZP-250),上海精宏实验设备有限公司;超纯水机(SYNS OOOCN),密理博有限公司;静电纺丝机(CS30K-H),郑州成越科学仪器有限公司;等离子体发射光谱仪(ICAP-6300),赛默飞世尔;扫描电镜(S4800),日本日立公司;中孔物理吸附仪(TriStar Ⅱ 3020),麦克默瑞提克(上海)仪器有限公司;傅里叶变换红外光谱仪(Equinox 55),德国布鲁克光学仪器有限公司;电子能谱仪(PHI 5000 VersaProbe),日本UlVAC-PHI公司。
1.2 试剂与材料
聚丙烯腈(PAN,Mw=150 000)和聚乙烯亚胺(PEI,Mw=70 000),麦克林化学试剂有限公司;二乙烯三胺(DETA)和四乙烯五胺(TEPA),上海阿拉丁生化科技股份有限公司;无水碳酸钠、氢氧化钠、硝酸、硝酸铅、硝酸钠、硝酸钾、硝酸镁、硝酸钙、氯化钠、硫酸钠和NN-二甲基甲酰胺(DMF)等均为分析纯,来自南京化学试剂有限公司;实验用水为超纯水。
1.3 D-PAN的制备方法
称取1.000 g PAN粉末溶解于10 mL DMF中,室温下搅拌至完全溶解,制得纺丝前体溶液,将其转移到玻璃注射器中,纺丝机正、负极分别与注射器针头和收集板相接,制备PAN纳米纤维。纺丝参数:正、负极间距16 cm、电压18 kV、流速1.0 mL/h。
在50 mL三口烧瓶中加入20 mL DETA和0.100 g PAN纳米纤维,室温下静置2 h,加入0.500 g无水碳酸钠,于393 K下搅拌反应3 h。待冷却至室温,滤出纤维,用超纯水洗涤至出水呈中性,经冷冻干燥后即可制得D-PAN。合成路径如图1所示。
1.4 静态吸附实验
量取一定浓度的Pb(Ⅱ)溶液于锥形瓶中,使用HNO3和NaOH调节pH值,随后加入一定量D-PAN,置于恒温振荡器中,以140 r/min的转速振荡24 h,测定平衡时溶液中Pb(Ⅱ)的浓度,并按式(1)计算平衡吸附量。
2 实验结果与讨论
2.1 形貌分析
图2为D-PAN的扫描电镜图(SEM)。单根纤维的直径约为360 nm,各根纤维相互缠绕穿插,形成具有多通道的网络结构。与PAN纳米纤维相比,D-PAN的比表面积、平均孔直径和孔体积均增加(表1),且表面粗糙,这是胺化反应的结果[22]。D-PAN形貌和孔径结构的变化利于更多吸附位点的暴露,用于Pb(Ⅱ)的捕集。
2.2 红外光谱
图3为D-PAN的红外光谱图(FTIR)。2 923、1 450 cm-1分别为—CH2-的反对称伸缩振动和面内弯曲振动吸收[23],对应于PAN基体的碳骨架和胺化试剂DETA中的亚甲基。2 239 cm-1处尖锐的峰为—C≡N的伸缩振动吸收,与PAN纳米纤维相比,D-PAN在此处的峰强明显减弱,反映出少量氰基被保留,而大量氰基发生胺化反应[24]。3 199 cm-1处较宽的峰和1 582 cm-1处尖锐的峰分别对应于伯(仲)胺基的N—H伸缩振动和面内弯曲振动吸收[25],1 075 cm-1处对应于伯(仲)胺基的C—N伸缩振动[23]。可见,DETA通过氰基的胺化反应被成功接枝到PAN基体上。
2.3 pH值的影响
Pb(Ⅱ)初始浓度1.0 mmol/L,吸附剂浓度0.4 g/L,温度为298 K,探究不同初始pH值(2.0、3.0、4.0、5.0)对D-PAN吸附Pb(Ⅱ)的影响,结果如图4所示。吸附量随着pH值升高而上升。在低pH值时,D-PAN上伯(仲)胺基发生质子化,占用氮原子上的孤对电子,不利于Pb(Ⅱ)的吸附。随着pH值升高,胺基去质子化,并恢复与Pb(Ⅱ)的配位能力,因此,吸附量显著提升[25]。
如图5所示,D-PAN在30 min内快速吸附,达到平衡吸附量的86.3%,120 min可达到吸附平衡,准一级动力学方程对实验结果拟合更优。D-PAN对溶液中Pb(Ⅱ)的快速吸附得益于内部相互穿插的网络和优化的孔隙结构,利于吸附质在纤维内部的快速扩散[16]。
2.6 常规无机离子的影响
Pb(Ⅱ)初始浓度1.0 mmol/L,分别与K+、Na+、Ca2+、Mg2+(2.5、10.0 mmol/L)共存和Pb(Ⅱ)初始浓度3.0 mg/L分别与Cl-、NO-3和SO42-(1.0 mmol/L)共存,吸附剂浓度0.4 g/L,pH=5.0,温度为298 K,探究无机阴阳离子共存对D-PAN吸附Pb(Ⅱ)的影响,结果如图7所示。常规阴阳离子共存均不同程度促进了D-PAN对Pb(Ⅱ)的吸附,吸附量最高可提升95.87%(K+共存)。“盐促”效应的主要机制在于:1)伴随碱(土)金属盐的加入,溶液中阴离子浓度增加,可以平衡D-PAN上正电荷,静电屏蔽作用促进D-PAN对Pb(Ⅱ)的吸附[31-33];2)根据软硬酸碱理论,Pb(Ⅱ)为交界酸,K+、Na+、Ca2+、Mg2+为硬酸,胺基与Pb(Ⅱ)的亲和力更强[34],碱(土)金属阳离子无法竞争吸附位点。3种阴离子共存的促进作用存在细微的差异,可能是因为Cl-与Pb(Ⅱ)发生配位作用,影响水体中Pb(Ⅱ)的形态,而SO2-4的电荷数高,静电屏蔽作用更明显,且可能存在吸附架桥作用[35]。
2.7 再生和稳定性能
利用0.1 mol/L的硝酸对D-PAN进行再生,随后进行再吸附实验,结果如图8所示。4次再生利用后,D-PAN的吸附量为1.10 mmol/g,证明其具有优良的再生和稳定性能。
2.8 吸附机理分析
2.8.1 XPS
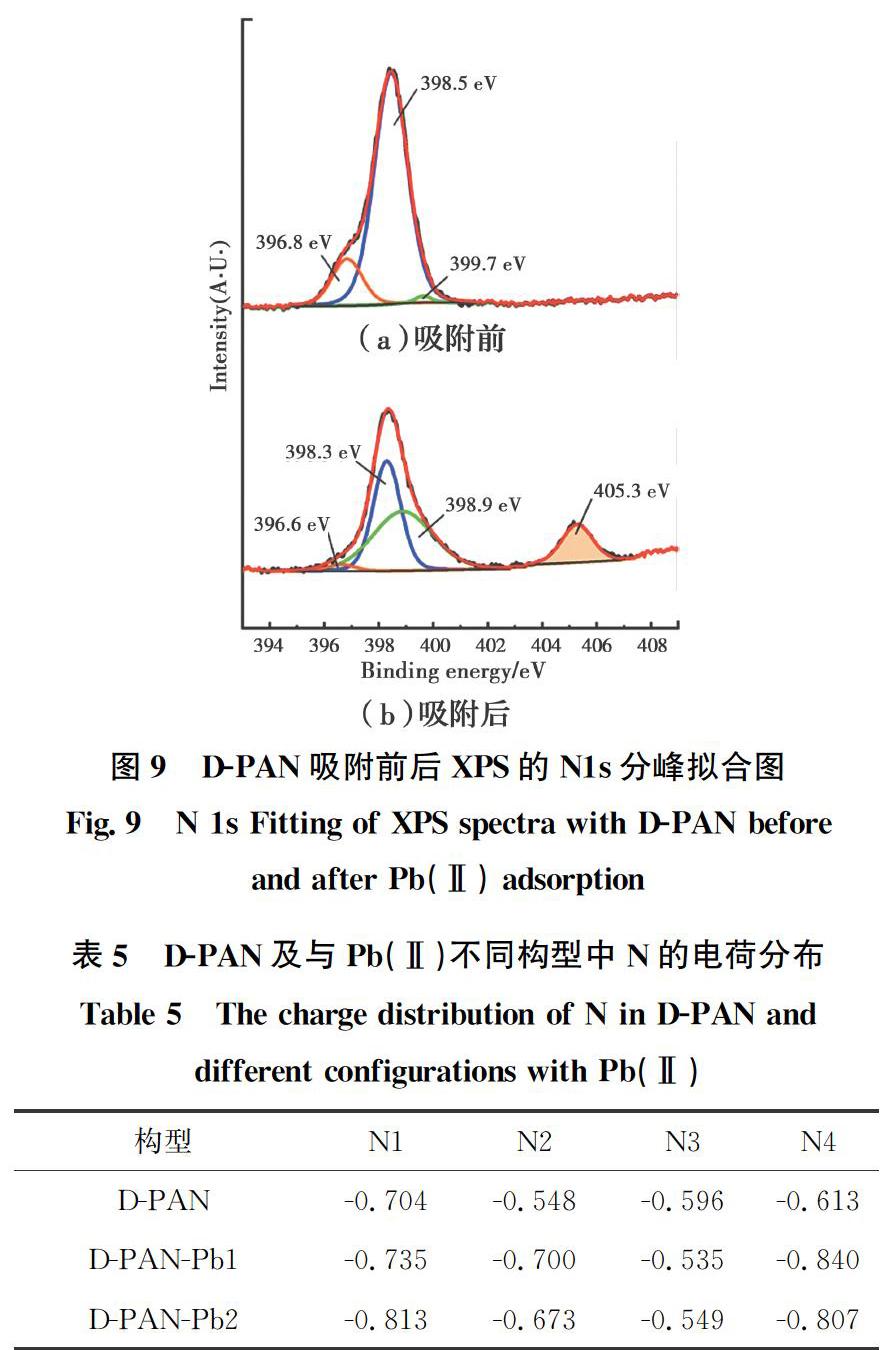
通过对比D-PAN吸附前后的XPS结果,探究吸附机理。对吸附后D-PAN的N1s进行分峰拟合,出现氮的4个XPS特征峰,如图9所示。其中,398.3 eV为—NH2/—NH—的結合能谱峰,相较于吸附前的398.5 eV发生了0.2 eV的负向位移,这是伯(仲)胺基中氮的孤对电子与Pb(Ⅱ)配位的结果,与DFT计算结果一致(见表5,与Pb(Ⅱ)配位后,N1、N2和N4的电荷密度均增加)。405.3 eV为硝态氮的结合能谱峰[36],证实NO-3进入D-PAN中,随着Pb(Ⅱ)的吸附,溶液中的NO-3也被吸附到D-PAN上以平衡电荷,与文献[37]结果一致。
2.8.2 DFT计算
通过DFT计算模拟吸附过程中可能出现的配合物构型[21]。如图10所示,D-PAN中的N原子通过D-PAN-Pb1(构型1)和D-PAN-Pb2(构型2)两种方式与Pb(Ⅱ)发生配位,具体计算结果见表6。配合物中Pb(Ⅱ)的电荷分别为1.298和1.164,表明吸附过程存在配体向Pb(Ⅱ)的电荷转移[38]。构型1中,Pb(Ⅱ)与两个N原子发生配位,键长分别为2.238、2.420 。构型2中,Pb(Ⅱ)与3个N原子配位,键长分别为2.314、2.433、2.409 。两种构型的结合能分别为-1 566.49、-1 690.83 kJ/mol,其中,构型2的结合能更低,可能为D-PAN和Pb(Ⅱ)的主要结合模式。
3 结论
通过二乙烯三胺改性聚丙烯腈纳米纤维成功制备多胺螯合纳米纤维吸附剂D-PAN。pH=5.0时,2 h内可达到Pb(Ⅱ)的吸附平衡,常温下最大吸附量为1.73 mmol/g。无机盐共存通过电荷屏蔽作用促进D-PAN对Pb(Ⅱ)的吸附,具有显著的“盐促”效应。经4次再生利用后,D-PAN的吸附量仍可达1.10 mmol/g。结合XPS表征和DFT计算结果分析吸附机理可知,Pb(Ⅱ)通过与多胺基团中N原子形成双齿和三齿配合物,构建稳定螯合结构实现Pb(Ⅱ)的去除。综上所述,D-PAN具有吸附快、容量大、易再生和“盐促”等优点,因而具有广阔的应用前景。
参考文献:
[1] SUN Y J, ZHOU S B, PAN S Y, et al. Performance evaluation and optimization of flocculation process for removing heavy metal [J]. Chemical Engineering Journal, 2020, 385: 123911.
[2] 韩颖. “血铅超标”事件追踪[J]. 劳动保护, 2011(4): 34-36.
HAN Y. The event tracking of "blood lead excess"[J]. Labour Protection, 2011(4): 34-36. (in Chinese)
[3] 吴龙贵. “吃铅笔也超铅”可怕在哪[J]. 环境教育, 2014(7): 30.
WU L G. The point in the phenomenon that eating pencils leads to excessive Pb [J]. Environmental Education, 2014(7): 30. (in Chinese)
[4] 叶伟雄, 叶艺娟, 黄振波, 等. 紫金县临江开发区1297名学生血铅检测结果分析[J]. 海峡预防医学杂志, 2013, 19(3): 29-30.
YE W X, YE Y J, HUANG Z B,et al. Analysis of blood lead test results of 1297 students in Linjiang Development Zone, Zijin County [J]. Strait Journal of Preventive Medicine, 2013, 19(3): 29-30. (in Chinese)
[5] FU J J, ZHANG A Q, WANG T, et al. Influence of E-waste dismantling and its regulations: temporal trend, spatial distribution of heavy metals in rice grains, and its potential health risk [J]. Environmental Science & Technology, 2013, 47(13): 7437-7445.
[6] TURNER A. Heavy metals in the glass and enamels of consumer container bottles [J]. Environmental Science & Technology, 2019, 53(14): 8398-8404.
[7] 辛玉婷, 花月, 沈小帅, 等. 铅蓄电池行业大气污染物现行排放标准在江苏省的适用性分析[J]. 环境科技, 2016, 29(5): 73-77.
XIN Y T, HUA Y, SHEN X S, et al. The applicability of air pollutants eemission standards for lead battery industry in Jiangsu Province [J]. Environmental Science and Technology, 2016, 29(5): 73-77. (in Chinese)
[8] 王宗爽, 徐舒, 安廣楠, 等. 铅大气污染物环境保护标准限值研究[J]. 环境科学学报, 2019, 39(9): 3163-3170.
WANG Z S, XU S, AN G N, et al. Research on the lead limit values of environmental protection standards [J]. Acta Scientiae Circumstantiae, 2019, 39(9): 3163-3170. (in Chinese)
[9] LUO J M, SUN M, RITT C L, et al. Tuning Pb(Ⅱ) adsorption from aqueous solutions on ultrathin iron oxychloride (FeOCl) nanosheets [J]. Environmental Science & Technology, 2019, 53(4): 2075-2085.
[10] SIRVI J A, VISANKO M. Lignin-rich sulfated wood nanofibers as high-performing adsorbents for the removal of lead and copper from water [J]. Journal of Hazardous Materials, 2020, 383: 121174.
[11] NIU Z R, FENG W L, HUANG H, et al. Green synthesis of a novel Mn-Zn ferrite/biochar composite from waste batteries and pine sawdust for Pb2+ removal [J]. Chemosphere, 2020, 252: 126529.
[12] 张雪彦, 金灿, 刘贵锋, 等. 希夫碱型木质素基吸附材料的制备及其对Pb2+吸附性能研究[J]. 离子交换与吸附, 2017, 33(5): 403-415.
ZHANG X Y, JIN C, LIU G F, et al. Preparation of schiff base-modified-lignin adsorbent and its adsorption performance of Pb2+ [J]. Ion Exchange and Adsorption, 2017, 33(5): 403-415. (in Chinese)
[13] 兰舫, 牛春梅, 李绍英, 等. 交联羧甲基罗望子胶对Pb2+的吸附研究[J]. 离子交换与吸附, 2014, 30(3): 242-249.
LAN F, NIU C M, LI S Y, et al. Adsorption performance of Pb2+ by crosslinked carboxymethyl tamarind [J]. Ion Exchange and Adsorption, 2014, 30(3): 242-249. (in Chinese)
[14] MA L J, WANG Q, ISLAM S M, et al. Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS2-4 ion [J]. Journal of the American Chemical Society, 2016, 138(8): 2858-2866.
[15] XUE J J, WU T, DAI Y Q, et al. Electrospinning and electrospun nanofibers: methods, materials, and applications [J]. Chemical Reviews, 2019, 119(8): 5298-5415.
[16] QIU J L, FAN P, YUE C L, et al. Multi-networked nanofibrous aerogel supported by heterojunction photocatalysts with excellent dispersion and stability for photocatalysis [J]. Journal of Materials Chemistry A, 2019, 7(12): 7053-7064.
[17] DENG S, LIU X H, LIAO J B, et al. PEI modified multiwalled carbon nanotube as a novel additive in PAN nanofiber membrane for enhanced removal of heavy metal ions [J]. Chemical Engineering Journal, 2019, 375: 122086.
[18] WANG G, WANG J R, ZHANG H, et al. Functional PAN-based monoliths with hierarchical structure for heavy metal removal [J]. Chemical Engineering Journal, 2017, 313: 1607-1614.
[19] 徐超, 劉福强, 巢路, 等. 新型多胺类螯合树脂的设计、制备及其对重金属离子吸附特性的研究[J]. 离子交换与吸附, 2013, 29(6): 481-495.
XU C, LIU F Q, CHAO L, et al. Synthesis of polyamine chelating resins and adsorption properties toward heavy metal ions from aqueous media [J]. Ion Exchange and Adsorption, 2013, 29(6): 481-495. (in Chinese)
[20] ZHOU H, ZHU H X, XUE F, et al. Cellulose-based amphoteric adsorbent for the complete removal of low-level heavy metal ions via a specialization and cooperation mechanism [J]. Chemical Engineering Journal, 2020, 385: 123879.
[21] ZOU L Z, SHAO P H, ZHANG K, et al. Tannic acid-based adsorbent with superior selectivity for lead(Ⅱ) capture: Adsorption site and selective mechanism [J]. Chemical Engineering Journal, 2019, 364: 160-166.
[22] XU X, ZHANG H J, AO J X, et al. 3D hierarchical porous amidoxime fibers speed up uranium extraction from seawater [J]. Energy & Environmental Science, 2019, 12(6): 1979-1988.
[23] NIE R F, MIAO M, DU W C, et al. Selective hydrogenation of C=C bond over N-doped reduced graphene oxides supported Pd catalyst [J]. Applied Catalysis B: Environmental, 2016, 180: 607-613.
[24] ALMASIAN A, GIAHI M, CHIZARI FARD G, et al. Removal of heavy metal ions by modified PAN/PANI-nylon core-shell nanofibers membrane: Filtration performance, antifouling and regeneration behavior [J]. Chemical Engineering Journal, 2018, 351: 1166-1178.
[25] HONG G S, LI X, SHEN L D, et al. High recovery of lead ions from aminated polyacrylonitrile nanofibrous affinity membranes with micro/nano structure [J]. Journal of Hazardous Materials, 2015, 295: 161-169.
[26] ZIMMERMANN A C, MECAB A, FAGUNDES T, et al. Adsorption of Cr(VI) using Fe-crosslinked chitosan complex (Ch-Fe) [J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 192-196.
[27] CHEN C, ZHANG M, GUAN Q X, et al. Kinetic and thermodynamic studies on the adsorption of xylenol orange onto MIL-101(Cr) [J]. Chemical Engineering Journal, 2012, 183: 60-67.
[28] HUI B, ZHANG Y, YE L. Preparation of PVA hydrogel beads and adsorption mechanism for advanced phosphate removal [J]. Chemical Engineering Journal, 2014, 235: 207-214.
[29] SAEED K, HAIDER S, OH T J, et al. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime)nanofibers and their applications to metal ions adsorption [J]. Journal of Membrane Science, 2008, 322(2): 400-405.
[30] ZOU M Y, ZHANG J D, CHEN J W, et al. Simulating adsorption of organic pollutants on finite (8, 0) single-walled carbon nanotubes in water [J]. Environmental Science & Technology, 2012, 46(16): 8887-8894.
[31] LI J, ZHANG S W, CHEN C L, et al. Removal of Cu(Ⅱ) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles [J]. ACS Applied Materials & Interfaces, 2012, 4(9): 4991-5000.
[32] KOSA S A, AL-ZHRANI G, ABDEL SALAM M. Removal of heavy metals from aqueous solutions by multi-walled carbon nanotubes modified with 8-hydroxyquinoline [J]. Chemical Engineering Journal, 2012, 181/182: 159-168.
[33] HU R, WANG X K, DAI S Y, et al. Application of graphitic carbon nitride for the removal of Pb(Ⅱ) and aniline from aqueous solutions [J]. Chemical Engineering Journal, 2015, 260: 469-477.
[34] KUMAR P A, RAY M, CHAKRABORTY S. Adsorption behaviour of trivalent chromium on amine-based polymer aniline formaldehyde condensate [J]. Chemical Engineering Journal, 2009, 149(1/2/3): 340-347.
[35] BRADL H B. Adsorption of heavy metal ions on soils and soils constituents [J]. Journal of Colloid and Interface Science, 2004, 277(1): 1-18.
[36] LIU W J, ZENG F X, JIANG H, et al. Adsorption of lead (Pb) from aqueous solution with Typha angustifolia biomass modified by SOCl2 activated EDTA [J]. Chemical Engineering Journal, 2011, 170(1): 21-28.
[37] ZHU C Q, LIU F Q, XU C, et al. Enhanced removal of Cu(Ⅱ) and Ni(Ⅱ) from saline solution by novel dual-primary-amine chelating resin based on anion-synergism [J]. Journal of Hazardous Materials, 2015, 287: 234-242.
[38] ZHU S, ASIM KHAN M, WANG F Y, et al. Rapid removal of toxic metals Cu2+ and Pb2+ by amino trimethylene phosphonic acid intercalated layered double hydroxide: A combined experimental and DFT study [J]. Chemical Engineering Journal, 2020, 392: 123711.
(編辑 胡玲)
