Rich B active centers in Penta-B2C as high-performance photocatalyst for nitrogen reduction
2021-03-14RanWangChaozhengHeWeixingChenChenxuZhaoJinrongHuo
Ran Wang,Chaozheng He,∗,Weixing Chen,∗,Chenxu Zhao,Jinrong Huo
a Institute of Environmental and Energy Catalysis,School of Materials Science and Chemical Engineering,Xi’an Technological University,Xi’an 710021,China
b Shaanxi Key Laboratory of Optoelectronic Functional Materials and Devices,School of Materials Science and Chemical Engineering,Xi’an Technological University,Xi’an 710021,China
c School of Sciences,Xi’an Technological University,Xi’an 710021,China
Keywords:Nitrogen reduction Photocatalytic Penta-B2C Mechanism B active center
ABSTRACT The photocatalytic nitrogen reduction reaction (NRR) has mild reaction conditions and only requires sunlight energy as a driving force to replace the traditional ammonia synthesis method.We herein investigate the catalytic activity and selectivity on Penta-B2C for NRR by using density functional theory calculations.Penta-B2C is a semiconductor with an indirect bandgap (2.328 eV) and is kinetically stable based on molecular dynamic simulations.The optical absorption spectrum of Penta-B2C is achieved in the ultraviolet and visible range.Effective light absorption is more conducive to generate photo-excited electrons and improving photocatalytic performances.Rich B atoms as activation sites in Penta-B2C facilitate capturing N2.The activated N2 molecule prefers the side-on adsorption configuration on Penta-B2C,which facilitates the subsequent reduction reaction.Among considered NRR mechanisms on Penta-B2C,the best pathway prefers the enzymatic mechanism,only required a low onset potential of 0.23 V.The hydrogen evolution reaction is inhibited when the hydrogen adsorption concentration is increased or N2 molecules first occupy the adsorption sites.Our results indicate Penta-B2C is a highly reactive and selective photocatalyst for NRR.Our work provides theoretical insights into the experiments and has guiding significance to synthesize efficient NRR photocatalysts.
Although the atmosphere contains sufficient nitrogen (N2) to account for about 78%,the chemical inertness of the N≡N triple bond makes it difficult to utilize [1].Ammonia (NH3) as N2reduction product is not only an important chemical in various fields but also a promising intermediate for energy storage [2-5].The industrial production of NH3depends on the Haber-Bosch process,which requires harsh environmental conditions,such as high temperature,high pressure,and heavy energy consumption [6,7].The photocatalytic nitrogen reduction reaction (NRR) requires only clean and abundant sunlight as an energy source to drive chemical reactions which reduces energy consumption and increases NH3yield [8-12].However,the ideal NRR photocatalyst should first overcome high energy barriers to cleave N≡N bonds [13–15].Moreover,the hydrogen evolution reaction (HER) as the competing reaction should be suppressed to improve NRR selectivity [1,6,16].To find and design suitably photocatalytic materials with high activity and high selectivity for NRR therefore is a great challenge so far.
Transition metal compounds are favorable candidates for various catalytic reactions due to occupied and unoccupied d-orbitals that can facilitateπback-donation [6,17-22].Moreover,the main group element boron (B) shows similar transition metal characteristics due to a lack of electrons and Lewis acid characteristics [23-25].In fact,B-doped two-dimensional (2D) materials have been widely investigated for catalyzing NRR [26,27].Experimental studies have reported the NH3generation rate is 9.8 μg h-1cm-1,and the Faraday efficiency at-0.5 Vversusreversible hydrogen electrode is 10.8% on 2D B-doped graphene [23].A single B atom supported on holey g-CN can serve as an efficient photocatalyst for NRR under visible and even infrared spectra [1].A single B atom decorated BN edge shows very low limiting potential for NRR,only-0.29 V [18].Those results show that B doping can change the local chemical environment to adjust the NRR preference.This is mainly attributed to the outer orbital of B is hybridized to generate sp2orbitals,unoccupied sp2orbitals can accept unpaired electrons of N2to enhance the B-N bond formation,while occupied 2p orbitals of B atom can donate electrons to the antibonding orbitals of N2to weaken the N≡N bond and activate the N2molecule[1,28].The B atom therefore is the core component that activates N2molecule,which in turn leads to high NH3productivity and Faraday efficiency [1,18,23,28].Although the B-doped 2D materials showed excellent NRR performance,the difference in the concentration of B doping and the uncontrollability of the B doping sites in the experimental synthesis were able to affect their NRR effi-ciency,which prompted us to find B-based compounds for suitable NRR catalysts.
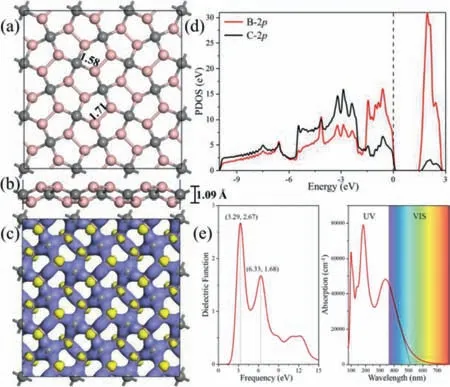
Fig.1.(a) Top view and (b) side view of the optimized Penta-B2C.Hereafter,the pink and gray spheres represent B and C atoms,respectively.(c) The deformation charge density of Penta-B2C,(charge depletion and accumulation are shown in blue and yellow).(d) Partial density of states for Penta-B2C.Fermi level is set to zero(denoted by the dotted line).(e) Dielectric function and optical absorption spectra of Penta-B2C."UV" and "VIS" mean ultraviolet light and visible light,respectively.
Some 2D pentagon materials exhibit novel physical and chemical properties due to their lower symmetry,including negative Poisson’s ratio,high carrier mobility and so on [29–34].The quasi-planar crystal structures and intrinsic electronic structures of Penta-TiP facilitate strong N2adsorption and exhibit a low limiting potential of-0.72 V for NRR [35].Previous studies have shown that the Penta-B2C monolayer as the B-based compound is thermally and dynamically stable [36].The potentially catalytic activity of Penta-B2C however is not clear so far.We therefore investigated the catalytic activity of NRR on Penta-B2C.Our investigation indicates Penta-B2C has excellent optical absorption capacity and stability.The side-on configuration of N2adsorption was screened out.Different mechanisms have shown that Penta-B2C can effectively promote the reduction of N2through the enzymatic pathway.And the competitive HER can be effectively inhibited.Our results indicate that Penta-B2C is an effective photocatalyst for NRR.
The 2D Penta-B2C with a typical Cairo pentagonal tile structure(Fig.1a) is a semiconductor with a bandgap of 2.328 eV.Each pentagonal ring is composed of three B atoms and two C atoms.The B-B and B-C bond lengths are 1.71 and 1.58 ˚A.Fig.1b shows that the side view of Penta-B2C is not a planar structure but has certain wrinkles.It is formed by a layer of C atoms sandwiched between two symmetrical B atom layers with 1.09 ˚A layer thickness.The formation of wrinkles is beneficial to maintain the symmetry of the pentagonal lattice and can provide abundant adsorption sites for chemical reactions.

Fig.2.(a) The optimal structure of N2 adsorption on Penta-B2C in two configurations,and corresponding charge density difference after N2 adsorption.Cyan and yellow represent charge depletion and accumulation,respectively.Hereafter,the blue spheres represent N atoms.(b) Spin-polarized partial density of states of Penta-B2C-2p orbitals (red and black curve) and N2-2p orbitals after N2 adsorption on Penta-B2C.The Fermi level is set to zero.
Moreover,the stability of Penta-B2C is an important precondition as catalysts [37].In order to reduce the size effect of Penta-B2C,we calculated the unconstrained and freely fluctuating Penta-B2C structure (Fig.S1 in Supporting information) by molecular dynamics simulations,in which temperature is gradually increased from 300 K to 2000 K.The corresponding potential energy diagram and structure are shown in Fig.S2 (Supporting information).Although the Penta-B2C structure fluctuates to varying degrees at high temperatures,it can be seen from the partial structure diagram that there is no substantial change in the bond structure.We can see that the potential energy is proportional to the temperature,which indicates that the Penta-B2C structure is relatively stable below 2000 K.
The deformation charge density (DCD) of Penta-B2C shows the charge redistribution of the forming Penta-B2C (Fig.1c).Upon the Mulliken charge analysis,each B atom is positively charged 0.30|e| and each C atom is negatively charged-0.59|e|.The positive charge of the B atom can be used as an ideal activation center,laying the foundation for the activation of N2.We further calculated the partial density of states (PDOS) of all B and C atoms in Penta-B2C as shown in Fig.1d.The existence of overlapping peaks between the B-2p and C-2p orbital indicates the covalent interaction between B and C atoms.The stronger B-2p orbital at valence band maximum (VBM) and conduction band minimum (CBM) indicates that the B atom can be as the activation site (i.e.,the electron donor and acceptor).
The imaginary part of the dielectric function for Penta-B2C is shown in Fig.1e.In the range of 0–15 eV,two distinct dielectric function peaks appear at 3.29 and 6.33 eV,and the maximum peak is at 3.29 eV.The appearance of the peak reflects the transition of electrons from the valence band to the conduction band.For the photocatalyst,the optical absorption activity is very important,which can affect the light conversion efficiency and photocatalytic performance [38].The optical absorption spectrum shows that Penta-B2C absorbs light less than 520 nm,which indicates that light absorption is achieved in the ultraviolet and visible range.Effective light absorption is more conducive to generate photo-excited electrons and improving photocatalytic performances.
The first bond cleavage of the N≡N triple bond is the most challenging kinetic step in the photochemical NRR [9,39].The effi-cient NRR photocatalyst should facilitate the chemisorption of the N2molecule and ensure that the inert N≡N triple bond is sufficiently activated.Therefore,we calculated the adsorption of N2molecules on Penta-B2C,including side-on and end-on adsorption configurations.For the side-on N2,two N atoms bond with two adjacent B atoms of the Penta-B2C surface.PDOS of the side-on N2on Penta-B2C show a stronger hybrid between the N-2p orbital and the B-and C-2p orbital.N2can be chemisorbed on the B atom of Penta-B2C with adsorption free energy (Gad) of-0.22 eV (Fig.2).The N–N bond is enlarged from 1.11 ˚A to 1.26 ˚A.Upon charge density difference (CDD) analysis,B atom and the N2molecule have charge accumulation and depletion regions,which indicates an acceptance-donation electron process between the B atom and the N2molecule.N2molecules get-0.35|e| by Mulliken charge analysis.Therefore,the N2molecule is activated on Penta-B2C.For the end-on N2,only one N atom bonds with the B atom of the Penta-B2C withGadof-0.54 eV.The N–N and B–N bond lengths are 1.13 and 1.49 ˚A.The charge depletion region is mainly in the B atom region,and a small amount is distributed in the N2molecule.N2molecules get 0.11|e| by Mulliken charge analysis.PDOS of the end-on N2on Penta-B2C do not show the hybrid states between the N-2p orbital and the B-and C-2p orbital.Therefore the end-on N2molecule has not been activated,which may stop the further reduction reaction.
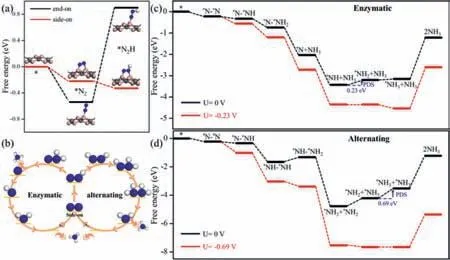
Fig.3.(a) Gibbs free energy diagrams for the first hydrogenation process of N2 molecules adsorbed on Penta-B2C with two configurations.(b) Schematic depiction of enzymatic and alternating mechanisms for N2 reduction to NH3.Blue and white spheres represent the N and H atoms,respectively.Gibbs free energy diagrams for NRR on Penta-B2C through (c) enzymatic and (d) alternating mechanisms at different applied potentials.
Although the side-on N2molecule is activated,Gadof the sideon N2molecule is smaller than that of the end-on N2molecule.The reason is attributed to the thermodynamic fluctuations in 2D limit [40,41].The charge transfers from Penta-B2C to the N2molecule after the N2adsorbed on Penta-B2C.The thermodynamic fluctuations of Penta-B2C are derived from the charge transfer and redistribution.Therefore,we define the energy change (Echange) of Penta-B2C between before and after the N2adsorption.Echangeof Penta-B2C between before and after the side-on N2adsorption is higher than that of the end-on N2adsorption (Table S2 in Supporting information).Therefore,the increasedEchangeof Penta-B2C between before and after the N2adsorption led to the decreasedEadof side-on N2.
For an efficient NRR photocatalyst,low onset potential and selectivity are necessary conditions.We first evaluated the catalytic performance of NRR on Penta-B2C.The N2molecules difficultly dissociate into two N atoms due to the endothermic process required 1.65 eV.Therefore,we only consider the associated hydrogenation process of N2molecules.The first hydrogenation step of side-on N2occurs easily with the free energy change (ΔG) to a downhill 0.11 eV.While the first hydrogenation step of end-on N2difficultly proceed with theΔGto an uphill 1.44 eV (Fig.3a).In the subsequent discussion,we would consider the enzymatic and alternating mechanisms from the side-on N2configuration (Fig.3b and Fig.S3 in Supporting information).

Fig.4.(a) Charge variation of two moieties along with the enzymatic mechanism.Each intermediate is divided into two moieties,where moiety 1 represents the Penta-B2C substrate and moiety 2 represents the adsorbed NxHy species.(b)Schematic illustration of band edge positions of Penta-B2C relative to the normal hydrogen electrode (NHE) and absolute vacuum scale (AVS).The dotted line represents the equilibrium potential of the reaction,and the solid line represents the reaction onset potential on Penta-B2C.
TheΔGdiagram of the enzymatic and alternating mechanisms from the side-on N2on Penta-B2C are shown in Figs.3c and d.In the enzymatic mechanism,the generation of∗NH2and∗NH3is an endothermic process.Other elementary reaction steps show a downward trend.The formation of∗NH2is the potential determination step (PDS) with 0.23 eV.The NRR onset potential in the enzymatic mechanism is only 0.23 V,which is much lower than the previously reported catalysts [3,42,43].It is well known that the last step∗NH3desorption process is independent of the onset potential because no hydrogenation is involved [1].The rapid removal of∗NH3facilitates the recycling of the catalyst.In the experiments,∗NH3is readily hydrogenated to NH4+in acidic media and released into the solution,thus effectively recycling the catalyst [44,45].Hence,the desorption of∗NH3cannot be the limiting factor in NRR.In the alternative mechanism,the formation of∗NH-∗NH2,∗NH2+∗NH3and∗NH3+∗NH3are endothermic processes,correspondingΔGof 0.33,0.56 and 0.69 eV.Other elementary reactions show a downward trend.The hydrogenation of∗NH-∗NH2into∗NH2+∗NH2is significant exothermic process due to the N–N bond broken.In the alternative mechanism,the last step of hydrogenation is PDS and the onset potential is 0.69 V.Our results indicate that the most favorable reaction mechanism of NRR on Penta-B2C is the enzymatic mechanism with a low onset potential of 0.23 V.
Penta-B2C has more abundant active sites than single B-doped carbon materials,such as B/g-C3N4[8],B/C2N [46],B/graphene[23],and B/g-CN [1].The wrinkled morphology of Penta-B2C increases the exposure area of active sites than other planar materials,which are more conducive to capturing and activating N2molecules.To further understand the catalytic performance of NRR on Penta-B2C,intermediates charge fluctuations for the enzymatic mechanism are shown in Fig.4.For the N2adsorption and first four exothermic hydrogenation steps,the∗NxHyadsorbed species are negatively charged.The Penta-B2C catalyst acts as an electron donor.For the final two endothermic hydrogenation steps,the∗NxHyadsorbed species are positively charged.The Penta-B2C catalyst acts as an electron acceptor.Therefore,the charge transfer is the driving force to propel the N2activation and reduction.
HER is the competing reaction for NRR.Since the adsorbed H may occupy the active site,causing alternative HER to NRR[47].We performed HER calculations to estimate the selectivity of Penta-B2C.ΔGof H atom is-0.17 eV,which is smaller than that of N2molecule.The N2molecule therefore is more conducive to occupy activation sites.ΔGof H atoms increase to about-0.3 eV after the N2molecules adsorbed,which effectively inhibit the HER process.Moreover,we found that the adsorption concentration of∗H affects the onset potential of the HER (Fig.S4 in Supporting information).When the adsorption concentration of∗H occupy 10% of the activation site,the onset potential of HER increases to 0.55 V.The HER is further inhibited with an increased adsorption concentration of∗H.Results indicate the high selectivity of Penta-B2C for NRR.
The photocatalyst generates electrons and holes by receiving solar energy and then undergoes a reduction reaction with the adsorbed N2molecules.The size and location of the catalyst bandgap are critical [48–50].Penta-B2C has a suitable bandgap of 2.328 eV.CBM of Penta-B2C is higher than the reduction potential for NRR(Fig.4b).Therefore,Penta-B2C with a suitable band edge can effectively photocatalyze the reduction of N2to NH3.
We herein investigate the photocatalytic NRR on Penta-B2C based on first-principles calculations.The stability of Penta-B2C is kinetically acceptable by molecular dynamics simulations.The Penta-B2C is a semiconductor with a bandgap of 2.328 eV at HES06 level.Rich B atoms in Penta-B2C can capture N2molecules.In particular,the activated side-on N2promotes further N2reduction.Therefore,the enzymatic and alternating mechanism for NRR is systematically investigated.Our results indicate that the enzymatic mechanism is the effective reduction pathway with an onset potential of 0.23 V.HER is inhibited when the hydrogen adsorption concentration is increased or N2molecules first occupy the adsorption sites.Therefore Penta-B2C is an effective catalyst for NRR.Moreover,Penta-B2C with a suitable bandgap and band edge can adsorb the ultraviolet and visible light and can photocatalysis the reduction of N2to NH3.Effective light absorption can generate photo-excited electrons to improve photocatalytic performances.Our work provides theoretical insights into the experimental development of NRR photocatalysts with high reactivity and selectivity.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the Natural Science Foundation of China (No.21603109),the Henan Joint Fund of the National Natural Science Foundation of China (No.U1404216),the Scientific Research Program Funded by Shaanxi Provincial Education Department (No.20JK0676).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.05.024.
杂志排行
Chinese Chemical Letters的其它文章
- Progress in mechanochromic luminescence of gold(I) complexes
- Spinel-type bimetal sulfides derived from Prussian blue analogues as efficient polysulfides mediators for lithium-sulfur batteries
- Alopecuroidines A-C,three matrine-derived alkaloids from the seeds of Sophora alopecuroides
- Boronic acid-containing diarylpyrimidine derivatives as novel HIV-1 NNRTIs:Design,synthesis and biological evaluation
- Diaminodiacid bridge improves enzymatic and in vivo inhibitory activity of peptide CPI-1 against botulinum toxin serotype A
- Peptide stapling with the retention of double native side-chains
