Constitutionally adaptive crown ether-based macrocyclic bolaamphiphile with redox-responsive switching of lower critical solution temperature behaviors
2021-03-14QiangqiangXuZhiliyuCuiJizhenYaoBoLiPingLvXinShenZhuoYuYanGeZhenhuiQi
Qiangqiang Xu,Zhiliyu Cui,Jizhen Yao,Bo Li,Ping Lv,Xin Shen,Zhuo Yu,Yan Ge,Zhenhui Qi
Sino-German Joint Research Lab for Space Biomaterials and Translational Technology,Synergetic Innovation Center of Biological Optoelectronics and Healthcare Engineering (BOHE),Xi’an 710072,China
Keywords:Macrocycle Crown ether Bola Amphiphiles Selenium
ABSTRACT Constitutionally adaptive chemistry of selenium-containing crown ethers (CEs) offers a new platform for controlling/switching the hydration of bolaamphiphile skeletons in water in an effective and simple manner by the virtue of covalent bonding.The adaptive behaviour of the macrocyclic bolaamphiphiles (transformations between C7SeBola and C7SeOBola) in response to redox environment was found to be a decisive factor.
Self-assembly of amphiphiles,a class of fascinating molecules bearing both hydrophilic and hydrophobic groups,in water has significant advantages in the designing and preparation of materials in terms of their biological applications and environmentally friendly processability.In 1984,Fuhrhop and co-workers reported the formation of bolaamphiphiles [1],which can be synthesized by connecting two hydrophilic end groups with a hydrophobic spacer(Scheme 1a,middle).In recent years,there has been a growing interest in the design of novel bolaamphiphiles (including reverse bolaamphiphiles,which possess opposite amphilicity;Scheme 1a,right) [2-5]owing to their unique assembly characteristics and potential applications in various fields,including membrane fabrication [2],drug/gene delivery [3],fabrication of responsive materials [4]and catalysis [5].Although various amphiphilic architectures have been studied,the introduction of selective responsiveness in bolaamphiphiles based on normal,irreversible covalent bonds remains challenging [6].A major challenge is to control/switch the hydration of the bolaamphiphile skeleton in water in an effective and simple manner [7].We envisioned that the formation of macrocyclic reverse bolaamphiphiles utilizing selenium-containing crown ethers (CEs) as end groups would present a unique opportunity to efficiently tune the hydration in a broad range of amphiphilic aggregates.
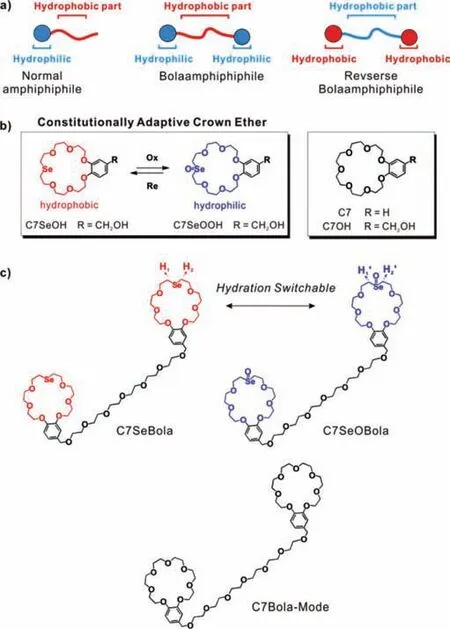
Scheme 1.Illustration of (a) amphiphile types,(b) constitutionally adaptive chemistry of crown ether (inset) with redox switchable hydration.Whereas the traditional crown ether cannot.(c) Chemical structures of redox switchable bolaamphiphile C7SeBola and C7SeOBola designed herein and its control compound C7Bola-Mode.
Crown ethers (CEs) are cyclic polyethers with a cavity of a specific size at the center.The discovery of CEs was seminal in the development of modern supramolecular chemistry [8].Compared to other macrocycles such as cucurbiturils [9],calixarenes [10],cyclodextrins [11]and pillararenes [12],CE-based amphiphiles in water are rare [13],probably due to their flexible structure and ambiguous aqueous solubility.However,in recent times,CEs have emerged as an intriguing class of model hosts for studying both water and aqueous supramolecular chemistries [14].CEs have high flexibility,allowing them to adopt different conformations for interacting with water molecules.For example,benzo-21-crown-7 ether (C7) has been identified as a new class of water-soluble macrocycles.The six ethylene glycol units provide an efficient polar surface for binding to water,rendering C7 highly water-soluble(solubility:4.21 mol/L) [14b];the solubility is even higher than that of the well-known water-soluble macrocycles (such as cucurbiturils andα-,β-andγ-cyclodextrins).Consequently,insight into the water-C7 interactions further led to the discovery of ‘structural water’[14a],which plays a vital role in the design of novel water-involved supramolecular adhesive materials.Moreover,the substitution of one chalcogen atom (e.g.,selenium) in CEs can induce dramatic changes in the shape,solvation (e.g.,hydration),and thermodynamics of guest binding [15].The selenium-substituted counterparts exhibit significantly low solubility in comparison to their original counterparts.Thus,based on only one small structural change,the CEs and their single selenium-substituted counterparts provide intriguing model systems to exclusively probe the dramatic changes in hydration of the entire molecule.
Elucidation of the crystal structure and theoretical modelling revealed that selenoxides are excellent hydrogen-bond acceptors[e.g.,H-bond distance of a selenoxide group (1.72 ˚A) [16]is much shorter than that of an ether (-O-) group (1.90 ˚A)].Therefore,as illustrated in Scheme 1b (inset),it is expected that the water solubility of the selenoxide-containing CE (e.g.,C7SeOOH) will be not only higher than that of the selenide-containing ether(e.g.,C7SeOH),but would also be higher than that of the ethercontaining compound (e.g.,C7OH).However,such comparisons have yet been reported,to the best of our knowledge.In such a case,it will be exciting to explore the reversible transformation between the selenide-and selenoxide-containing CE.This would not only generate a switchable pair of macrocycles differing dramatically in hydration but would also allow to sensitively vary its ring constitution in response to the chemical environment.Such constitutionally adaptive chemistry of CEs remains unexplored;however,it may offer an elegant platform for controlling/switching the hydration of bolaamphiphile skeletons in water in an effective and simple manner by the virtue of covalent bonding.Nevertheless,from the viewpoint of novel molecular designs,seleniumcontaining constitutionally adaptive CE-based bolaamphiphiles capable of exhibiting redox-responsiveness have not been reported so far,probably because investigation of the aqueous behaviours of CEs and selenium-containing CEs is a challenging task and remains rare.
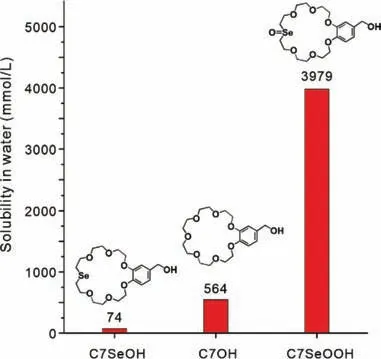
Fig.1.Solubility of C7SeOH,C7OH and C7SeOOH determined by quantitative 1H NMR measurements.
In this study,we designed an unprecedented reverse bolaamphiphile based on selenium-containing constitutionally adaptive CE (C7SeBola).As illustrated in Scheme 1c,the seleniumsubstituted benzo-21-crown-7 ethers were linked by a flexible glycol chain that acted as a hydrophilic spacer.Thus,two constitutionally adaptive CEs not only serve as the ditopic hydrophobic end groups but also render selective redox responsiveness in these bolaamphiphiles.The C7-substituted compound C7Bola-Mode was prepared as the control sample.We present the adaptive behaviour of the macrocyclic bolaamphiphiles (transformations between C7SeBola and C7SeOBola) (Scheme 1c) in response to redox environment,which was found to be a decisive factor for the switching on/off of its lower critical solution temperature (LCST)behaviour.
In our preliminary test,we found that the water solubility of C7SeOOH (Fig.1) reached 1851 g/L (3979 mmol/L) at room temperature.Similarly,the water solubility of C7OH was 217.9 g/L(564 mmol/L) while that of C7SeOH was 33.25 g/L (74 mmol/L)[10].As expected,the hydration capability of C7SeOOH was significantly enhanced.This significantly enhanced solvation/hydration capability of C7SeOOH relative to that of C7SeOH (57-fold difference in water solubility) is sufficient to design novel CE-based bolaamphiphiles.Indeed,computationally predicted (Fig.S20 in Supporting information) molecular electrostatic potential (MEP) revealed that the selenoxide segment of C7SeOOH (-0.085 e) is comparatively higher in value than that of C7SeOH (-0.053 e).The MEP shows the relative polarity on a molecule,and the polarity impacts the solubility-high polarity often correlates to high hydration capability.Thus,the selenoxide-substituted crown moiety has higher polarity than the selenide-substituted region,thereby promoting the binding of water moleculesviahydrogen bonds.This suggested that the computational modelling results were in good agreement with the experimental observations.
The simple addition of water to C7SeBola and subsequent vigorous shaking ensured the formation of a clear solution (1 mg/mL,0.87 mmol/L) (Fig.3a,inset).After sonication for 10 min,welldispersed aggregates with a size of 500-2000 nm were identified by dynamic light scattering (DLS,Fig.2a).Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were performed to study the formation of the presumed self-assembled aggregates (Figs.2b,c,and Figs.S14,S15 in Supporting information).Both SEM and TEM images confirmed the formation of aggregates with an average diameter;these findings were consistent with the DLS results.The small angle X-ray diffraction patterns of the freeze-dried supramolecular aggregates showed prominent diffraction peaks (Fig.S16 in Supporting information) that suggested a sheet-like structure.Furthermore,the water conductivity(ρ) was measured as a function of the concentration of C7SeBola(C) to determine the critical aggregate concentration (CAC) of C7SeBola in water [17].
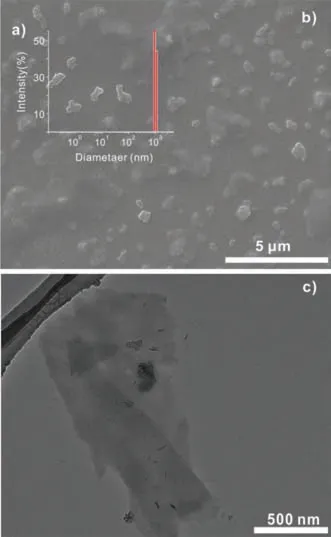
Fig.2.(a) DLS of a solution of C7SeBola (1 mg/mL) in water;(b) SEM images of compound C7SeBola (1 mg/mL) in water;(c) TEM images of the aggregate formed from C7SeBola in water (1 mg/mL).
A clear aqueous solution of C7SeBola at room temperature(Fig.3a) turned opaque on heating and became clear again when the solution was cooled.Thus,the change in turbidity was reversible.This indicated that C7SeBola exhibited LCST behaviour in water.Although linear and cyclic flexible oligo(ethylene glycol) derivatives have been reported to exhibit sharp order-disorder phase transitions at the LCST [18,14b-c,19],this phenomenon was not observed for C7Bola-Mode even on heating (Fig.3c),suggesting that the selenium-containing constitutionally adaptive CE segment was responsible for imparting the LCST behaviour.Ultravioletvisible measurements revealed that the cloud-point (Tcloud,with 50% transmittance) of C7SeBola at 1 mg/mL (0.87 mmol/L) was 52.4 °C.Nonetheless,hysteresis of this system was very small(1.4 °C,Fig.2a) and much lower than that of many polymeric thermoresponsive systems [19].The extremely small hysteresis indicates that the system response time is short and subsequent experiments will be performed under equilibrium conditions.Furthermore,the overall LCST transition processes of C7SeBola were highly reversible and showed no signs of fatigue even after ten repeated heating and cooling cycles (Fig.3b),thereby demonstrating the strong structural integrity of C7SeBola for further solution studies.Moreover,a typical salting-out effect was observed after the addition of sodium chloride,suggesting that the increasing cation concentration led to a decrease inTcloud(Fig.3d).
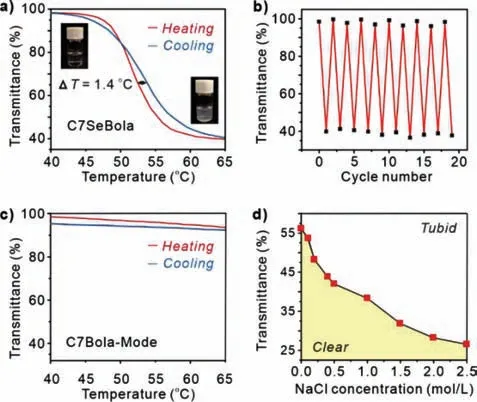
Fig.3.(a) LCST transition behavior of C7SeBola in water (1 mg/mL,0.87 mmol/L).(b) The transmittance of 0.87 mmol/L C7SeBola when the temperature was switched between 25 and 70 °C in cycled manners.(c) LCST transition behavior of C7Bola-Mode in water (1 mg/mL).(d) Concentration dependent of Tcloud with varied ratio of C7SeBola and NaCl appeared in (a).The heating rate was constantly kept as 1 K/min.
Interestingly,constitutionally adaptive CEs led to the first successful case of redox-controlled “ON-OFF” thermoresponsive macrocyclic bolaamphiphiles without the aid of any decorated thermoresponsive segments [20].Redox-responsive switching of C7SeBola was investigated by1H NMR spectroscopy.Upon adding 6 equiv.of hydrogen peroxide (H2O2),the peak corresponding to the protons neighbouring the Se atom (H1and H2) was split into two sets of peaks that were shifted downfield from 2.7 ppm to 3.2 and 3.4 ppm (Fig.4a,pink curve).This is consistent with the previously reported splitting pattern for selenide oxidation,indicating the formation of C7SeOBola.The peak splitting occurs because theR2-Se=O unit of selenoxide is not planar.The top two hydrogen atoms on the adjacentCH2groups arecisto the oxygen atom while the bottom two aretrans.Consequently,the two hydrogen atoms on eachCH2group couple with each other,generating doublets or triplets.Indeed,in the mass spectrum of the oxidized solution,the peak atm/z1201.3571 further confirms the formation of C7SeOBola in solution.Since the reversible transformation between selenide and selenoxide can be easily controlled by the alternate addition of H2O2and vitamin C (Vc),the transformation between C7SeOBola and C7SeBola was also probably reversible in a similar manner.During the two oxidation and reduction cycles,the transformation ratio between C7SeOBola and C7SeBola was nearly 100%,which was also supported by the1H NMR analysis (Fig.4a bottom and Fig.4b).
Cyclic voltammetry (CV) provides more quantitative information for comparing the oxidation behaviours of C7SeBola and its counterpart C7Bola-Mode.The results in Fig.5b of the two compounds without selenium just showed one oxidation peak at the potential~1.18 V (vs.Ag/AgCl) and~1.25 V (vs.Ag/AgCl),which were due to the water decomposition.In the compounds containing selenium,additional oxidation peak~0.95 V (vs.Ag/AgCl) appeared because of the oxidation of selenium.Therefore,we concluded that the selenide compounds were more sensitive to oxidation than their ether analogues.
Based on the redox-responsive transformation between C7SeBola and C7SeOBola by the alternating addition of H2O2or Vc,we demonstrated the chemically responsive,reversible,turbid-to-clear and clear-to-turbid transitions in an “ON-OFF”manner.When a solution of C7SeBola (1 mg/mL) was heated to 60 °C (Fig.5c),the solution became turbid (Fig.5c) because the sample was heated above itsTcloud(52.4 °C).When H2O2was added to this turbid solution (Fig.5c),it turned clear due to the formation of fully hydrated selenoxide-substituted CE [14c].Addition of Vc to this clear solution at 60 °C made it turbid again due to the regeneration of C7SeBola.
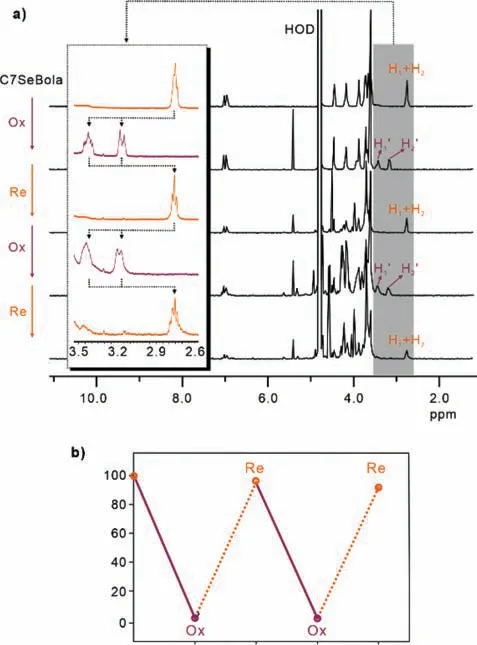
Fig.4.(a) 1H NMR spectra of C7SeBola (0.87 mmol/L) upon alternating addition with 6 equiv.H2O2 or 6 equiv.more Vc in D2O at 25 °C.(b) The ratio of C7SeBola in the presence (purple circle) H2O and Vc (organge circle) of C7SeBola upon alternating addition with H2O2 or 3 equiv.more Vc in D2O at 25 °C.
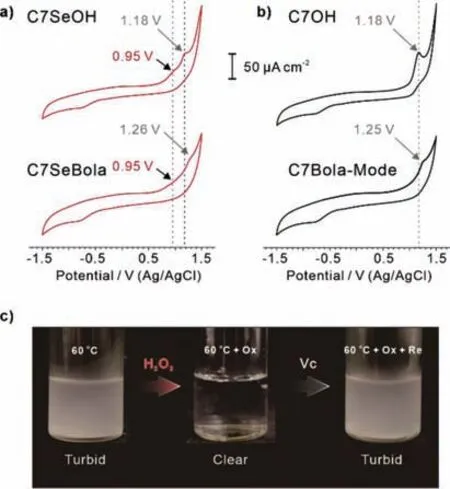
Fig.5.The cyclic voltammetry curves of (a) selenium-containing CEs C7SeOH,C7SeBola and (b) CEs C7OH,C7Bola-Mode.(c) Photograph of (i) C7SeBola (0.87 mmol/L),(ii) 6 equiv.mixture of C7SeBola (0.87 mmol/L) and H2O2 (5.22 mmol/L),and (iii) a mixture of C7SeBola (1 mg/mL),H2O2 (5.22 mmol/L) and Vc(5.22 mmol/L) in aqueous solution at 60 °C,the temperature is higher than Tcloud of C7SeBola (52.4 °C).
In conclusion,a new class of macrocyclic bolaamphiphile was prepared from a constitutionally adaptive CE.The compound exhibited good LCST behaviour,including highly sensitive phase separation and excellent thermal reversibility.It exhibited a redoxresponsive switch in the turbidity at a certain temperature upon the addition of H2O2and Vc.Moreover,unlike the redox-sensitive guest and host-guest complexation [12g]for the construction of redox-responsive macrocyclic amphiphiles,the reversible transformations between selenide and selenoxide in CEs not only generate a switchable pair of macrocycles differing dramatically in their hydration behaviour but also allow to sensitively vary the ring constitution in response to the chemical environment.This constitutionally adaptive chemistry of CEs provides an alternative for designing redox-responsive material.Since there are versatile routes for the chemical modification of benzo-crown ethers,diverse supramolecular structures can be designed on the basis of macrocyclic amphiphiles and other responsive materials.Moreover,since the hydrogen bond strength of selenoxide is higher than that of ether and selenide,various combinations of C7SeOOH,C7OH,and C7SeOH in pairs may serve as a new easy-to-access model system to exclusively probe water-solute interactions and consequently understand how the host pocket wettability is controlled by the one atom-substitution approach.
Declaration of competing interest
All authors declare that No conflict of interest exists.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos.52001255,21901209,21901210,22071196,22007078),Key R&D Program of Shaanxi Province (No.2021KWZ-18),Aeronautical Science Foundation of China (No.ASFC-2020Z061053001),Fundamental Research Funds for the Central Universities (Nos.3102019smxy001,3102019ghxm005) and Fellowship from CSC Innovative Team Program (No.CXXM20190099).We appreciate Ms.Hu Sijun for the assistance of MS measurements.We thank the Analytical &Testing Center of NPU for the characterization of materials.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.05.058.
杂志排行
Chinese Chemical Letters的其它文章
- Progress in mechanochromic luminescence of gold(I) complexes
- Spinel-type bimetal sulfides derived from Prussian blue analogues as efficient polysulfides mediators for lithium-sulfur batteries
- Alopecuroidines A-C,three matrine-derived alkaloids from the seeds of Sophora alopecuroides
- Boronic acid-containing diarylpyrimidine derivatives as novel HIV-1 NNRTIs:Design,synthesis and biological evaluation
- Diaminodiacid bridge improves enzymatic and in vivo inhibitory activity of peptide CPI-1 against botulinum toxin serotype A
- Peptide stapling with the retention of double native side-chains
