Synthesis of tetrasubstituted thiophenes from pyridinium 1,4-zwitterionic thiolates and modified activated alkynes
2021-03-14TiminWngXuehengZhuQingqingToWeiXuHiynSunPingWuBinChengHongbinZhi
Timin Wng,Xueheng Zhu,Qingqing To,Wei Xu,Hiyn Sun,Ping Wu,Bin Cheng,Hongbin Zhi,b,∗
a Institute of Marine Biomedicine/Hoffmann Institute of Advanced Materials,Shenzhen Polytechnic,Shenzhen 518055,China
b State Key Laboratory of Chemical Oncogenomics,Shenzhen Engineering Laboratory of Nano Drug Slow-Release,Peking University Shenzhen Graduate School,Shenzhen 518055,China
c Key Laboratory of Coordination Chemistry and Functional Materials in Universities of Shandong,Dezhou College,Dezhou 253023,China
Keywords:1,4-Zwitterionic thiolate Activated alkyne Formal [3+2] Thiophene Metal-free Catalyst-free
ABSTRACT Pyridinium 1,4-zwitterionic thiolates were applied to a formal [3+2]annulation reaction with modified activated alkynes,affording various tetrasubstituted thiophenes with aryl,alkenyl,alkyl or silyl group at the special position.The structural modification of alkyne substrates enabled the synthesis of diverse thiophenes to be achieved using the pyridinium 1,4-zwitterionic thiolates as the sulfur-containing building blocks.This approach is metal-free and catalyst-free.
Recently,we embarked on a program to extensively explore the annulation reactions of one kind of novel pyridinium 1,4-zwitterionic thiolate A (Scheme 1) [1],which could introduce a sulfur atom to organic molecular frameworks or use the sulfur atom to assist the bond-forming process.Thiolate A is air-stable,odourless,easy-to-handle,and readily available.In addition,its annulation reactions are often metal-free and catalyst-free,and only a base or heating is required for some special cases.Generally,it could mainly participate in two classes of reactions [i.e.,(5+m,path a) and (3+m,path b),Scheme 1],which have been demonstrated in our previous reports [2].Among them,the [3+2]reaction mode could be applied to the synthesis of a variety of tetraand trisubstituted thiophenes [2b].For this approach,the Y group is confined to suitable electron-withdrawing groups or hydrogen,as the alkyl or aryl group-substituted internal alkynes (e.g.,methyl but–2-ynoate and 4-phenylbut-3-yn-2-one) gave no reaction at all.Thus,how to install other groups (e.g.,amodified substrates would undergoryl,alkenyl,alkyl and silyl) in the corresponding position of Y is the problem always on our mind.Although there are already many synthetic approaches to access polysubstituted thiophenes reported in the literature [3],many of them involve metal catalysts,malodorous starting materials,and/or harsh reaction conditions.Thiophenes,one class of sulfur-containing fivemembered heterocycles,widely present in pharmaceuticals,bioactive molecules [4],and functional materials [5],and they are often used as vital organic synthetic intermediates in organic chemistry and materials chemistry [6].
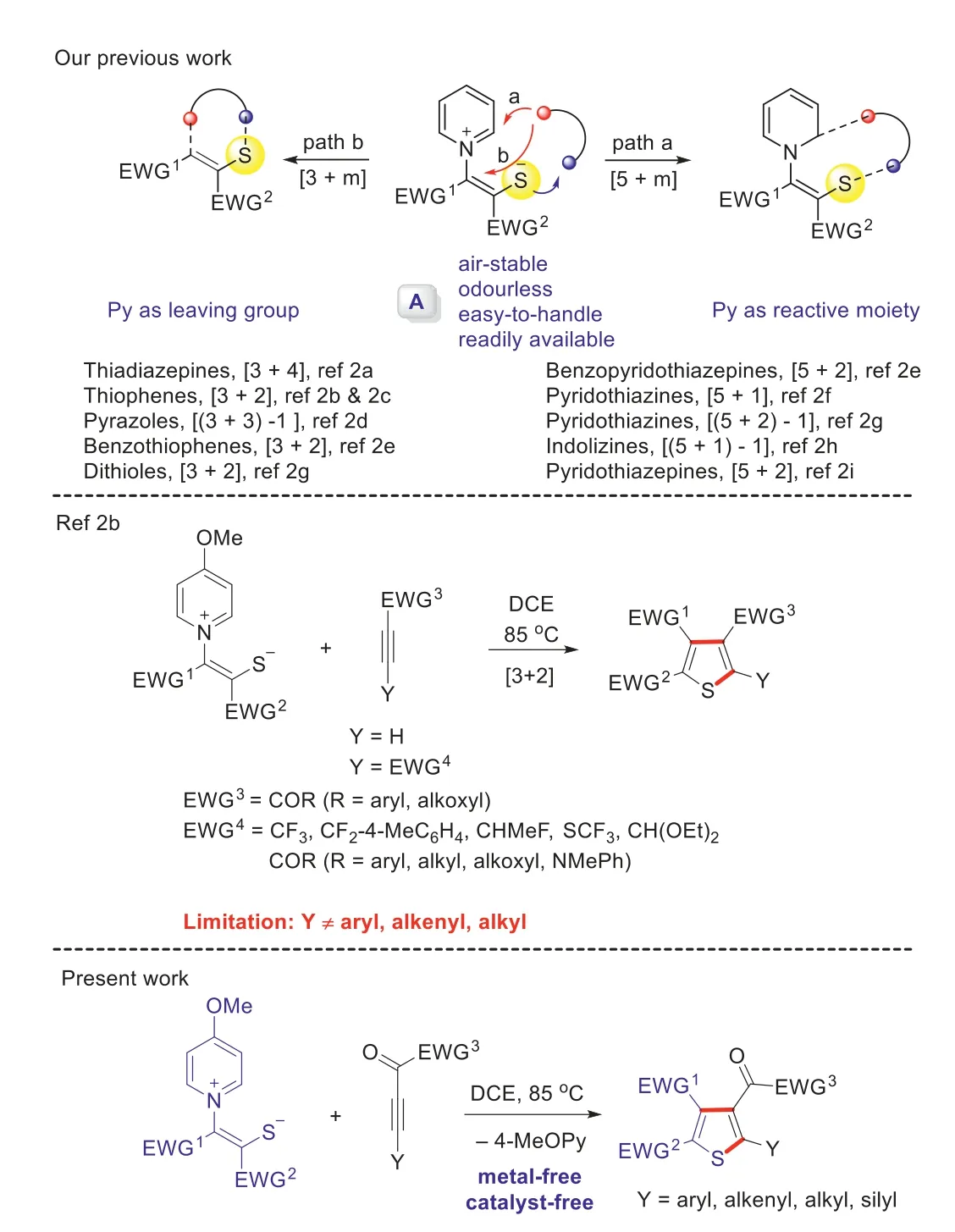
Scheme 1.Reaction modes of pyridinium 1,4-zwitterionic thiolates and this work.
Given our ongoing interest in sulfur chemistry [7],especially pyridinium 1,4-zwitterionic thiolate A,we hope to use this versatile building block to synthesize more diverse thiophene derivatives and break through the limitation of substrate scope in our previous report [2b].Inspired by the work of Hou [8],we envisaged that the reactivity of the original failed substrates (e.g.,methyl but–2-ynoate and 4-phenylbut-3-yn-2-one) could be improved by introducing an additional carbonyl group between the alkynyl and EWG3moieties,and the modified substrates would undergo a formal [3+2]annulation reaction,affording polysubstituted thiophenes with aryl,alkenyl,alkyl,or silyl moiety at the position of Y group.Herein,we will describe our preliminary explorations on this subject.
Based on our previous experience of the formal [3+2]annulation reaction,pyridinium 1,4-zwitterionic thiolate 1a and methyl 2-oxo-4-phenylbut-3-ynoate (2a) were chosen as the model substrates to verify the design under the optimal reaction conditions in our previous report [2b].Delightfully,when 1a and 2a were simply mixed,stirred,and heated at 85 °C in 1,2-dichloroethane without any additives,the desired annulation reaction occurred smoothly,delivering tetrasubstituted thiophene 3 in 81% yield(Scheme 2),which demonstrated that the rational structural modification is effective for the previous failed substrate and an aryl group could be installed at the position of Y.Subsequently,other aryl and heteroaryl (e.g.,thienyl) group-substituted alkynes were examined and all of them reacted well,delivering the annulation products in high yields (4–13).The substrates with substituents at theo,mandp-position of phenyl ring were all competent.Using this carbonyl insertion strategy,alkenyl and alkyl chains,even silyl moiety could be fixed on the thiophenes (14–19) and the steric hindrance of alkyl moiety had no obvious influence on the yield.The silyl group could be used to conduct further derivatizationviathe Hiyama coupling reaction.Except for the substrates with ester function as the EWG3,the ketone or amideactivated alkynes were also investigated.Substrates bearing ketone groups (20 and 21) performed well as those with ester moiety,while amide substrate (22) afforded a low yield of 40%.In contrast,N1,N4-dimethyl-N1,N4-diphenylbut-2-ynediamide and 1-(piperidin-1-yl)prop–2-yn-1-one did not work in our previous report [2b].Finally,the scope of pyridinium 1,4-zwitterionic thiolate was investigated (23–26).The annulation reactions of other thiolates (1b–1e) proceeded smoothly but diketone and ketoester substrates only gave low yields.It is worth noting that thiolate 1e could prepare CF3-containing thiophene (e.g.,26),and thiophenes 25 and 26 were decorated with four different substituents.In addition,it should be mentioned that 1,1,1-trifluoro-4-phenylbut-3-yn-2-one was also a viable alkyne substrate,and 4-(2,2,2-trifluoro-1-hydroxyethyl)thiophene derivative 27 was obtained in 83% yieldviaa two-step transformation.
To further demonstrate the utility of the present synthetic protocol,a series of derivatization reactions were conducted (Scheme 3).The inserted carbonyl moiety could be reduced to a hydroxyl group,and then the resulting product 28 could undergo a lactonization reaction to afford 29 under the acidic conditions.The carbonyl moiety could also be readily transformed into the double bond (compound 30) or reactive intermediate hydrazone 31.In addition,the carbonyl and ester moieties could simultaneously be condensated with hydrazine hydrate to afford fused thiophene 32.
The present protocol follows the same mechanism proposed in the previous report (Scheme 4) [2b].The annulation process is triggered by anS-Michael addition of pyridinium to activated alkyne followed by aC-Michael addition,and then a retro-Michael addition delivers the desired thiophene accompanied with 4-MeO-Py extrusion.

Scheme 2.Substrate scope of the formal [3+2]annulation reaction.Reaction conditions:1 (1.5 equiv.),2 (0.3 mmol),DCE (3 mL),85°C,in air.

Scheme 3.Further transformations of thiophenes.

Scheme 4.Proposed mechanism for [3+2]cascade cyclization reaction.
In summary,the synthesis of diverse tetrasubstituted thiophene derivatives was achievedviaa formal [3+2]annulation of pyridinium 1,4-zwitterionic thiolates and modified activated alkynes.Using the carbonyl insertion strategy could produce thiophenes with aryl,alkenyl,alkyl or silyl group at the position of Y group.The annulation reaction is metal-free and catalyst-free.The other explorations of pyridinium 1,4-zwitterionic thiolates are in progress in our laboratory and will be reported in due course.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank National Natural Science Foundation of China (Nos.21971092,21901014,21472072,21871018,21732001 and 21672017),Shenzhen Science and Technology Innovation Committee (No.JCYJ20200109141808025),and Characteristic Innovation Project of Guangdong Provincial Education Department (No.2020KTSCX295)for the financial support.
杂志排行
Chinese Chemical Letters的其它文章
- Progress in mechanochromic luminescence of gold(I) complexes
- Spinel-type bimetal sulfides derived from Prussian blue analogues as efficient polysulfides mediators for lithium-sulfur batteries
- Alopecuroidines A-C,three matrine-derived alkaloids from the seeds of Sophora alopecuroides
- Boronic acid-containing diarylpyrimidine derivatives as novel HIV-1 NNRTIs:Design,synthesis and biological evaluation
- Diaminodiacid bridge improves enzymatic and in vivo inhibitory activity of peptide CPI-1 against botulinum toxin serotype A
- Peptide stapling with the retention of double native side-chains
