Distinguishable multi-substance detection based on three-channel NIR fluorescent probe in physiology and pathology of living cells and zebrafish
2021-03-14LiFngjunHuoYongkngYueKiqingYingWenCixiYin
S h Li,Fngjun Huo,Yongkng Yue,Kiqing M,Ying Wen,Cixi Yin,∗
aInstitute of Molecular Science,Key Laboratory of Chemical Biology and Molecular Engineering of Ministry of Education,Shanxi University,Taiyuan 030006,China
b Research Institute of Applied Chemistry,Shanxi University,Taiyuan 030006,China
Keywords:Mitochondrial-targeted Discrimination detection Distinguishable Multi-substance Three channels
ABSTRACT Mitochondria is the main organelle for the production of reactive sulfur species (RSS),such as homocysteine (Hcy),cysteine (Cys),glutathione (GSH) and sulfur dioxide (SO2).These compounds participate in a large number of physiological processes and play an extremely important role in maintaining the balance of life systems.Abnormal concentration and metabolism are closely related to many diseases.Due to their similarities in chemical properties,it is challenging to develop a single fluorescent probe to distinguish them simultaneously.Here,we synthesized the probe PI-CO–NBD with three fluorophores,NBD-Cl and benzopyranate as the reaction sites of GSH/Cys/Hcy and SO2,respectively.Three biothiols all could cleavage ether bond to release benzopyrylium and coumarin moiety,which emitted red and blue fluorescence,but Cys/Hcy also could do intramolecular rearrangement after nucleophilic substitution,resulting in yellow fluorescence.Thus the probe can distinguish Cys/Hcy and GSH.Subsequently,only SO2 could quench red fluorescence by adding C=C of benzopyrylium.The probe also could localize well in mitochondria by oxonium ion for all kinds of cells.The probe not only could detect above sulfur-containing active substances of intracellular and extracellular but also monitor the level of them under oxidative stress and apoptosis process in living cells and zebrafish.
Reactive sulfur species (RSS) mainly includes three biological thiols,such as homocysteine (Hcy),cysteine (Cys),glutathione(GSH) and sulfur dioxide (SO2) [1–3].This compound is ubiquitous in cells and even organisms,participates in the regulation of many physiological processes,and plays an extremely important role in maintaining the balance of life systems [4].The concentration of active sulfur and abnormal metabolism are closely related to many diseases [5–9].Pathological studies have shown that excessive sulfite/bisulfite can cause toxic reactions in cells and certain tissues[10,11],and cause respiratory diseases [12],cardiovascular diseases and neurological diseases,etc.[13,14].Cys is the center of sulfur metabolism in cells,and its lack is related to skin diseases,liver damage,mental retardation and other diseases [15].Hcy is an intermediate product produced during the production of Cys,and its unbalanced concentration level can cause diseases,such as neurological diseases,cardiovascular diseases,osteoporosis and enteritis[16,17].GSH is the most abundant thiol in cells [18–21],and GSH can maintain the redox activity in cells [22–25].When the GSH concentration is not balanced,it can cause other diseases,such as Alzheimer’s disease and lung disease [26–29].Therefore,it is necessary to develop a new method for distinguishing detection.In recent years,a large number of literatures have reported fluorescent probes to detect one of the active sulfur [30].However,most fluorescent probes are difficult to effectively distinguish Cys,Hcy,GSH and SO2,and the wavelengths of many probes are mainly concentrated in the short-wave region.Therefore,it is of great significance to develop functionally specific fluorescent probes for detecting biologically reactive sulfur species (RSS).
It is well known that mitochondria are the main source of intracellular RSS,such as biothiols,and play a vital role in redox homeostasis,drug metabolism,signal transduction,and other physiological and pathological processes [31,32].Interestingly,positively charged molecules with fluorophores can target mitochondria well.More importantly,we used this probe to visualize thiol fluctuations during oxidative stress and apoptosis,which will prove that it is very valuable for elucidating the pathophysiological process of living organisms.This result will provide an effective means for studying the pathological process of thiol-related diseases.
I n our work,we synthesized the probe PI-CO–NBD with three fluorophores,NBD-Cl and benzopyranate as the reaction sites of GSH/Cys/Hcy and SO2,respectively.Three biothiols all could cleavage ether bond to release benzopyrylium and coumarin moiety,which emitted red and blue fluorescence,but Cys/Hcy also could do intramolecular rearrangement after nucleophilic substitution,resulting in yellow fluorescence.Thus the probe can distinguish Cys/Hcy and GSH.Subsequently,only SO2could quench red fluorescence by adding C=C of benzopyrylium.The probe also could localize well in mitochondria by oxonium ion for all kinds of cells.The probe not only could detect above sulfur-containing active substances of intracellular and extracellular but also monitor the level of them under oxidative stress and apoptosis process in living cells and zebrafish.
The probe PI-CO–NBD was obtained and characterized through a series of experiments which could be found in Supporting information.The synthesis route of PI-CO–NBD was shown in Scheme 1.
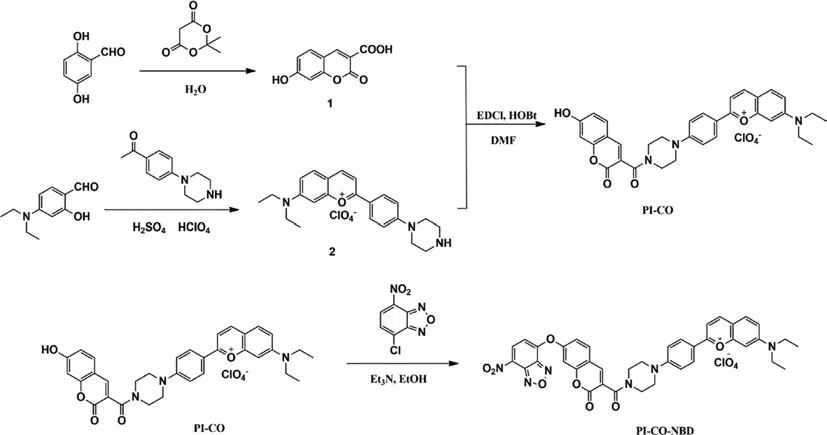
Scheme 1.The synthesis of the probe PI-CO–NBD.
A series of spectral experiments were carried out.The fluorescence changes of PI-CO–NBD in response to Cys,Hcy,GSH and SO2was tested in PBS buffer (pH 7.4,containing 20% CH3CN,v/v).As shown in Fig.1,when Cys-or Hcy addition,the ether bond was broken to releasing 640 nm red fluorescence (under 565 nm excitation,the intensity was prominent).At the same time,the fluorescence intensity at 455 nm gradually increased as the concentration increased,and a new emission peak appeared at 550 nm,their fluorescence intensity gradually increaseed with the increase of Cys/Hcy.The significant increase in fluorescence intensity was due to the release of NBD-Cl.However,the addition of GSH could only induce fluorescence enhancement at 456 nm.Based on this,in the presence of GSH,Cys/Hcy could be monitored in three channels.In order to test the response of PI-CO–NBD to SO2,when using 565 nm excitation,an emission peak appeared at 640 nm,and with the addition of SO2,the fluorescence intensity gradually decreases.The results showed that PI-CO–NBD can achieve rapid detection of SO2.The UV spectral experiment,pH,the time dependence,selective test,detection limit and linear correlation were carried out as shown in Figs.S1-S5 (Supporting information).
It was speculated that the recognition mechanism of PICO–NBD was as following (Scheme 2).The reaction mechanism was shown in Scheme 2.NBD-Cl linked by ether bond is the recognition site of thiol.The sulfhydryl groups of Cys/Hcy and GSH are nucleophilic reagent and could react with PI-CO–NBD to force the break of the ether bond and release the red fluorophore PI-CO.NBD-Cl and Cys/Hcy can undergo intramolecular rearrangement to display three-channel fluorescence,while GSH could not produce intramolecular rearrangement due to steric hindrance.After SO32-was added,the double bond on the benzopyrrole was broken and the red fluorescence was quenched.In order to further study the responding mechanism,we measured the corresponding HRMS characterization of the PI-CO–NBD + Cys/Hcy and GSH (Fig.S7 in Supporting information).The corresponding product NBD-GSH and PI-CO were formed after PI-CO–NBD reacted with GSH (calcd.for[NBD-GSH]-:m/z469.0783,found 469.0789).When treated with Cys/Hcy,the corresponding product were NBD-Cys/NBD-Hcy and PI-CO (calcd.for [NBD-Cys]-:m/z283.0143,found 283.0143;calcd.for [NBD-Hcy]-:m/z297.0299,found 297.0300).These results provided support for our proposal that probe (PI-CO–NBD) could discriminate Cys/Hcy and GSH.
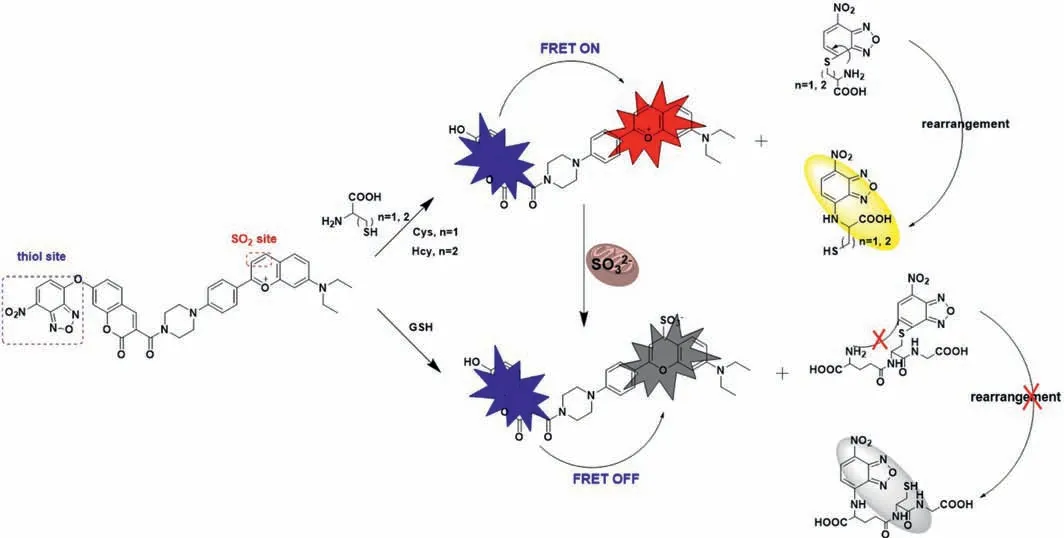
Scheme 2.Response mechanisms of PI-CO–NBD with Cys/Hcy/GSH and SO2.
HeLa cells were incubated with PI-CO–NBD in PBS buffer at 37 °C,and the time-varying signals of the three channels were recorded.Over time,blue,yellow and red fluorescence gradually increased and stabilized (Fig.S8 in Supporting information).This indicated that PI-CO–NBD can discriminate between endogenous Cys/Hcy and GSH in cells.Encouraged by the above-mentioned advantageous properties of PI-CO–NBD probein vitro,we investigated the application of PI-CO–NBD in fluorescence imaging of thiols in living cells.The HeLa,HL-7702 and MCF-7 were used as test models [33-37].We evaluated the organelle targeting ability of the probe.A commercially available mitochondrial dye (Mito-Tracker Green) was used as the target dye for dyeing experiments.MCF-7 cells,HL-7702 cells and HeLa cells containing PI-CO–NBD were incubated with Mito-Tracker Green for 30 min.As we guessed,Figs.2C,G and K show that the fluorescence signals of the green and red channels overlap well.In addition,Pearson’s colocalization coefficient was 0.90,0.86,0.87.Therefore,the probe has the function of targeting mitochondria (Fig.2).

Fig.1.Fluorescence spectra of PI-CO–NBD (10 μmol/L) with (A) Cys (0–50 μmol/L),(B) Hcy (0–50 μmol/L),(C) GSH (0–50 μmol/L),(D) SO32- (0–50 μmol/L) in the PBS buffer(pH 7.4,containing 20% CH3CN,v/v). λex=395 nm,Ex/Em slit:5/2 nm.
Thiols are closely related to many physiological and pathological processes.Abnormal content and metabolism can lead to serious consequences [38,39].Therefore,we choose HeLa cells as a cell model to study oxidative stress and apoptosis in cells.Before incubating cells,PMA (phorbol 12-myristic acid 13-acetate that causes oxidative stress in cells) and BSO (Lththionine sulfoximine,a sulfinimide that reduces GSH levels is used to induce apoptosis)were used for changing intracellular thiol concentration [40].First,the cells were incubated with 10 μmol/L PI-CO–NBD at 37 °C for 10 min,and the blue,yellow and red fluorescence was observed,indicating that PI-CO–NBD has successfully detected abundant intracellular thiols (Figs.3A-E).Then HeLa cells were stimulated with NEM (a thiol specific scavenger) for 30 min,and then incubated with PI-CO–NBD for 10 min.The fluorescence signal in the channels was inhibited,revealing the endogenous specific fluorescence response of thiols (Figs.3F-J).In addition,as shown in Figs.3K-T,HeLa cells first was incubated with PMA or BSO for 30 min before addition with the PI-CO–NBD probe,a significant decrease in fluorescence occurred,indicating a significant reduction intracellular mitochondrial thiol levels,oxidative stress and apoptosis of cells took place.Similarly,we used HL-7702 and MCF-7 as cell models(Figs.S9 and S10 in Supporting information),and monitored the changes of intracellular thiols according to the above method,and these results were consistent with that of HeLa cells.Therefore,the PI-CO–NBD could successfully monitor oxidative stress and apoptosis of cells.

Fig.2.Intracellular localization of PI-CO–NBD in living MCF-7,HL-7702 and HeLa cells.(A,E,I) All the tested cells were prestained with MitoTracker Green FM (1 μmol/L),(λex=488 nm and λem range:498–651 nm).(B,F,J) All the tested cells were treated with PI-CO–NBD (10 μmol/L). λex=565 nm and λem range 610–670 nm.(C,G,K) Merged images.(D,H,L) Intensity profiles of regions of interest.
Next,we studied the response of PI-CO–NBD to SO2in the presence of thiols at the cellular level.When cells cleared by NEM were incubated with PI-CO–NBD and Cys,fluorescent signals were obtained in the blue,yellow,and red channels (images B and C in Fig.S11(i) in Supporting information).Then,continued to incubate the cells with SO32-,and observed the quenching of the red fluorescent channel signal (image I in Fig.S11(i) in Supporting information).For PI-CO–NBD and the GSH system,only the blue and red channels have fluorescent signals (images B and D in Fig.S11(ii) in Supporting information),and the subsequent incubation of SO32-could quench the red fluorescence (image I in Fig.S11(ii)in Supporting information).Experiments have shown that,due to the presence of Cys and GSH,PI-CO–NBD at the cellular level could detect SO2through different fluorescent emission.
To assess the permeability of PI-CO–NBD at the living level,we conducted experiments in model biozebrafish.As shown in Fig.S12 (Supporting information),zebrafish incubated with PI-CO–NBD emitted faint fluorescence in blue,yellow and red channels.Zebrafish were incubated with NEM (a mercaptan remover) as the control group,showing minimal fluorescence in three channels.After the addition of Cys/Hcy,the fluorescence intensity of the three channels was significantly enhanced again.However,with the addition of GSH,only the blue and red emission channels changed significantly.These results showed that PI-CO–NBD was able to detect Cys/Hcy without interference from GSH by special yellow channel.Therefore,the probe was an effective molecular tool for rapidly detecting biothiol levels in living animals.Similarly,we investigated the probe’s ability to detect thiol in inflammatory processes induced by PMA or BSO in living animals.The model zebrafish were divided into four groups.One group was treated with PI-CO–NBD,while the other group was pretreated with NEM,PMA or BSO,respectively,before PI-CO–NBD addition.As shown in Fig.4,the live zebrafish treated with PI-CO–NBD showed an obvious fluorescence signal,whereas in the zebrafish pretreated with NEM,the fluorescence signal was significantly blocked due to NEM.The zebrafish was incubated with PMA or BSO for 30 min,the fluorescence signal was reduced,indicating lower bithiol levels during inflammatory responses and apoptosis.These results provide an idea for further applications of the probe in biology.

Fig.3.Fluorescence images of endogenous thiols in living HeLa cell.(A-E):Cells were treated with the PI-CO–NBD probe,(F-T):Cells were respectively pretreated with NEM(1 mmol/L),PMA (1 μg/mL),or BSO (100 μmol/L) for 30 min and then incubated with PI-CO–NBD (10 μmol/L) for 10 min.Blue channel: λex=405 nm, λem=450-510 nm;yellow channel: λex=451 nm, λem=520–580 nm;red channel: λex=561 nm, λem=610–670 nm.
In a word,the PI-CO–NBD,a multicolor fluorescent probe was constructed based on FRET regulation.This probe contained mutisite to discriminate Cys/Hcy,GSH and SO32-.Among them,NBDCl connected by ether bonds is a potential site to promote thiol tandem reactions.On the one hand,the nucleophilic substitution of the sulfhydryl group with PI-CO–NBD led to the release of PICO and emitted red fluorescence at 640 nm.On the other hand,the substitution of amino and sulfhydryl groups in Cys/Hcy could produce yellow fluorescence emission.Due to steric hindrance,GSH could not induce intramolecular rearrangement,so the yellow channel had no fluorescence emission.At the same time,after adding SO32-,the double bond of the benzopyridine unit was broken,the fluorescence intensity at 640 nm decreased rapidly.In addition,due to the special benzopyrrole structure,PI-CO–NBD could specifically target mitochondria.Therefore,the probe was used to visualize the thiol fluctuations under oxidative stress and apoptosis,indicating that the probe can elucidate the pathophysiological process of organisms.We hope that this research can be used in the analysis of diseases caused by thiols and SO2.

Fig.4.Fluorescence imaging of Cys/Hcy and GSH with three-channel in zebrafish:(A-E):Zebrafish were treated with the PI-CO–NBD probe;(F-T):Cells were respectively pretreated with NEM (1 mmol/L),PMA (1 μg/mL),or BSO (100 μmol/L) for 30 min and then incubated with PI-CO–NBD (10 μmol/L) for 10 min.Blue channel: λex=405 nm,λem=450–510 nm;yellow channel: λex=451 nm, λem=520–580 nm;red channel: λex=561 nm, λem=610–670 nm.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the National Natural Science Foundation of China(Nos.21775096,22074084),One Hundred People Plan of Shanxi Province,Shanxi Province "1331 Project" Key Innovation Team Construction Plan Cultivation Team (No.2018-CT-1),2018 Xiangyuan County Solid Waste Comprehensive Utilization Science and Technology Project (No.2018XYSDJS-05),the Shanxi Province Foundation for Selected (2019),Innovative Talents of Higher Education Institutions of Shanxi,Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No.2019L0031),Key R&D Program of Shanxi Province (No.201903D421069),the Shanxi Province Science Foundation (No.201901D111015),and Scientific Instrument Center of Shanxi University (No.201512).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.05.026.
杂志排行
Chinese Chemical Letters的其它文章
- Progress in mechanochromic luminescence of gold(I) complexes
- Spinel-type bimetal sulfides derived from Prussian blue analogues as efficient polysulfides mediators for lithium-sulfur batteries
- Alopecuroidines A-C,three matrine-derived alkaloids from the seeds of Sophora alopecuroides
- Boronic acid-containing diarylpyrimidine derivatives as novel HIV-1 NNRTIs:Design,synthesis and biological evaluation
- Diaminodiacid bridge improves enzymatic and in vivo inhibitory activity of peptide CPI-1 against botulinum toxin serotype A
- Peptide stapling with the retention of double native side-chains
