Nickel-catalyzed asymmetric arylative cyclization of N-alkynones:Efficient access to 1,2,3,6-tetrahydropyridines with a tertiary alcohol
2021-03-14JingynTinWendinLiRuihoLiLinHeHuiLv
Jingyn Tin,Wendin Li,Ruiho Li,Lin He,Hui Lv,,∗
a key Laboratory of Biomedical Polymers of Ministry of Education &College of Chemistry and Molecular Sciences,Engineering Research Center of Organosilicon Compounds &Materials,Ministry of Education,Sauvage Center for Molecular Sciences,Wuhan University,Wuhan 430072,China
b Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan,School of Chemistry and Chemical Engineering,Shihezi University,Shihezi 832000,China
Keywords:Asymmetric catalysis Alkynones Cyclization Nickel
ABSTRACT Nickel/(S)-t-Bu-PHOX complex catalyzed asymmetric arylative cyclization of N-alkynones has been achieved,delivering 1,2,3,6-tetrahydropyridines containing a chiral tertiary alcohol in high yields and excellent enantioselectivities,which provides efficient access to chiral tetrahydropyridine and piperidine analogues.
Enantioselective construction of high valuable chiral heterocycles in an atom economy and step economy manner is one of the most important goals of chemists pursued [1,2].To this end,the transition-metal-catalyzed intramolecular cyclization of alkynals/alkynones,one of the most straightforward methods for the efficient construction of five-to six-membered heterocycles bearing a tertiary alcohol,has been widely investigated [3-5].As a result,cyclization of alkynals/alkynones has been achieved by various kinds of transition-metal catalysts,such as rhodium [6-15],ruthenium [16],nickel [17-23]or palladium complexes [24-28],which greatly promoted the development of intramolecular cyclization of alkynals/alkynones.However,most of these approaches involved anexo-trig pathway,affording five-membered heterocycles containing an exocyclic olefin (Scheme 1a) [29-31].By comparison,theendo-trig cyclization of alkynals/alkyones to generate endocyclic alkenes was rarely reported,although it provides concise access to some valuable molecules.In 2016,Lam group reported their pioneer work on Ni-catalyzed desymmetrization of 1,3-diketones,which achievedendo-trig cyclization of alkynones,giving fused bicycles efficiently [32].Nevertheless,only cyclic 1,3-diketones with relatively high activity could be tolerated in this transformation,which limited its applications in construction of chiral heterocycles.Thus,it is highly desirable to develop new methodology to expand the generality ofendo-trig cyclization of alkynones [33].
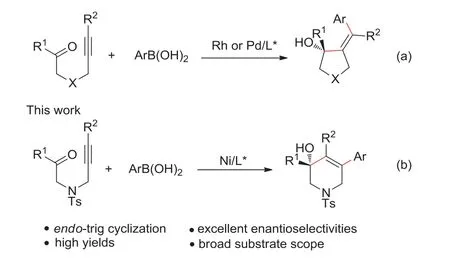
Scheme 1.Transition metal catalyzed arylative cyclization of alkynones.
In the past decades,nickel catalyzed asymmetric reactions has emerged as a powerful strategy for construction of chiral molecules[34-38].In this context,our group reported nickel-catalyzed intramolecular asymmetric reductive cyclization of aryl halides with unactivated ketones through the addition of aryl nickel species to carbonyl group [39].Inspired by this result,we envision that the vinyl nickel species may also react with unactivated ketones to generate chiral heterocycles efficiently.Herein,we report a highly enantioselective intramolecular arylative cyclization ofN-alkynones to furnish chiral tetrahydropyridines containing a tertiary allylic alcohol in high yields and excellent enantioselectivities (Scheme 1b),which are important structural motifs widely existed in natural products and biologically active compounds [40-42].
Our initial study began with nickel-catalyzed arylative cyclization ofN-alkynone (1a) with phenylboronic acid (2a) in the presence of 10 mol% Ni(OAc)2·4H2O in DCE.Firstly,a series of commercial available chiral oxazoline ligands were evaluated.As shown in Table 1,Pybox (L1),Pyox (L2),and Box (L3) did not exhibit any catalytic activity in this transformation (entries 1–3).When phosphine-oxazoline ligand L4 was employed,it delivered target product in 50% yield with 14%ee(entry 4).Increasing the steric hindrance of oxazoline,the enantioselectivity was greatly proved,but the yield was decreased gradually (entries 5 and 6).Considering the excellent enantiocontrol ability of (S)-t-Bu-PHOX,it was chosen as the best ligand for further optimization.Subsequently,the solvent effects were investigated,and the results disclosed that toluene and methanol were detrimental to the conversion,which lead to a totally inhibition of this transformation.The yields increased when 1,4-dioxane,CH3CN and 2-methyltetrahydrofuran (2-MeTHF) were used as solvent,while the enantioselectivities decreased to some extent (entries 7–11).Interestingly,a little water can improve the yield and has little impact on the enantioselectivity (entry 12).To our delight,the yield was increased to 95% when Ni(TFA)2was used as metal precursor in presence of 2 equiv.water(entry 13).Increasing the temperature to 90 °C,a full conversion was obtained,affording target product 3a in 99% yield with 99%ee(entry 14).
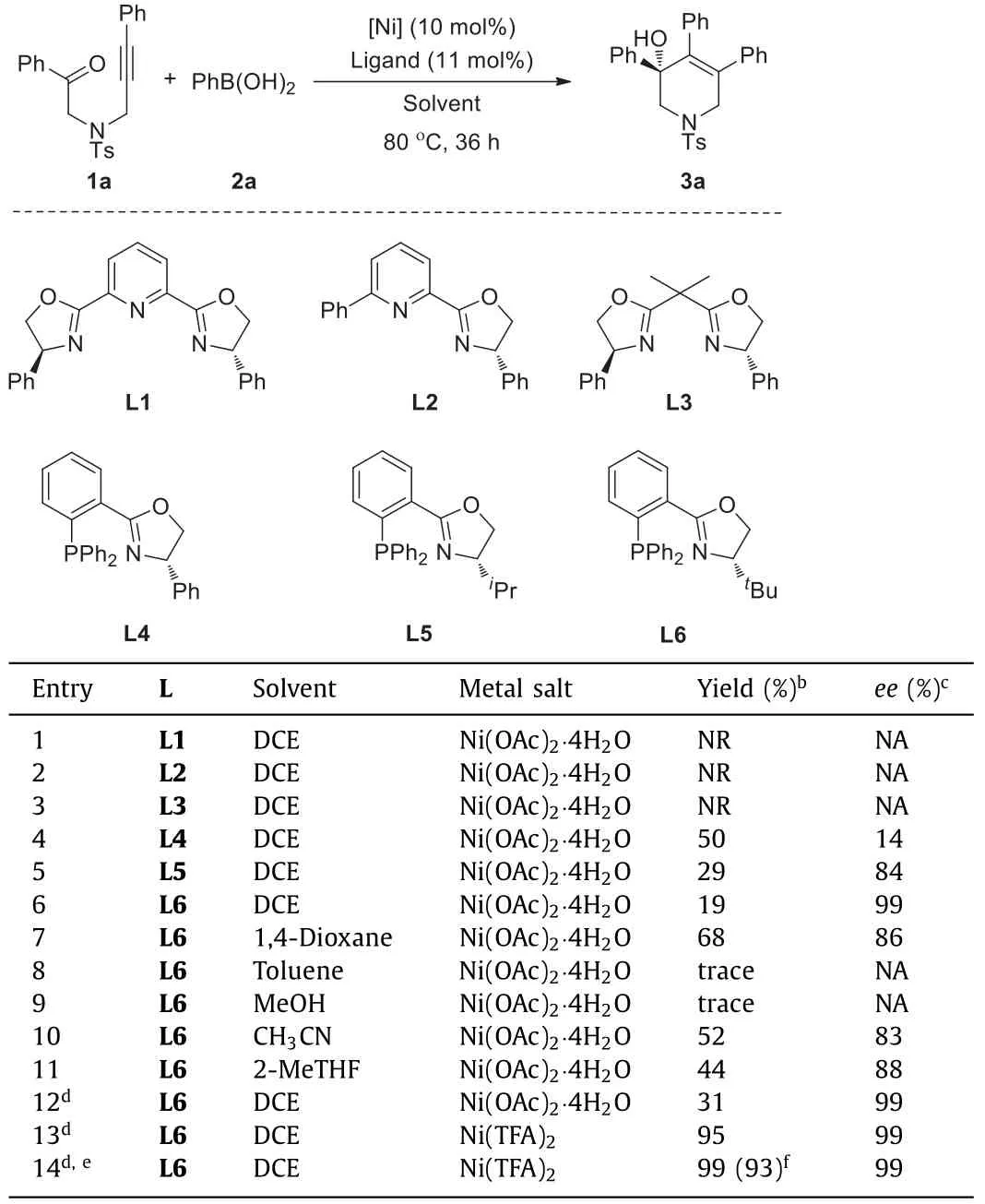
Table 1 Optimization for nickel-catalyzed arylative cyclization of 1a and 2a.a
With the optimal conditions in hand,we surveyed the generality of nickel-catalyzed arylative cyclization ofN-alkynones.Generally,the reaction had a broad substrate scope and exhibited good tolerance to various substituted arylboronic acids andN-alkynones.As shown in Scheme 2,different kinds of arylboronic acids,no matter the position and the electronic nature of substituent,are well tolerated in this transformation,giving target products in high yields and excellent enantioselectivities (3a-3l).Notably,the reaction shows excellent compatibility to a series of functional groups,such as aldehyde (3f),ester (3g),cyano group (3h),hydroxyl group(3i),and there was no significant impact on the yield and enantioselectivity.When R1is substituted aryl group or naphthyl group,the reaction proceeded very smoothly,delivering desired products 3m-3r with high yields and excellent enantioselectivities.It is worth noting that the reaction was also compatible with alkyl substitutedN-alkynone,furnishing 3s in 87% yield with 87%ee.When R2is substituted benzene group,it afforded target products with high yields and excellenteevalues (3t-3v).Replacing R2by a heteroaryl,the yield and enantioselectivity decreased to some extent(3w).The ether-tethered substrate was also tolerated,but the ee value dropped dramatically (88% yield and 66%ee,see Supporting information).
To demonstrate the synthetic utility of the current methodology,a gram-scale reaction was conducted under the standard condition (Scheme 3),affording desired product 3a in 88% yield with 98%ee,which indicated that the method has a potential application in construction of chiral molecules with a tetrahydropyridine motif.
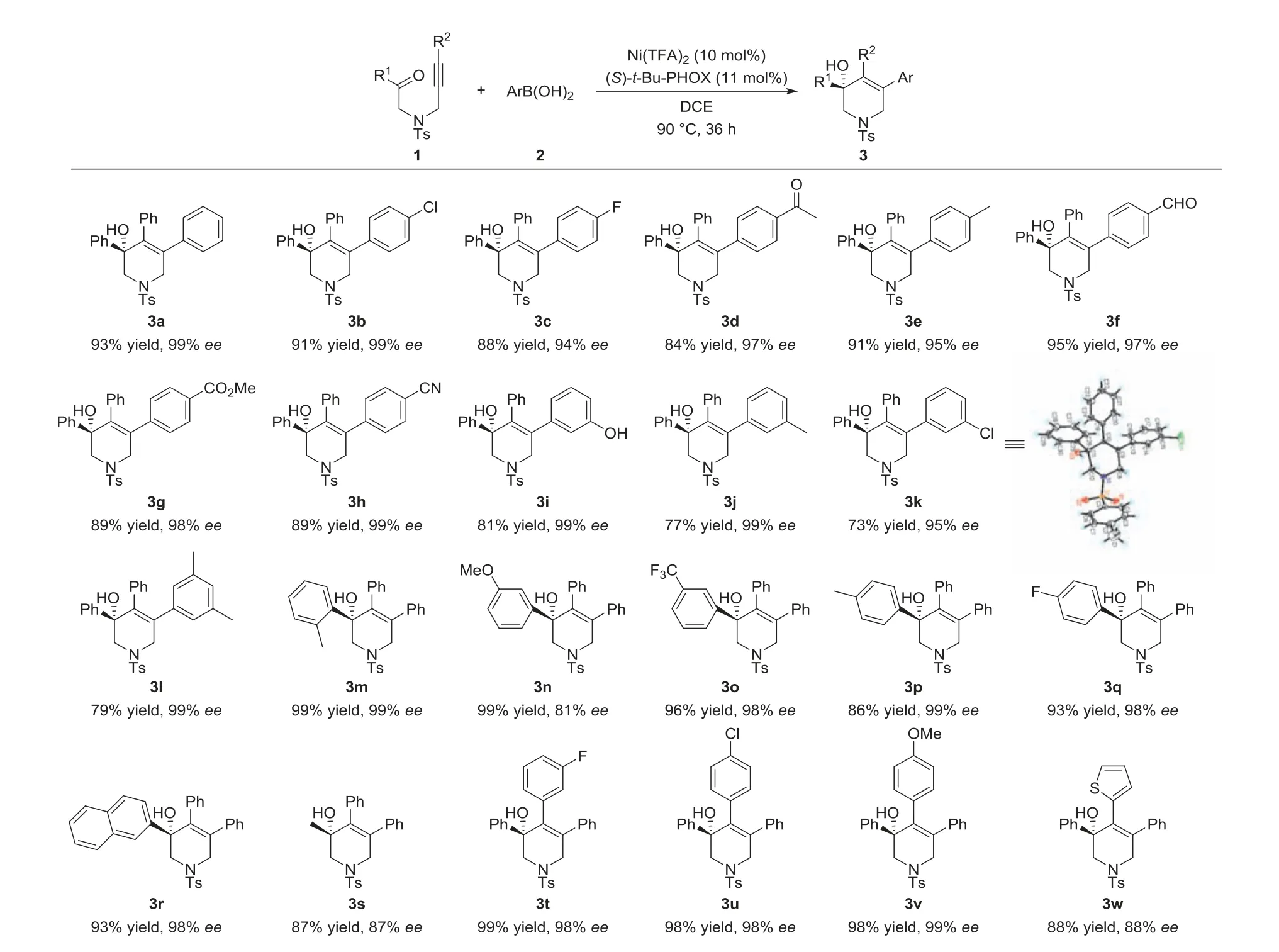
Scheme 2.Substrate scope.Unless otherwise noted,the reactions were performed with 1 (0.1 mmol),2 (0.3 mmol),Ni(TFA)2 (10 mol%),(S)-t-Bu-PHOX (11 mol%) and H2O(0.2 mmol,5.5 mol/L in dioxane) in DCE (2 mL) at 90 °C for 36 h under Ar atmosphere.Yields of isolated product 3.Enantiomeric excess was determined by chiral HPLC.The absolute configuration of 3k was confirmed to be R by X-ray,the other configurations follows that of 3k.
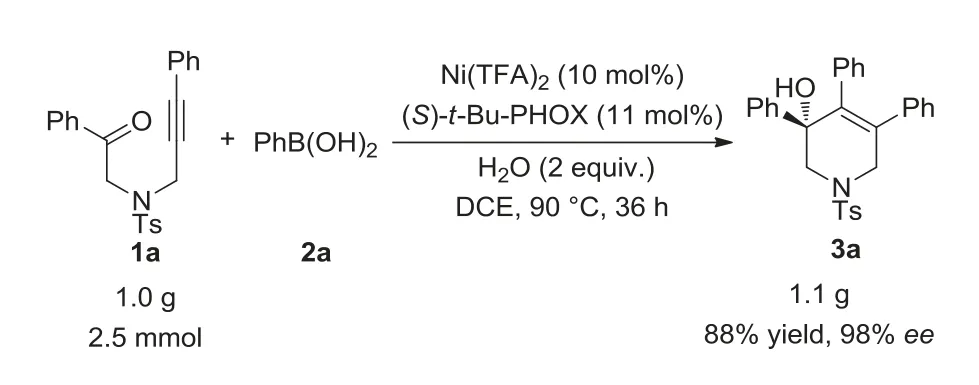
Scheme 3.Gram scale reaction.
In conclusion,nickel-catalyzed asymmetric cyclization ofNalkynones with arylboronic acids has been achieved,offering 1,2,3,6-tetrahydropyridines bearing a chiral tertiary alcohol in excellent yields with excellent enantioselectivities (up to 99% yield,up to 99%ee).This reaction proceeded through anendo-trig pathway,which provides efficient access to heterocycles with an endocyclic allylic alcohol.Moreover,the potential utility of this method was demonstrated by a gram-scale reaction without loss of yield and enantioselectivity.Further development and application of this reaction is underway in our laboratories.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We are grateful for financial support from the National Natural Science Foundation of China (Nos.22071188,21871212),the open foundation of CAS Key Laboratory of Molecular Recognition and Function,the "Double First-Class" Project of Shihezi University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.06.006.
杂志排行
Chinese Chemical Letters的其它文章
- Progress in mechanochromic luminescence of gold(I) complexes
- Spinel-type bimetal sulfides derived from Prussian blue analogues as efficient polysulfides mediators for lithium-sulfur batteries
- Alopecuroidines A-C,three matrine-derived alkaloids from the seeds of Sophora alopecuroides
- Boronic acid-containing diarylpyrimidine derivatives as novel HIV-1 NNRTIs:Design,synthesis and biological evaluation
- Diaminodiacid bridge improves enzymatic and in vivo inhibitory activity of peptide CPI-1 against botulinum toxin serotype A
- Peptide stapling with the retention of double native side-chains
