Preparation and characterization of HMX/EVA/hBNNSs microcomposites with improved thermal stability and reduced sensitivity
2021-03-23YueYngXiodongLiYntoSunJinTinHuiminLiuBidongWuJingyuWng
Yue Yng ,Xio-dong Li ,b,*,Yn-to Sun ,b,Jin-n Tin ,Hui-min Liu ,Bi-dong Wu ,b,Jing-yu Wng ,b
a School of Environment and Safety Engineering,North University of China,Taiyuan,030051,Shanxi,China
b Shanxi Engineering Technology Research Center for Ultrafine Powder,North University of China,Taiyuan,030051,Shanxi,China
Keywords:Energetic materials 2D(two dimensional)materials HBNNSs(hexagonal boron nitride nanosheets)HMX(1,3,5,7-Tetranitro-1,3,5,7-tetrazocane)Thermal stability Mechanical sensitivity
ABSTRACT Hexagonal boron nitride nanosheets(HBNNSs)have huge potential in the field of coating materials owing to their remarkable chemical stability,mechanical strength and thermal conductivity.Thin-layer hBNNSs were obtained by a liquid-phase exfoliation of h-BN powders and incorporated into EVA coatings for improving the safety performance of 1,3,5,7-tetranitro-1,3,5,7-tetrazocane(HMX).HBNNSs and ethylene-vinyl acetate copolymer(EVA)were introduced to HMX by a solvent-slurry process.For comparison,the HMX/EVA and HMX/EVA/graphene(HMX/EVA/G)composites were also prepared by a similar process.The morphology,crystal form,surface element distribution,thermal decomposition property and impact sensitivity of HMX/EVA/hBNNSs composites were contrastively investigated.Results showed that as prepared HMX/EVA/hBNNSs composites were well coated with hBNNSs and EVA,and exhibited better thermal stability and lower impact sensitivity than that of HMX/EVA and HMX/EVA/G composites,suggesting superior performance of desensitization of hBNNSs in explosives.
1.Introduction
Explosives,as metastable materials with high energy density,are able to perform rapid decomposition and release large amounts of heat and gases when subjected to some certain external stimuli[1-3].However,the rapid improvements of this material are greatly limited by some intrinsic deficiencies in safe performance and thermal property[4].Desensitization is one of the main strategies used to control the sensitivity of explosives to thermal stimulation and mechanical loading[5].Recently,the application of two dimensional(2D)materials shows great potential as desensitizers in explosives to improve security and thermal performance[6-8].
Among 2D crystals,hexagonal boron nitride nanosheets(hBNNSs)as a promising coating material[9],possess high oxidation resistance[10],high thermal conductivity[11-13],stable dielectric properties[14],high operation temperature and wide band gap[15],accompanied by remarkable physicochemical stability[16-18].This characteristics makes hBNNSs attractive fillers in composites for a wide range of applications,including energetic materials.Vovusha studied the adsorption characteristics of explosive molecules with hexagonal boron nitride(h-BN)and graphene flakes using first principles density functional theory(DFT)and found that binding between h-BN flakes and the explosive molecules is stronger than that of graphene flakes due to higher charge transfer in the BN-complexes[19].However,investigations on the effects of exfoliated h-BN nanosheets for modifications of energetic materials has hitherto been lacking in the literature.
Herein,we aim to develop polymer bonded explosives(PBXs)with enhanced thermal stability and safety property through incorporating non-covalently exfoliated h-BN nanosheets.To achieve this goal,hBNNSs were prepared by a liquid-phase exfoliation of h-BN.1,3,5,7-tetranitro-1,3,5,7-tetrazocane(HMX)surfacecoated with hBNNSs and ethylene-vinyl acetate copolymer(EVA)was successfully fabricated by a simple solvent-slurry process.For comparison,the HMX/EVA and HMX/EVA/graphene(HMX/EVA/G)composites were also prepared by a similar process.The morphology,crystal form,surface element distribution,thermal decomposition process and impact sensitivity of HMX/EVA/hBNNSs composites were contrastively investigated.
2.Experiment
2.1.Materials
Raw HMX was provided by Liaoning Qingyang Chemical Industry Corporation(Liaoyang City,China).1,2-dichloroethane(AR grade)was purchased from Tianjin Tianda Chemical Industry Ltd.(Tianjin,China).EVA was obtained from DuPont Engineering Polymers Co.Ltd.H-BN powder(98%)and graphene(99%)were purchased from Sigma Aldrich and Nanjing JCNANO Co.Ltd,respectively.
2.2.Preparation of HMX/EVA/hBNNSs micro-composites
HBNNSs were exfoliated by a sonication-centrifugation process.The submicrometer-sized h-BN powder(1.0 g)was extensively tiptype sonicated(650 W,10 h)in N,N-Dimethylformamide(DMF,40 mL)at room temperature[20-22].After sonication,the solution was centrifuged for 10 min with a high rate of 8000 rpm.The whitish supernatant was separated and dried at 80°C for 24 h.The schematic of liquid exfoliation of h-BN crystals into hBNNSs is depicted in Fig.1a and the photograph of hBNNSs suspensions is shown in Fig.1b.
EVA(0.25 g)was dissolved into 1,2-dichloroethane(3.97 mL)to form a 5.0 wt% EVA solution firstly.HMX(4.70 g)and hBNNSs(50 mg)were added into deionized water(DI water,20 mL)to form a dispersed slurry after vigorously stirring and ultrasonic treatment.Then EVA solution was slowly injected into the suspension while stirring at 60°C and vacuum(-0.04 MPa).The same experimental conditions were kept until the solvent was completely volatilized.After filtering,washing by DI water,and freezing drying,the HMX/EVA/hBNNSs(95:4:1,wt%)micro-composites were fabricated.The process is illustrated in Fig.2.For comparison,the micro-composites of HMX/EVA(95:5,wt%)and HMX/EVA/G(95:4:1,wt%)were prepared by a similar process,respectively.

Fig.1.(a)Schematic of liquid exfoliation of h-BN crystals into hBNNSs by sonication and centrifugation.DMF(green spheres in Fig.1a)was used for liquid exfoliation.(b)The image of resulted hBNNSs suspension.
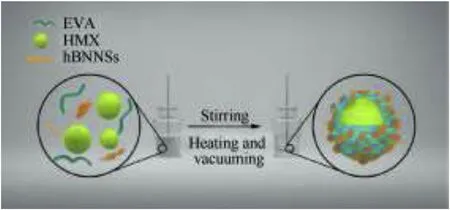
Fig.2.Illustration of the overall preparation procedures of HMX/EVA/hBNNSs microcomposites.
2.3.Characterization
The morphology and thickness of preprared hBNNSs were investigated by transmission electron microscope(TEM,JEOL JEM-2100 F,Japan)and atomic force microscope(AFM,Agilent 5500,USA),respectively.The morphology of HMX,graphene,h-BN,HMX/EVA,HMX/EVA/G,HMX/EVA/hBNNSs micro-composites were characterized by a field-emission scanning electronic microscope(FE-SEM,Tescan Mira3 LMH,Czech).The crystal form of the samples was investigated with a X-ray diffractometer(XRD,Dandong Haoyuan DX-2700,China)at 40 kV and 30 mA using Cu-K-αradiation.Surface elemental analysis of the samples was performed by an ESCALAB 250XI X-ray photoelectron spectrometer(XPS,Thermo Electron Corporation,USA)to explore the coating state of samples.Thermal decomposition analysis was performed on a differential scanning calorimeter(DSC,Yingnuo DSC800,China)at linear heating rates of 5,10,15,and 20 K min-1,respectively.According to the GJB772 A-1997 standard method 601.2[23],the samples were tested for impact sensitivity using type 12 drop hammer apparatus.Each sample(35 mg)was subjected to an impact of a 2.50 kg hammer at corresponding heights,and the ambient temperature is 20-25°C(see supporting information).The special drop height(H50)indicates that in 50%of the tests,the 2.5 kg drop hammer will cause the explosion event:the higher the value of H50,the lower impact sensitivity.
3.Results and discussion
3.1.Morphological characterization
As can be seen in Fig.3a,the raw HMX particles were hexagonal short rod-shaped crystal with an average particle size about 50100μm.As shown in Fig.3b,the lateral size of original h-BN platelets were estimated in the range of 0.51μm with a thickness of 100 nm,showing a distinct layered structure.Due to ultimately thin shapes,the hBNNSs were entirely transparent to an electron beam in Fig.3d,the low-magnification TEM images of the sheets.The thickness of the hBNNSs was approximately 1.83.5 nm,and the number of layers(layer-layer distance 0.33 nm)was about 511,which were significantly exfoliated than pristine h-BN particles as shown in Fig.3e and f.This phenomenon implies that sonication is able to exfoliate hBNNSs from h-BN particles due to interactions between DMF solvent molecules and h-BN particle surfaces.The SEM image of graphene is shown in Fig.3c,where graphene sheets exhibited a curved layered structure,since their structure becomes thermodynamically stable via bending[24].Fig.3g,h,and i display typical SEM images of HMX/EVA,HMX/EVA/G and HMX/EVA/hBNNSs micro-composites respectively,the particle diameters were about 500700μm.The shapes of them were nearly spherical or ellipsoidal,without obvious crakes or voids.The smooth and dense surface of HMX/EVA/G and HMX/EVA/hBNNSs indicated a high degree coverage of HMX and the complete coating,suggesting the addition of 2D materials had no side effect on coating process.The EVA closely combined hBNNSs with HMX crystal.Thus the compact and coherent coating would strengthen the interaction between HMX crystals and hBNNSs,providing toughening effect to PBXs structure.
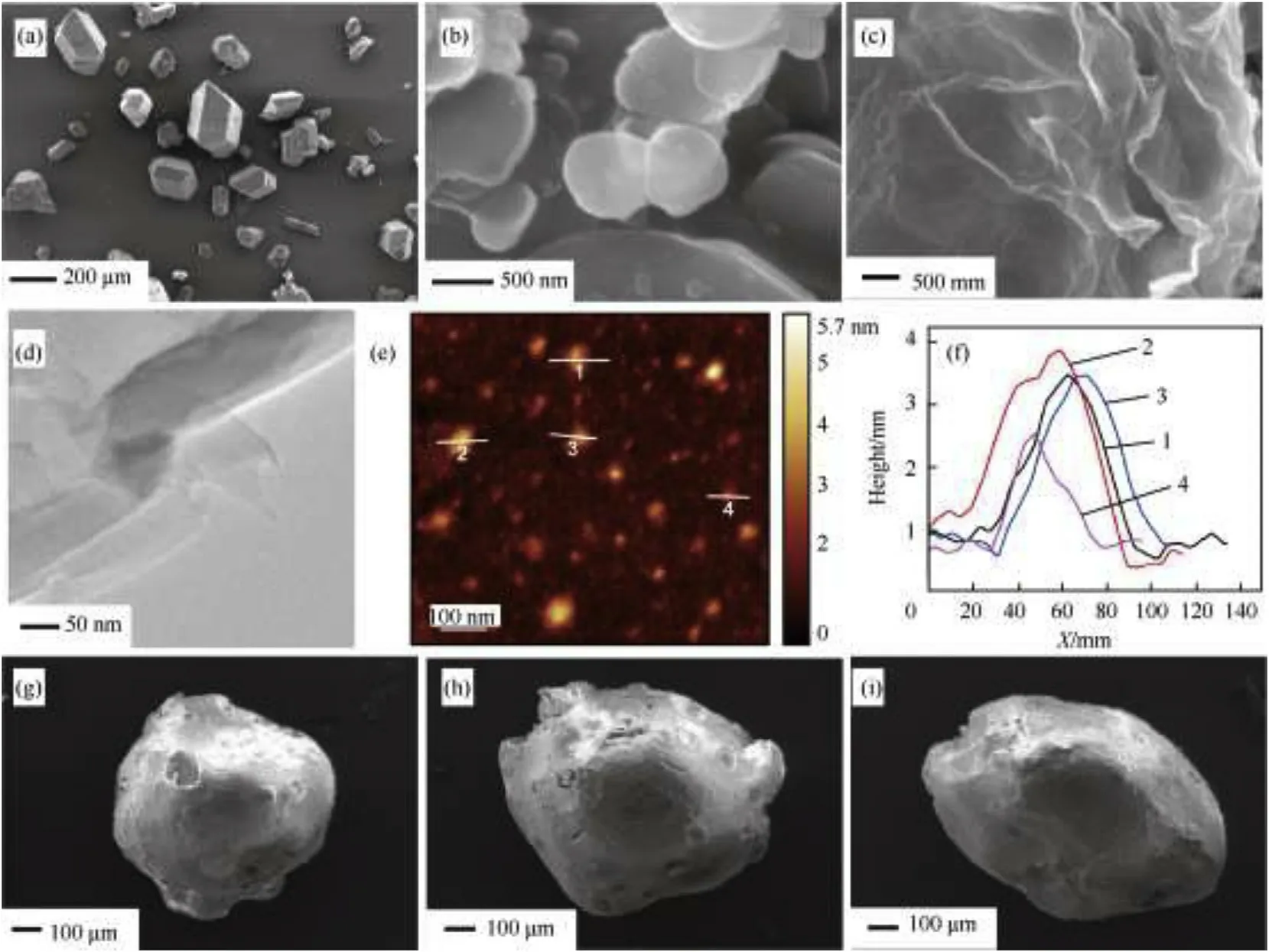
Fig.3.(a)SEM image of raw HMX;(b)SEM image of h-BN;(c)SEM image of graphene;(d)TEM image of prepared hBNNSs;(e)and(f)AFM image and typical height profiles of hBNNSs;(g)SEM image of HMX/EVA;(h)SEM image of HMX/EVA/G and(i)SEM image of HMX/EVA/hBNNSs.
3.2.X-ray diffraction characterization
The crystal structure of HMX raw material,HMX/EVA,HMX/EVA/G and HMX/EVA/hBNNSs was characterized by XRD and the XRD patterns are shown in Fig.4a.The XRD patterns of the samples were consistent with that ofβ-HMX(monoclinic,P21/c)shown in JCPDS Card No.45-1539[25].Sharp and intensive diffraction peaks of raw HMX suggested the intact grains and high crystallinity.The typical diffraction peaks of HMX were detected in HMX/EVA,HMX/EVA/G and HMX/EVA/hBNNSs composites,indicating a wellformed crystal structure reserved in the coated composites.It is interesting to notice that the diffraction intensity of some peaks of the three coated samples were subtly altered comparing to HMX raw material.For example,the peak intensity of HMX/EVA increased at 14.69°(011)and decreased at 22.08°(-112);for HMX/EVA/G and HMX/EVA/hBNNSs:increased intensity at 2θof 16.02°(020)and 23.00°(120),and decreased at 31.91°(-132)and 37.27°(-232),respectively.Such phenomenon may ascribe to the preferred orientation of HMX crystal,which could cause different adhesion effects of coating materials on different crystal faces[26,27].
Fig.4b shows partially enlarged XRD pattern of raw HMX,HMX/EVA/hBNNSs and JCPDA Card No.34-0421 of h-BN[12,28].Compared with that of raw HMX,the peak intensity of HMX/EVA/hBNNSs composites slightly increased at 2θof 41.60°and 43.87°,which corresponds to the typical peak at 41.597°(100)and 43.873°(101)of h-BN JCPDA Card[27],indicating the existence of exfoliated hBNNSs in HMX/EVA/hBNNSs.
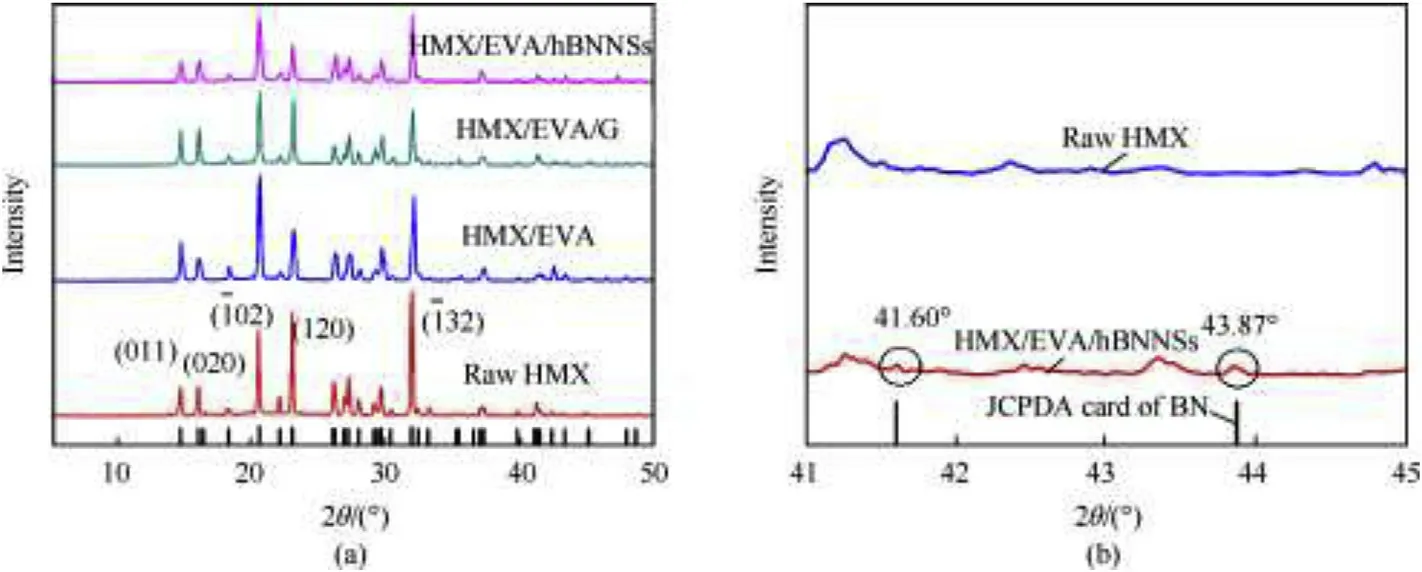
Fig.4.(a)X-ray diffraction patterns of samples.(b)Partially enlarged XRD pattern of raw HMX,HMX/EVA/hBNNSs composites and JCPDA Card of h-BN.
3.3.XPS characterization
XPS analysis was applied to determine the elemental compositions of the sample surfaces and the results are presented in Fig.5 and Table 1.The characteristic peaks at the binding energies of 287.91,406.97 and 533.18 eV for HMX corresponded to C1s,N1s and O1s,respectively[29].Compared to the XPS spectrum of raw HMX,the contents of N and O decreased a little with HMX/EVA,HMX/EVA/G and HMX/EVA/hBNNSs composites since the coating materials had no N element and less content of O than that of HMX surface.Meanwhile,compared to raw HMX,the elemental content of C of HMX/EVA increased by 4.61%since the adhesion of 1.0 wt%graphene.And there was a new peak at the binding energy of 190.52 eV for HMX/EVA/hBNNSs with content of 6.89% of B element,which corresponds to the presence of B1s in hBNNSs[30,31].After mixing of HMX,hBNNSs and EVA,the coated composites became an adhesive film,adhering and imparting sufficient strength to core material by stirring shear.The loose HMX and hBNNSs were bonded together tightly by binder to make them molding material for further explosive forming.
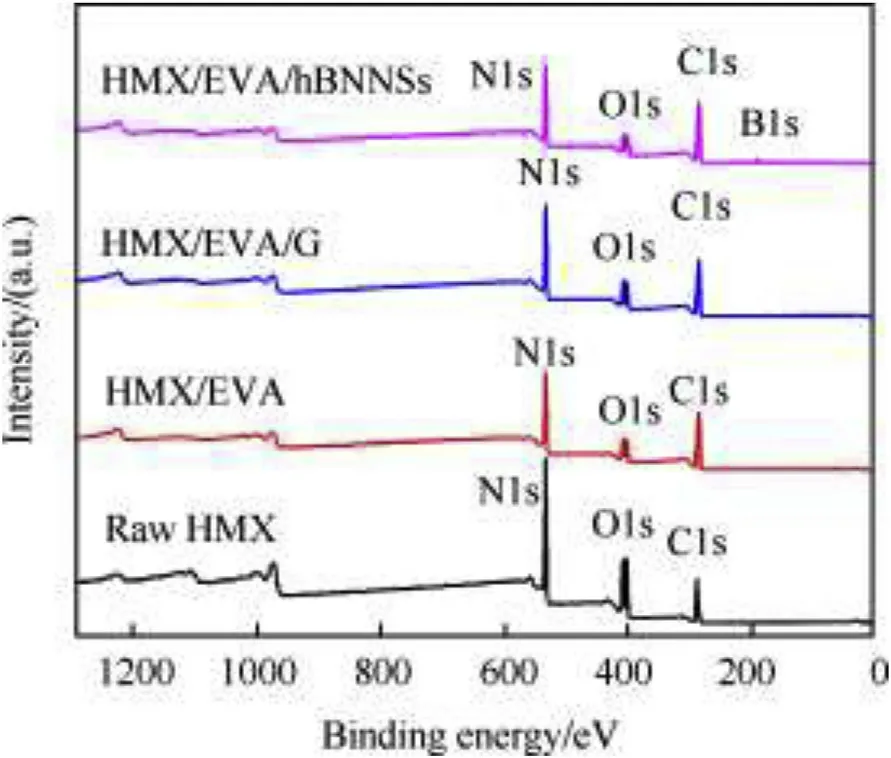
Fig.5.XPS spectra of raw HMX and coated samples.

Table 1Element contents of samples obtained from XPS results.
To confirm the mechanism of hBNNSs coating process,highresolution data for C,N and B elements were also recorded,as shown in Fig.6.There were two peaks at 284.80 eV and 287.75 eV corresponding to the groups of-CH2and N-C-N in the C1s spectrum of HMX(Fig.6 a1),respectively.The appearance of peaks at 285.20 eV(O=C-H3),286.65 eV(C-O)and 289.10 eV(O2-CH3)in HMX/EVA/hBNNSs further verifeid the coating of EVA on HMX crystal surface(Fig.6 b1)[32].Moreover,the N1s spectrum of raw HMX(Fig.6 a2)showed typical components of N-O2(at 406.47 eV)and C-N-C(at 401.16 eV)[29].After the addition of hBNNSs in HMX/EVA/hBNNSs,a new peak centered at 397.68 eV could be found(Fig.6 b2),attributing to the N atoms from h-BN nanomembrane[30].The existence of hBNNSs in HMX/EVA/hBNNSs composites could also be verified by the appearance of peak centered at 190.04 eV in Fig.6 b3,the high resolution B1s XPS spectrum.The above analysis further suggested that the coating process of HMX with hBNNSs and EVA was complete and successful.
3.4.Thermal decomposition Properties
DSC analysis at 10 K/min heating rate was applied to investigate the thermal characteristics of samples.And DSC patterns of samples were depicted at Fig.7.From the DSC curves of pure HMX,it possessed two endothermic peaks(461.94 K and 552.89 K)and one exothermic peak(556.02 K),which represents the polymorphic phase transition(βtoδ),melting point and thermal decomposition of raw HMX,respectively[24].Compared to the DSC curve of raw material,the temperature of endothermic peaks of other samples’DSC curves substantially unaltered,which indicates the additives has good compatibility with HMX crystal.
To evaluate the thermal decomposition behaviors of different samples,non-isothermal DSC patterns recorded at different heating rates are obtained in Fig.S1,the decomposition temperatures are tabulated in Table 2 and the thermal decomposition kinetic parameters of different samples are summarized in Table 3.
Eais apparent activation energy,A is the pre-exponential factor,Tp0is the peak temperature point corresponding toβ→0,Tbis the critical temperature of thermal explosion,ΔH≠,ΔG≠andΔS≠are activation enthalpy,activation Gibbs free energy and activation entropy,respectively(the calculation process is in supporting information).
In the four DSC patterns at different heating rate of 5,10,15,and 20 K/min,the same trend,that is,the decomposition peak temperature of samples shifted to the higher temperature with an increased heating rate,could be noticed in each curve,which is consistent with Eq.(1)(see supporting information).As listed in Table 3,three methods,Kissinger,Ozawa and Starink methods[33-35],offered high accuracy of the Eavalues determination.The Eaof three coating samples were higher than that of raw HMX,indicating increased thermal decomposition barriers of three coating samples.After the coating process,the samples obtained higher value of Tb,implying that coated HMX micro-composites owned better thermal stability than raw HMX.Furthermore,compared to HMX/EVA/G,the Tbof HMX/EVA/hBNNSs further increased,which attributes to the higher oxidation resistance of hBNNSs than that of graphene[36].The improved thermal stability also derives from the uniform distribution of hBNNSs in the PBXs matrix,efficiently confining the neighboring HMX particles against thermal decomposition.Under the heating condition,the insensitive additive hBNNSs delayed the thermal decomposition of HMX,improving the thermal stability of PBXs.
3.5.Mechanical sensitivity
Safety is the most important property for energetic materials.Mechanical sensitivity,as one of the crucial criteria for measuring safety of explosives,is critical for further application of energetic composites in solid propellants and shaped charge.We measured the impact sensitivity of raw HMX material and coated samples,and the results(H50)are listed in Table 4.As shown in Table 4,the impact sensitivity of raw material was rather high,revealing its tobe-improved safety property.After coating with EVA,the H50was enhanced from 28.2 cm to 42.3 cm,which forcefully explained that EVA is a suitable polymer to form HMX-based PBXs for reducing mechanical sensitivity.After adding graphene and hBNNSs,H50of samples was further improved to 56.5 cm and 65.3 cm,respectively.It should be noticed that hBNNSs and graphene were both good desensitizers in HMX-based PBXs.
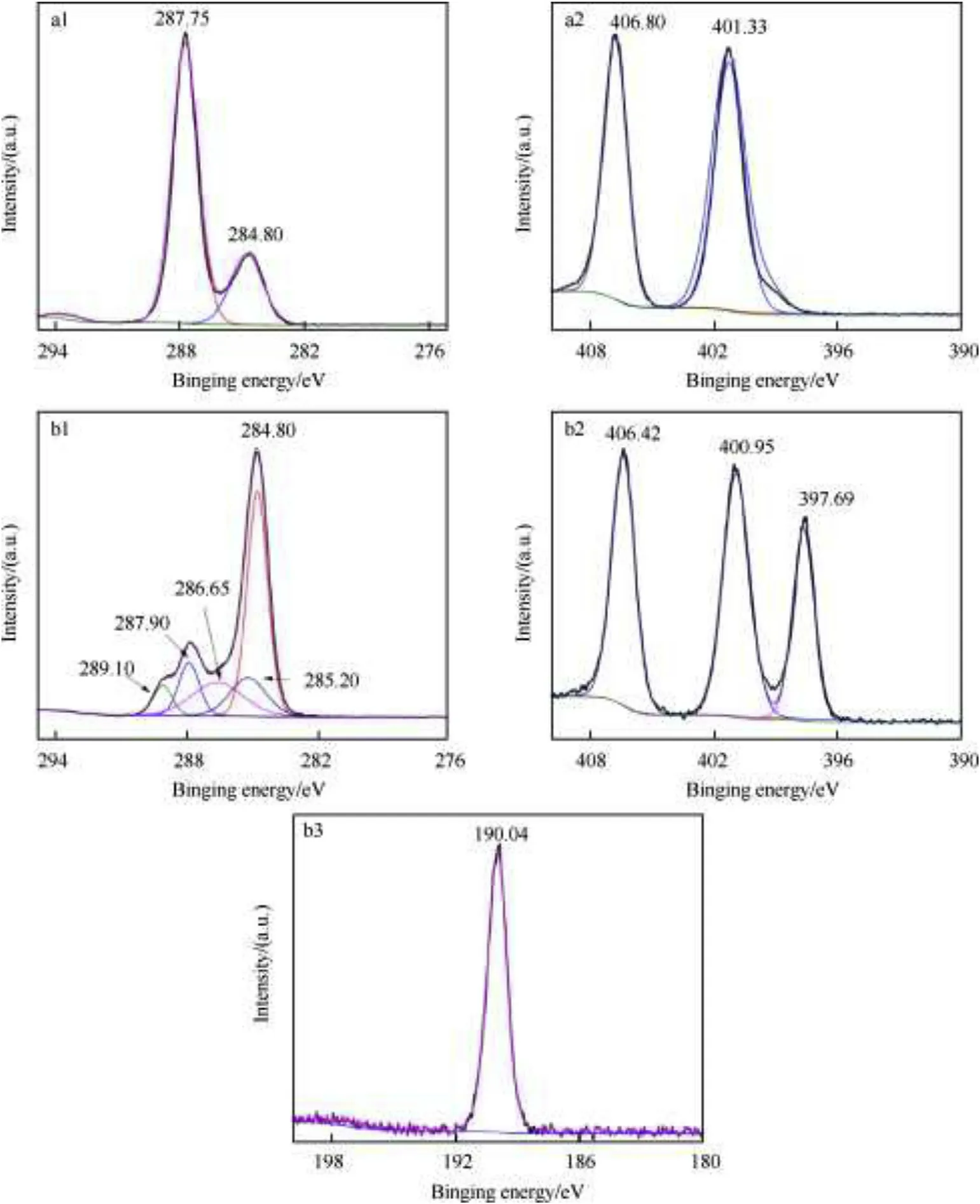
Fig.6.High resolution XPS spectra of samples:(a1),(a2)C1s and N1s spectrums of HMX;(b1),(b2)and(b3)C1s,N1s and B1s spectrums of HMX/EVA/hBNNSs.
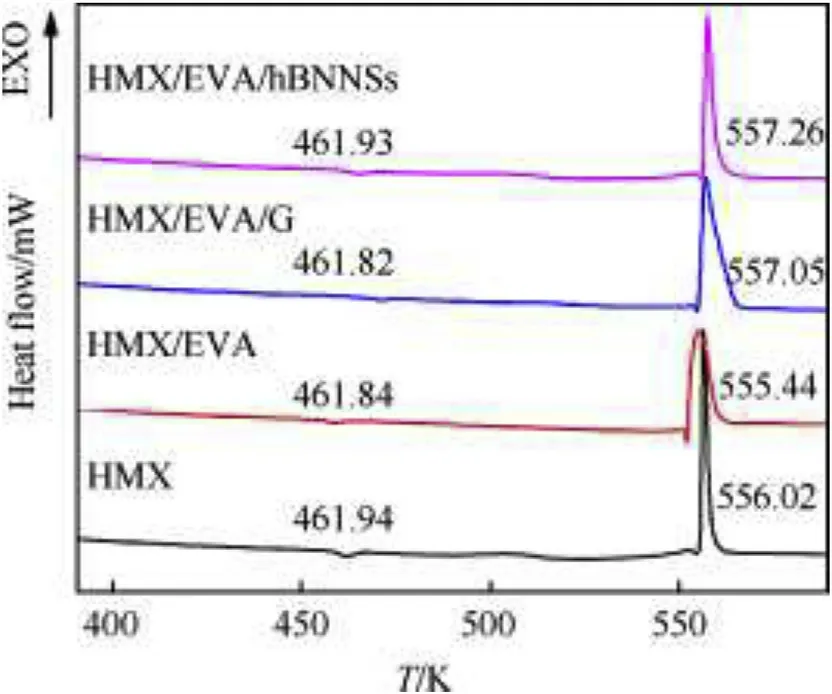
Fig.7.DSC curves of different samples at heating rate of 10 K/min.
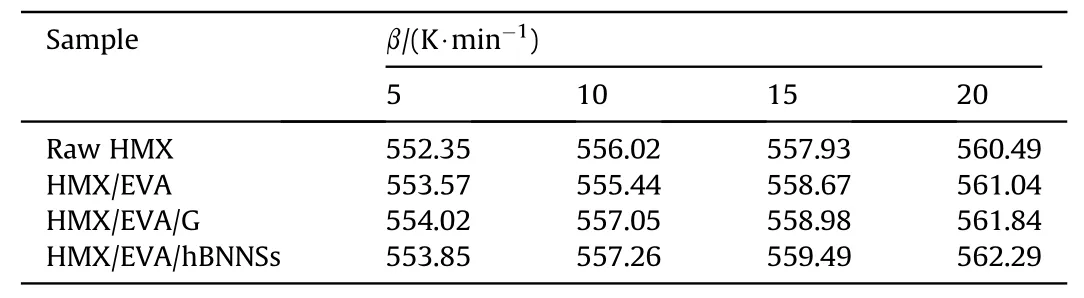
Table 2Thermal decomposition temperatures of samples at different heating rates.
With coating effect from EVA,its inherent plastic and elastomeric effect conferred to PBXs matrix.Due to softer phonon modes for hBNNSs and the mass difference between B and N,the thermal conductivity for hBNNSs are somewhat lower than graphene[37].However,under large in-plane strain and out-of-plane compression caused by impact,the distinct interlayer interactions in hBNNSs provided different mechanical behaviors from graphene[38],which could provide strong support for matrix to avoid the occurrence of hot spot.Besides,the binding between HMX and hBNNSs molecules is stronger than graphene due to higher charge transfer[19]in the HMX/EVA/hBNNSs composites which also offers desensitization effect.The coating structure would slip and form a thermal conductivity network to dissipate energy and reduce effects from external impact stimuli,causing less hot spots[39]than pure HMX crystal.Moreover,the significant improvement of mechanical property is reasonably attributed to the uniform distribution of robust hBNNSs in HMX-based PBXs structure,causing efficient load transfer.
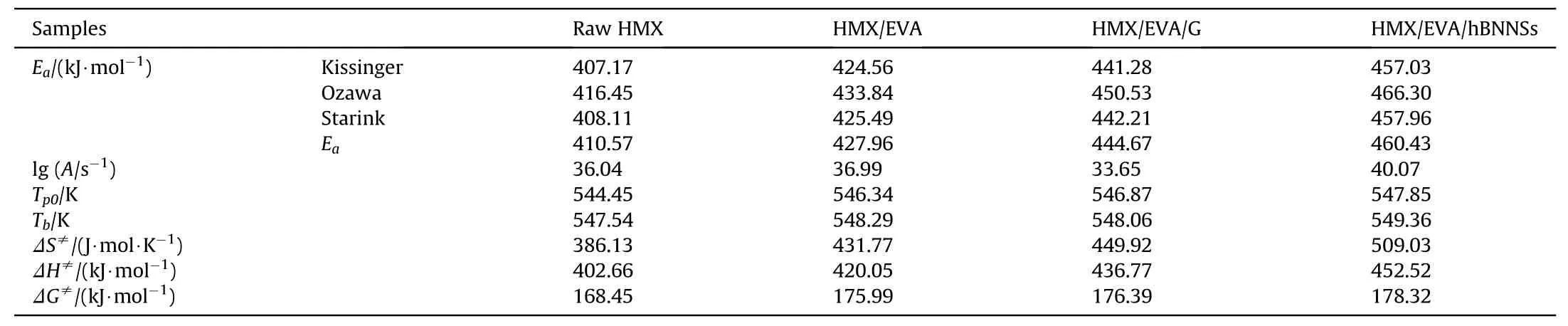
Table 3Thermal decomposition kinetic parameters of samples.
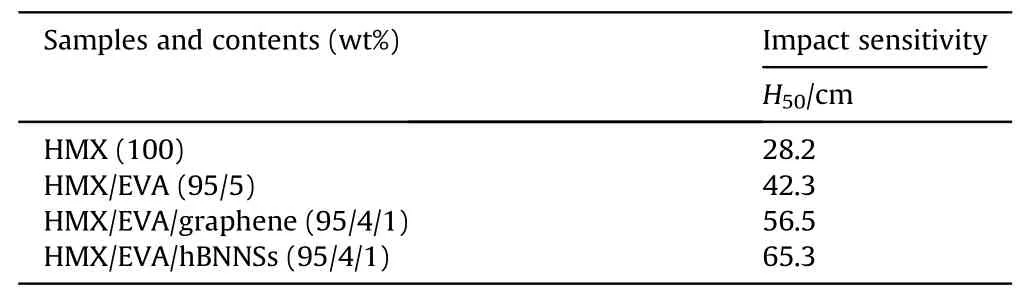
Table 4Impact sensitivity of raw HMX and HMX-based composites.
4.Conclusions
(1)HBNNSs was prepared and the effect of its addition to HMX/EVA/hBNNSs matrix was investigated.
(2)The formed HMX/EVA/hBNNSs composites had intact surface and good spherical morphology.Compared with HMX/EVA/graphene composites,the increased apparent activation energy and H50of the HMX/EVA/hBNNSs composites indicated that hBNNSs provided better thermal stability and lower mechanical sensitivity to HMX-based PBXs than graphene.
(3)Our work may offer a promising desensitized hBNNSs to improved thermal and safety properties when utilize in advanced weapon system.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
Acknowledgements
The project was supported by Equipment Pre-research Key Laboratory Fund(No.6142020305).The authors would like to thank Shiyanjia Lab(www.shiyanjia.com)for the support of XPS test.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2020.09.002.
杂志排行
Defence Technology的其它文章
- Ricochet quantification using a multiple sensor approach☆
- Reaction degree of composition B explosive with multi-layered compound structure protection subjected to detonation loading
- Effects of nano-sized aluminum on detonation characteristics and metal acceleration for RDX-based aluminized explosive
- Increasing of photostability of HNS explosive in the presence of UV photostabilizers
- Numerical investigation on cooling performance of filter in a pyrotechnic gas generator
- Decision support model for effects estimation and proportionality assessment for targeting in cyber operations
