Single-atom site catalysts supported on two-dimensional materials for energy applications
2021-03-14QiXuJianZhangDingshengWangYadongLi
Qi Xu,Jian Zhang,Dingsheng Wang,Yadong Li
Department of Chemistry,Tsinghua University,Beijing 100084,China
Keywords:Single-atom site catalysts Two-dimensional materials Synthesis Characterization Energy applications
ABSTRACT Single-atom site catalysts (SACs) and two-dimensional materials (2DM) have gradually become two hot topics in catalysis over the past decades.Their combination with each other can further endow the derived SACs with extraordinary properties such as high loading,identical active sites,uniform coordination environment,distinctive metal-support interaction,and enhanced catalytic activities.In this review,we highlight the recent development in this specific research topic according to the types of substrates and focus on their applications in energy conversion field.Additionally,we also make a brief introduction to the synthesis and characterization methods for SACs supported on 2DM (SACs/2DM).Finally,the challenges and perspectives are summarized based on the current development status.It is believed that this work will make contributions to the rational design and fabrication of novel SACs/2DM,promoting their practical energy applications in the future.
1.Introduction
With the aggravating energy crisis and environmental issues,it is demanded to develop the sustainable and clean energy conversion technologies imminently [1,2].In the past decades,among various attempts,electrochemical energy conversion such as water splitting,CO2conversion,N2reduction,fuel cells and metal–air batteries,has been considered as one of effective and promising ways to replace traditional fossil energy in the future [3-13].To some extent,the energy conversion performance mainly depends on catalysts employed at the electrode which could simultaneously affect both reaction rate and efficiency.The effective catalysts chosen hitherto comprising noble transition-metal nanoparticles (such as Pt/C,RuO2,IrO2) exhibit high activities and superior stability [14-18].However,the widespread use of such catalysts is hampered by the scarcity and high cost of the noble metal [19,20].Hence,minimizing the usage of such metal without sacrificing the catalytic performance would become a feasible and effective approach to solve this problem.
Among developed strategies,downsizing nanoparticles into single atoms especially for noble metal catalysts is a crucial route to achieve the target [21-25].During general catalytic cycles,only atoms on the surface can get access to reagents.This suggests that the portion of inner part metal atoms is wasted and useless,which seriously reduces the mass activity and atom utilization of catalysts.In SACs,each atom can be thoroughly exposed and deemed as an active center,greatly improving the atom utilization up to nearly 100% [26-29].In addition,when metal atoms are monodispersed,the electronic and spatial structure can also be modified,which could bring about unexpectedly enhancement of catalytic performance [30-35].However,it is noteworthy that the surface free energy would increase with the decrease of size,resulting in the metal species aggregation [36-38].It means that the preparation of SACs is not easy,and specific support materials with high surface area providing adequate anchoring sites are required to stabilize these isolated single atoms [39-42].Traditional support materials applied to fabricate SACs usually possess threedimensional structures which are non-uniform in spatial distribution.This makes the homogeneity of active sites doubtful,hindering us from getting access to the essence of catalytic process.
2D nanomaterials (such as graphene [43,44],MXenes [45,46],graphitic carbon nitride (g-C3N4) [47],layered double hydroxides(LDHs) [48,49],transition metal dichalcogenides (TMDs) [50-52],2D metal-organic framework (MOF) [53,54]and 2D covalent organic framework (COF) [55]) gradually become a research hotspot in recent years which are potential excellent supports for anchoring single atoms.Plenty of advantages can be offered when selecting 2D materials as supports:(1) 2D materials usually possess large surface area and can provide sufficient anchoring sites for stabilizing isolated single atoms which benefit the preparation of high-loading SACs;(2) the strong metal-support interaction can modify the electronic and structural properties of active centers which is conducive to enhance the catalytic activity and stability;(3) the uniform coordination environment created by simple structures makes it an ideal platform to exploit the intrinsic mechanism of catalytic process by combining density functional theory (DFT)calculations and experimental techniques.Nonetheless,it is undeniable that current studies on SACs/2DM are still insufficient.More efforts should be devoted to explore novel efficient systems,new applications,and more accurate mechanism,thus providing new impetus to promote the development of SACs/2DM.
In this review,the advantages of SACs/2DM are firstly illustrated and their developments including synthesis and characterization methods are subsequently discussed.The catalytic performances of SACs/2DM concentrating on various important reactions in energy conversion field are demonstrated thoroughly according to recent reports classified by the types of substrates.Finally,the challenges and perspectives are summarized based on the current development status.This review aims to provide a deep and comprehensive understanding of the current development in this research field,ultimately promoting the generation of more novel high-efficiency SACs/2DM.
2.The emergence of SACS/2DM
The development of 2D materials is started with the discovery of graphene,an ultrathin carbon material which is derived from the delamination of graphite with outstanding anisotropic physical and electronic properties.Subsequently,other classes of 2D materials are explored including g-C3N4,LDHs,TMDs,metal carbides and nitrides (collectively known as MXenes),and others [56].These materials usually consist of single or several layers of atoms with strong covalent bonds connecting atoms and unique interaction existing between layers.The distinct structure endows 2D materials with different properties compared to parent bulk materials such as large specific surface area,exceptional conductivity and special mechanical properties [57].Based on these features,2D materials have attracted enormous attention and became one hot research topic in energy conversion field.
However,most pure 2D materials show low catalytic performance without additional treatments [58].Taking graphene for example,the hydrogen evolution reaction (HER) performance is very poor due to the weak hydrogen adsorption ability of graphene.Hence,diverse optimization protocols have been proposed to enhance the catalytic activities of 2D materials such as:(1) defect engineering.The defects existing in 2D materials are usually recognized as the real active sites during catalytic process.Therefore,it has been adopted as a powerful strategy to enhancing the activity of 2D materials.(2) regulating lateral size and thickness.When reducing the lateral size and thickness,more surface area can be exposed to make more active sites accessible and the electronic structures can also be tuned to acquire better performance.(3) strain engineering.Lattice strain can effectively tune the electronic states of 2D materials and such method has been verified to be an efficient way for regulating the catalytic performance of 2D materials.(4) interface engineering.It is demonstrated that interactions at the interface between two different materials will alter their electronic states and chemical properties,which will have an effect on the catalytic activity.This strategy commonly involves combining two different materials together by chemical bonds or physical contact to generate novel interface.(5) heteroatom doping.It has been demonstrated that heteroatom doping is an effective approach to improve catalytic activity of 2D materials.Especially metal-atom doping,which is mainly used to synthesize SACs,has been widely developed to promote the performance for various reaction process.Owing to the simplicity of preparation process and diversity of selected metal or supports,combining with SACs is verified to be an effective approach to meet the standard of practical applications [58].On one hand,the large exposed surface could furnish abundant anchoring sites to trap isolated metal atoms,achieving an extremely high metal loading which is difficult for 3D substrates.For instance,the loading of Pd on TiO2nanosheets could be up to 1.5 wt% [59],while Ag SAC supported on g-C3N4could achieve an incredible Ag loading of 10.21 wt%[60].Moreover,the ultrathin structure can make almost all the active center accessible to the reagents realizing truly high atom utilization which is usually hampered by stereo skeleton of 3D supports.On the other hand,SACs supported on 2D materials are ordinarily less coordinatively saturated and the electronic structures can be regulated by the interaction with 2D substrates,which means they can more efficiently interact with reactants to achieve an exceptional performance.In addition,compared to 3D supports,SACs/2DM are more suitable and simpler for investigating the catalytic mechanisms of some vital heterogeneous reactions by in situ characterization techniques and theoretical calculations,owing to the well-defined and uniform active sites.
3.Synthesis and characterization methods
Precisely fabricating and controllably synthesizing uniform SACs anchored on 2D materials is an infinitely challenging and attractive subject,owing to the tendency of agglomeration of metal species caused by the high surface energy.Hence,multiple factors are supposed to be taken into consideration for making the metal atoms stabilized and isolated,avoiding the formation of clusters and nanoparticles.These factors include the loading of metal atoms,the variety of support materials,the metal-support interactions,and the density of anchoring sites on support.Based on considerations above,various synthetic approaches have been explored and developed in last decades.Furthermore,in order to better understand the structure-performance relationship and provide more accurate guidance for developing novel efficient SACs,it is significant to confirm the successful synthesis of SACs and reveal active sites with the help of various characterization methods.Scanning transmission electron microscopy (STEM) and X-ray absorption spectroscopy (XAS) techniques are two powerful tools which are ordinarily combined to investigate the dispersion form of metal species on the support and supplement each other to offer comprehensive information about electronic structures,oxidation states,and coordination configurations of single active metal sites.Herein,we will make a brief summary and introduction of these commonly used methods for preparing and characterizing SACs on 2D materials.
3.1.Synthesis methods
3.1.1.Wet-chemical method
The wet-chemical method is one of the most widely used methods for fabricating SACs currently due to its easy operation and low requirements for equipment which makes it most promising to be applied to large scale production.It usually contains several experimental steps composed of:(1) loading to metal precursor onto the support materials by impregnation,precipitation,or ion exchange;(2) drying and calcination;and (3) reduction or activation.Considering that the metal species are generally monodispersed in precursor and often protected by functional ligands,it is easy to obtain isolated metal species dispersed on support materials during the synthesis process.However,because of the necessity for exposing the active sites to reagents,the ligands must be removed by post-treatment which will bring about the possibility of metal agglomeration.As a result,enhancing the strong metal-support interaction and reducing the loading to an extremely low value (less than 1%) are two effective solutions to the problem for preventing the metal species aggregating into clusters or large particles.From this perspective,2D materials are one of the excellent candidate for support owing to their numerous anchoring sites and strong interaction with surface target metal atoms.
3.1.2.Photochemical method
Ultraviolet light is also an efficient auxiliary tool for synthesizing SACs.A facile method has been explored with the help of ultraviolet light.Zheng’s group [59]firstly reported this method for synthesizing Pd SACs over 2D TiO2nanosheets.It is found that the ethylene glycolate radicals generated by the ultraviolet light on the surface of TiO2nanosheets play an important role in promoting the formation of crucial intermediate,PdCl1/TiO2,which can be consequently converted into Pd1/TiO2SAC.The loading of Pd on derived Pd SAC can be up to 1.5% and the Pd1/TiO2SAC displays superior catalytic activity in hydrogenation of C=C and C=O groups,far exceeding the performance of commercial Pd catalysts.Another example is demonstrated by Wu’s group [61]in which an atomically dispersed frozen solution is generated by irritating the solution with ultraviolet light at low temperature.The aggregation of platinum atoms is amazingly prevented by this way and the isolated platinum atoms can be deposited on diverse supports materials such as graphene which exhibits high catalytic activity.Although this synthesis method is relatively simple and can be operated under mild condition,the universality and resistance to extreme conditions are further to be confirmed.
3.1.3.Pyrolysis and thermal reduction
Pyrolysis and thermal reduction are the most widely adopted strategies to fabricate SACs anchored on 2D graphene and graphene-like materials.The simplicity of the methods means no complicated instruments are required and scalable application can be easily achieved.Importantly,various precursors such as rGO,small organic molecules and polymers could be chosen to stabilize the single atoms in high temperature condition,finally acquiring the SACs.During the thermal process,doped heteroatoms such as N atoms in the abundant functional groups could grab the isolated atoms and coordinated with them generating a strong chemical interaction.The loading amount of single atoms could also be regulated by changing the ratio of precursors and inorganic metal salts,in which case a high loading can be achieved.For example,Li’s group [62]fabricated a Pt SAC supported on N-doped graphene through a one-pot pyrolysis strategy.The loading amount of Pt atoms could reach as high as 5.3 wt% while maintaining atomically dispersed form.Moreover,due to harsh synthesis condition under high temperature,the obtained SACs ordinarily tend to have superior thermal stability and conductivity which means the SACs can play a big role in electrochemical energy conversion field.
3.1.4.Electrodeposition method
For the synthesis of superior single-atom electrocatalysts,electrodeposition method has become an emerging and effective route.The advantages of this process can be summaried:(1) the derived SACs are exactly supported on the electrode eliminating complicated post-treatment;(2) the concentration of deposited atoms on the support can be tuned by controlling the anode potential and deposited time;(3) comparing to others,it is eco-friendly and costefficient for just consuming a certain amount of electricity.Besides,the approach can be applied to fabricate SACs on diverse 2D support materials such as graphdiyne,MXene,and MoS2.Taking the work reported by Sun’s group [63]for example,a threeelectrode electrochemical cell is utilized to operate the single atom Pt deposition experiment.The cell system equips a prefabricated MoS2/graphite paper as the counter electrode and a pure Pt foil as the working electrode.After modulating the voltage of the working electrode,the Pt atoms gradually flee from the foil and migrate to the counter electrode driven by the electrical field,finally anchoring on the MoS2and precisely positioning at generated Mo-and Svacancies.The obtained Pt/MoS2catalyst exhibits higher HER activity than the pristine MoS2flake.Moreover,this synthetic method can also be applied to other noble metals such as Au and Pd which display high HER activity as well.Nevertheless,the applications of SACs synthesized in this way are mostly limited to electrocatalysis which deserve further exploration in applications.
3.2.Characterization method
Electron microscopy is one of the most widely used and powerful techniques to directly detect the existence of single atoms or nanoparticles.Normal transmission electron microscopy (TEM)whose detection limit is about 1 nm is usually applied to observe the presence of nanoparticles.It is often combined with X-ray diffraction (XRD) to exclude the existence of nanoparticle preliminarily.However,due to high detection limit of TEM and high requirement for metal loading of XRD,it is doubtful to conclude that the metal species are completely dispersed into single-atom form without clusters or small nanoparticles.High-angle annular darkfield scanning transmission electron microscopy (HAADF-STEM) is further used to confirm the sole presence of individual atoms.Because the central atoms are usually heavier than the atoms in the support,it can be easily observed if there are metal atoms agglomeration occurring.Furthermore,the equipped energy-dispersive Xray spectroscopy (EDS) is able to furnish information about the composition of the SACs to prove the presence of the desired metal.Especially,the aberration-corrected HAADF-STEM make it possible to intuitively observe the bright dots which are corresponding to isolated metal atoms due to the difference in Z contrast,which is conducive to explore the coordination and distribution of metal atoms.Nevertheless,the issue we have to admit is that we could not examine all the area in the sample and assure no nanoparticles are formed out of the inspected field.
In consequence,in order to obtain comprehensive information about the whole sample,XAS is usually performed by using synchrotron facilities.XAS can be subdivided into two parts including X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) according to energy range which could provide different structural information.Analyzing the XANES data can identify the chemical state and geometric coordination structure of metal species on the basis of white-line intensity and the finger-print peaks features.Interatomic distance,the species and number of local coordinated atoms and structural disorder can be further detected by the Fourier-transformed (FT)k2-weighted EXAFS spectra.For SACs,if no signal on the metalmetal shell can be observed in the FT-EXAFS spectra,it could be concluded that metal atoms exist in atomic dispersion form.The quantitative coordinating results is reflected by the result fitting and further detected by the simulation of XANES curve to accurately understand the geometric configurations of the active sites.
4.SACs supported on diverse 2D materials
In this part,the discussion will be focused on the development of SACs/2DM according to the type of substrates.SACs supported on graphene,MXenes,C3N4,LDHs,TMDs,2D MOF,and 2D COF including their applications focusing on energy conversion field will be introduced respectively.
4.1.SACs/graphene
Carbon materials have been widely used as substrates to anchor single atoms owing to large surface,stable chemical properties,and high conductivity [64,65].Especially,graphene which possesses a monolayer planar structure has attracted much attention for its outstanding performance.The functional groups and defects embedded in the graphene sheets can modulate the surface and act as anchoring sites to stabilize the single atoms.In addition,the rapid mass and electron transport kinetics brought by the unique structure and high conductivity make it possible to achieve a superior electrocatalytic activity in energy field.Touret al.[66]fabricated a Ru SAC supported on N-doped graphene matrix (denoted as Ru-N/G-750) through the annealing of graphene oxide precursor with trace Ru element in the presence of NH3(Fig.1a).The atomic dispersion form of Ru atoms was identified by STEM techniques (Figs.1b-d).In the NH3atmosphere,the nitrogen doping and reduction of graphene oxide were accomplished simultaneously.The obtained Ru-N/G-750 showed high activity and excellent stability toward oxygen reduction reaction (ORR)viaa four-electron pathway with a relatively low potential in acidic media.The mass activity was calculated to be 7.5 times higher than commercial Pt/C (Fig.1e).After 10,000 cycles durability test,only negligible decay could be observed and 90% activity were reserved with well-preserved Ru single atomic species,showing its remarkable stability (Fig.1f).Moreover,the Ru-N/G-750 showed extraordinary tolerance to carbon oxide and methanol which could not be witnessed in commercial Pt/C catalyst (Fig.1g).The X-ray adsorption fine structure analysis (XAFS) revealed that Ru element existed in form of Ru-N4moieties.However,the DFT calculations demonstrated that ORR activity preferably originated from the Ruoxo-N4moieties rather than Ru-N4under the oxidative electrocatalytic condition.It is worth noting that the unique ordered structure of graphene also makes it an ideal platform to predict the performance of SACs combining theoretical calculations.Huang’s group [67]synthesized a series of 3d metal SACs (Fe,Co and Ni)supported on nitrogen-doped holey graphene frameworks (which were denoted as M–NHGFs,M=Fe,Co or Ni) through a rational and general approach.It was revealed that all the M–NHGFs shared the same coordination configuration in the form of MN4C4by thorough analyses of XAFS results and images of annular darkfield scanning transmission electron microscope.Based on this ideal model system,DFT calculations were utilized to predict the catalytic activity of M–NHGFs towards oxygen evolution reaction(OER),deducing the activity trend was Ni–NHGF>Co–NHGF>Fe–NHGF.The speculation was further confirmed by electrochemical measurement,showing that Ni–NHGF displayed the most outstanding catalytic activity and stability.The overpotential of Ni–NHGF at a current density of 10 mA/cm2(η10) was as low as 331 mV,which was superior to Co–NHGF,Fe–NHGF,and most of the documented nanoparticulate Ni catalysts.

Fig.1.(a) Schematic illustration of the synthesis route for the Ru-N/G-750 annealed at 750 °C in NH3 atmosphere.(b) TEM image,(c) high-resolution TEM image,and (d)aberration-corrected HAADF-STEM image of Ru-N/G-750.(e) Mass activity and current density of the Ru-N/G-750 and commerical Pt/C catalysts at 0.70 V (vs. RHE) for the ORR.(f) Stability test of the Ru-N/G-750 for 10,000 cycles in O2-saturated 0.1 mol/L HClO4.(g) Chronoamperometric curves of a methanol crossover test with Ru-N/G-750 and Pt/C at 0.70 V (vs. RHE).1 mol/L methanol was added at t=400 s.Reprinted with permission [66].Copyright 2017,American Chemical Society.
Electrochemical reduction of CO2is also another key application for SACs/graphene.Wanget al.[68]devised a novel approach to synthesize a Fe SAC anchored on N-doped graphene through a thermal pyrolysis method.Confirmed by spherical-aberrationcorrected scanning transmission electron microscopy–annular dark field and XANES analysis,the structure of active sites was identified to be a unique Fe-N5coordination with an extra axial ligand coordinated to FeN4.At a low overpotential of 0.35 V,the Fe-N5catalyst exhibited a high CO Faradaic efficiency (FE) (ca.97%)which surpassed the contrast Fe-N4and outperformed all reported Fe-N-C based catalysts (Fig.2a).The Fe-N5catalyst showed superior current density and CO production rate as well (Figs.2b and c).DFT calculations indicated that the axial pyrrolic nitrogen ligand of the FeN5site could facilitate the desorption of CO molecules,resulting in the high selectivity of CO.Xie and her co-workers[69]developed a facile ion adsorption strategy to construct a Ni SAC (denoted as Ni2+@NG) based on graphene (Fig.2d).The FE was achieved more than 80% in the potential range of-0.68 V to-0.88 V and reached the maximum value of 92% at-0.68 V,which was comparable to the best reported carbon materials in aqueous media (Fig.2e).It is noteworthy that this strategy could be easy to scale up and achieve practical applications for the absence of conventional harsh pyrolysis and acid leaching procedures in this field.

Fig.2.(a-c) Comparison of the electro-catalytic activity of as-synthesized catalysts:(a) FE,(b) partial current density,and (c) production rate of CO versus potential.Reprinted with permission [68].Copyright 2019,Wilety-VCH Verlag GmbH &Co.KGaA,Weinheim.(d) Schematic illustration of surface immobilization of transition metal ions on Ndoped graphene through ion adsorption strategy.The inset shows the structure comparison of Ni2+@NG and Ni(II)-cyclam complex.(e) FE for CO production of Ni2+@NG at different potentials.Reprinted with permission [69].Copyright 2018,Wiley-VCH Verlag GmbH &Co.KGaA,Weinheim.
4.2.SACs/MXenes
MXenes,2D transition metal carbides/nitrides,have attracted much attention for applications in energy field owing to their excellent metallic conductivity,chemical stability,hydrophilic properties and unique layered structure [70].These special properties make MXenes promising candidates for facilitating lots of electrochemical applications.Utilizing MXenes as substrates to anchor single atoms has also been explored widely and applied to various energy conversion reactions.Wanget al.[71]reported the fabrication of a Pt SAC supported on double transition metal MXene nanosheets—Mo2TiC2Txwith superior HER activity.In the preparation process,by electrochemical exfoliation method,ample surface Mo vacancies were generated to immobilize the single Pt atoms.Consequently,the single Pt atoms were anchored by the adjacent C atoms in the MXene,forming covalent Pt–C bonds to obtain the final catalyst (Mo2TiC2Tx-PtSA).The resultant Mo2TiC2Tx-PtSAshowed exceptional catalytic performance in HER measurement and required low potentials of 30 and 77 mV to achieve 10 and 100 mA/cm2respectively,which is comparable to Pt benchmark catalyst (Fig.3a).The stability was tested for 10,000 cycles(or 100 h) and almost no decay was observed (Fig.3b),indicating the excellent stability of Mo2TiC2Tx-PtSA.In particular,the mass activity of Pt SAC was about 40 times greater than the commercial Pt/C catalyst (Fig.3c).DFT calculations revealed that the excellent activity and stability were attributed to the strong covalent interactions between positively charged Pt single atoms and the support.
In another study,Yang’s group [72]introduced single atom zinc implanted MXene (denoted as SA-Zn MXene) to a sulfur cathode.It was delighted to find that not only the conversion reactions of polysulfides from Li2S4to Li2S2/Li2S could be catalyzed due to the decrease of the energy barriers,but also a strong interaction with polysulfides could be achieved owing to the high electronegativity of single Zn atoms on MXene (Figs.3d and e).Moreover,benefiting from the homogenously dispersed Zn atoms,the nucleation of Li2S2/ Li2S on the largely exposed 2D surface could accelerated during the redox reactions.Based on these unique features,the sulfur cathode exhibited a high reversible capacity of 1136 mAh/g.Besides,after further electrode optimization,the cathode was endowed with a high areal capacity (5.3 mAh/cm2),high rate capability (640 mAh/g at 6 C),and good cycle stability (80% capacity preservation after 200 cycles at 4 C).This example demonstrated that single atom metal immobilized MXenes could effectively accelerate the redox reaction of polysulfides and promote the performances of lithium–sulfur batteries.
4.3.SACs/C3N4
g-C3N4is known to be the most stable allotrope of carbon nitride and could be deemed as one special case of graphene due to its unique structure composed of s-triazine or tri-s-triazine structural motives with sp2C and sp2N atoms.It has been emerging as an excellent substrate to confine single atoms for its uniform N-coordinating cavities to trap isolated atoms and special optical properties,which has been deeply investigated over the past years.For example,Wu and co-workers [73]introduced Pt atoms into g-C3N4with high dispersion and stability through a simple impregnation method.The single Pt atoms are atomically immobilized by rich Pt-N/C bonds confirmed by FT-EXAFS curves.The obtained Pt SAC (at 0.16 wt% Pt loading) exhibited tremendously enhanced photocatalytic activity towards H2evolution reaction,showing a remarkable H2generation rate of 318 μmol/h which was 8.6 times than Pt nanoparticles and nearly 50 times than bare g-C3N4.The turnover frequency (TOF) of Pt SAC could reach as high as 775 h-1which was nine times of that for Pt nanoparticles,indicating that SAC could extremely accelerate the reaction.The ultrafast transient absorption spectroscopy revealed that the performance improvement should be ascribed to the intrinsic change of the surface trap states of g-C3N4induced by the introduced Pt atoms.Other than photocatalysis,in another report,Liuet al.[74]constructed a new type of Au1Nxsingle-site confined in g-C3N4at atomic level which exhibits good activity in both OER and ORR process.Benefiting from the special reduction procedure,the atomic-scale Au1Nxsite could firmly embedded into the periodic cavity structure of g-C3N4through strong Au–N bonds.The resultant precise valence of Au1+endowed the catalyst with latent redox activity for effective OER performance.Notably,probably owing to poor conductivity of g-C3N4,the performance of SACs supported on g-C3N4was usually inferior to that on graphene.
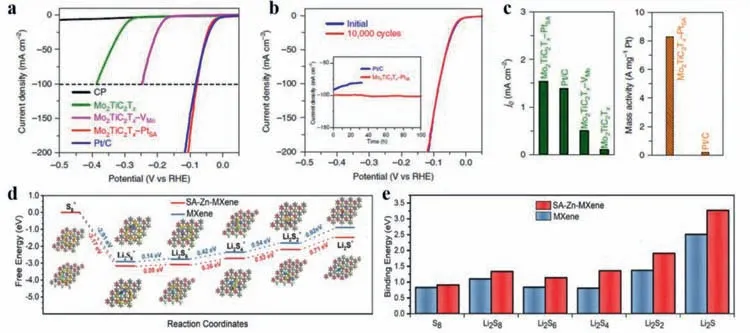
Fig.3.(a) HER polarization curves of carbon paper (CP),Mo2TiC2Tx,Mo2TiC2Tx–VMo,Mo2TiC2Tx–PtSA and Pt/C (40%),acquired utilizing graphite rod as the counter electrode in 0.5 mol/L H2SO4 solution.(b) Stability test of Mo2TiC2Tx–PtSA through potential cycling,before and after 10,000 cycles.Inset is chronoamperometry curve of Mo2TiC2Tx–PtSA and Pt/C.(c) Exchange current densities of the catalysts,and the mass activity of exceptional Pt/C and Mo2TiC2Tx–PtSA.Reprinted with permission [71].Copyright 2018,Nature Publishing Group.(d) The Gibbs free energy profiles of lithium polysulfides on SA-Zn-MXene and MXene,showing a much lower reaction free energy from Li2S2 to Li2S on SA-Zn-MXene than that on MXene.(e) Binding energies between lithium polysulfides and SA-Zn-MXene layers as well as between lithium polysulfides and MXene layers,showing that SA-Zn-MXene has a higher binding energy with all lithium polysulfides than that with MXene.Reprinted with permission [72].Copyright 2020,Wiley-VCH Verlag GmbH &Co.KGaA,Weinheim.
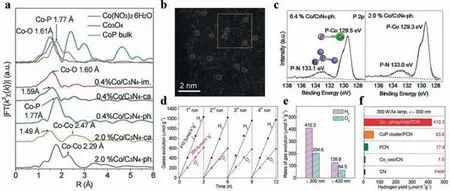
Fig.4.(a) The corresponding k3-weighted FT spectra of Co/C3N4 with different Co loadings (0.4% and 2.0%) at three prepared stages:impregnation (noted as “im.”),calcination (“ca.”),and phosphidation (“ph”).The data of Co(NO3)2•6H2O,Co3O4,and CoP bulk are displayed for reference.(b) Representative HAADF-STEM image of Co1-phosphide/PCN.(c) P 2p XPS spectra of 0.4%Co/C3N4-phosphidation and 2.0%Co/C3N4-phosphidation samples.(d) Typical time course of H2 and O2 productions from water splitting under light irradiation (λ > 300 nm) by Co1-phosphide/PCN photocatalyst (20 mg).The reaction system was evacuated after each run.(e) Photocatalytic watersplitting activities under simulated sunlight (λ > 300 nm) and visible light (λ > 420 nm) irradiation.(f) H2 yield rates of Co1-phosphide/PCN,CoP cluster/PCN,PCN,Co1-oxo/C3N4 and g-C3N4 (where CN=C3N4).Reprinted with permission [75].Copyright 2020,Wiley-VCH Verlag GmbH &Co.KGaA,Weinheim.
Apart from utilizing the intrinsic structure of g-C3N4to stabilize single atoms,doped heteroatoms could also be used to modulate the coordination environment of isolated active metal centers.For instance,Wei’s group [75]designed a novel Co SAC with single Co1-P4site embedded in g-C3N4nanosheets for overall water splitting through a facile phosphidation approach.The synthetic route included two steps:(1) loading Co single atoms onto g-C3N4after impregnation and calcination and (2) subsequently phosphated under PH3atmosphere to transfer the active sites into Co1-P4centers which was identified by XAS,electron microscopy,and XPS spectra (Figs.4a-c).The as-prepared Co SAC showed outstanding and steady water-splitting activity with high H2evolution rate whose maximum could reach 410.3 mmol h-1g-1under simulated sunlight irradiation,which is superior to most reported carbon nitrides-based and oxynitride-based materials (Figs.4d-f).It was demonstrated that the distinct coordinatively unsaturated Co centers could effectively prevent charge recombination and prolong the lifetime of carriers by almost 20 times when compared to pure g-C3N4,further boosting water molecular adsorption and activation for oxygen evolution.This work suggested that phosphidation treatment of single active site was a potential strategy to achieve significant enhancement in overall water-splitting reaction.
4.4.SACs/LDHs
Layered double hydroxides,another typical 2D material,are also a promising candidate for confining metal atoms due to their rigid laminates structures and abundant OH-groups.Attributed to their special structures,they usually show low overpotential for OER and have attracted much attention over the past years.Especially,it is investigated that the OER process can be boosted and a more exceptional performance will be achieved when a third metal element is introduced.For example,Sunet al.[76]fabricated a Ru SAC anchoring on the surface of cobalt-iron LDHs (denoted as Ru/CoFe-LDHs) by a hydrolysis deposition method (Fig.5a).Proved by highresolution STEM (HR-STEM) and XAS (Figs.5b-d),Ru atoms was singly dispersed on the surface stabilized by the formation of Ru–O–M (M stands for Co or Fe) bonds,suggesting the strong electronic interaction between Ru atoms and the support.Facilitated by the unique interaction,at the loading of 0.45 wt% of Ru element,the obtained Ru/CoFe-LDHs required only 198 mV to reach the current density of 10 mA/cm2with a Tafel slope of 39 mV/dec(Figs.5e and f).The stability test demonstrated that the current density could be maintained with negligible decay (less than 1%)up to 24 h (Fig.5g),further affirming the excellent performance of Ru/CoFe-LDHs which is greatly surpass the pristine CoFe-LDHs and commercial RuO2catalyst.Confirmed by XAS and DFT+U techniques,the existence of Ru atoms directly acted as active sites which played a significant part in the reaction.Moreover,the CoFe-LDHs also participated into the OER reaction as co-catalyst,accelerating the process by decreasing the kinetic barrier to form∗OOH group from∗O group.
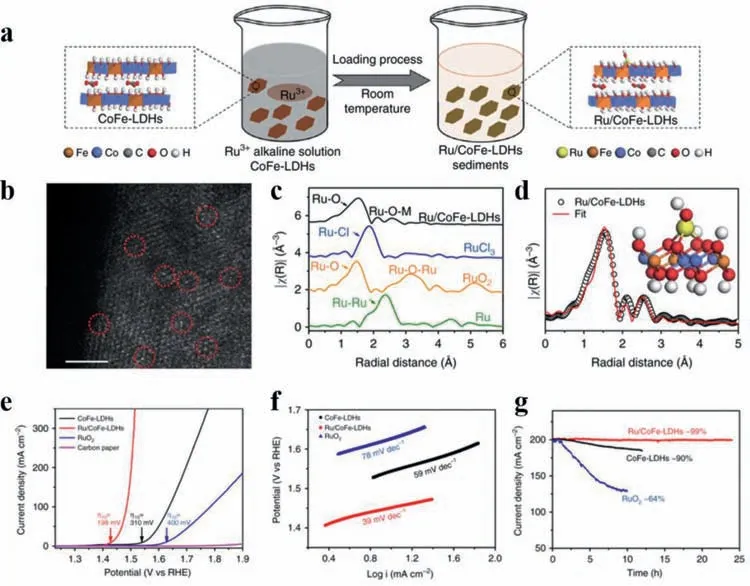
Fig.5.(a) Schematic illustration of the hydrolysis-deposition to form Ru/CoFe-LDHs.(b) The spherical aberration corrected STEM image of Ru/CoFe-LDHs nanosheets shows that Ru is atomically dispersed on the surface of LDHs.Scale bar:2 nm.(c) FT Ru K-edge EXAFS spectra of Ru/CoFe-LDHs,RuO2,RuCl3,and Ru metal.(d) Corresponding fittings results of Ru EXAFS for Ru/CoFe-LDHs and simulated EXAFS spectra from Ru–O and Ru–O–M (M=Co or Fe) bonds (the inset represents the enlarging local structure of Ru/CoFe-LDHs),indicating the exclusive existence of Ru–O–M bonds in Ru/CoFe-LDHs.(e) Comparison of iR compensated polarization curves of Ru/CoFe-LDHs with CoFe-LDHs,carbon paper,and the commercial RuO2 catalyst.(f) The corresponding Tafel plots of Ru/CoFe-LDHs,CoFe-LDHs,RuO2.(g) The potentiostatic curves of above mentioned catalysts under a certain overpotential with an initial current density of 200 mA/cm2.Reprinted with permission [76].Copyright 2019,Nature Publishing Group.
Apart from OER,applications to CO2hydrogenation system are also exploited.Yamashita and co-workers [77]proved that Ru SAC supported on LDHs could be an effective heterogeneous catalyst to promote the hydrogenation reaction of CO2to formic acid while no reaction occurred on the pure LDHs.The authors synthesized various LDHs to stabilize Ru species and found that Ru/LDH(Mg2+/Al3+=5) displayed the maximum turnover number (TON)of 461.It was revealed that there was a strong correlation between the catalytic performance and the CO2adsorption capacity around the Ru centers based on the experimental and theoretical considerations.The substantial strong Brønsted OH-ligands with a special location could make the Ru active sites electron-rich,which was crucial and conducive to the CO2adsorption.In addition,the CO2adsorption capacity could be tuned by the composition of LDHs as well.These pivotal factors enabled the fabricated Ru SAC to achieve outstanding performance under mild reaction conditions,even under low-pressure conditions.
4.5.SACs/TMDs
In past decades,transition metal dichalcogenides especially MoS2have attracted much attention of researchers for their unique structures,electronic properties,and improved activities toward HER,which are deemed as a promising substitute for Pt [78].Plenty of trials have been made to further improve the activity and confining SACs is tested to be an effective approach to achieve the target.In the realistic case,single atoms ordinarily disable to act as active sites but they can assist as auxiliaries in enhancing the HER activity.It is demonstrated that true active sites lie in the S atoms on the edges with unsaturated coordination number.For example,Bao and his co-workers [79]reported a novel strategy to trigger the HER activity by single-atom metal doping method for the first time.After replacing Mo atoms in plane with Pt atoms,the activity and stability were distinctly enhanced compared with pristine MoS2.Based on the result of DFT calculations,the adsorption behavior of H atoms on the S atoms in plane were tuned by doped neighboring Pt atoms,bringing about the performance elevation.Apart from noble metal atoms,this strategy can also be utilized to non-precious metals.Guet al.[80]discovered that when introducing single Ni atoms to the pure MoS2,the HER activity can be increased in both alkaline and acidic conditions.Revealed by experimental and theoretic results,the doped single Ni atoms tended to modify the adsorption behavior of H atoms on their adjacent S atoms,resulting in the promotion in HER activity.
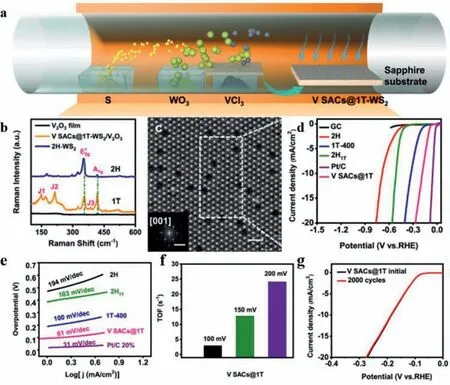
Fig.6.(a) Schematic illustration of the synthetic route for V SACs@1T-WS2.(b) Raman spectra of V2O3 film (black),V SACs@1T-WS2/V2O3 (orange),and 2H-WS2 (blue).(c)HR HAADF-STEM image of the V SACs@1T-WS2.(Inset is the corresponding fast Fourier transform).(d) LSV curves of GC,2H (2H-WS2),1T-400 (V SACs@1T-WS2 annealed at 400 °C in H2/Ar for 2 h),2H1T (V SACs@2H-WS2,transformed by V SACs@1T-WS2),Pt/C (Pt/C 20%) and V SACs@1T-WS2 electrodes in 0.5 mol/L H2SO4 with a scanning rate of 5 mV/s.(e) Tafel plots of 2H,1T-400,2H1T,Pt/C 20% and V SACs@1T electrodes.(f) TOF values of V SACs@1T-WS2 acquired at diverse overpotentials.(g) Electrochemical stability test of V SACs@1T-WS2 for 2000 cycles.Reprinted with permission [81].Copyright 2021,Nature Publishing Group.
Besides popular MoS2,some other TMDs are also exploited to confine single atoms for the purpose of improving HER activity.For instance,Li’s group [81]fabricated a V SAC supported on 1T-WS2monolayers (V SACs@1T-WS2) through a direct one-step chemical vapor deposition method (Fig.6a).Confirmed by STEM images and Raman spectroscopy,V species were atomically dispersed on 1TWS2monolayers while the energetically favorable 2H-WS2monolayers were derived under the same condition without the VCl3precursor (Figs.6b and c).By further experimental and theoretical investigations,it was discovered that the 1T-VS2intermediates played a vital role and acted as a template in the formation of V SACs@1T-WS2.Compared to the 2H-WS2counterparts,the V SACs@1T-WS2exhibited an outstanding HER activity,remarkably low Tafel slope of 61 mV/dec,high TOF of 3.01 s-1at 100 mV,and excellent durability (Figs.6d-g).DFT calculations highlighted that the superior HER performance should be attributed to the activated V-atom sites.
4.6.SACs/2D MOF
MOF is another commonly used template to fabricate SACs for its unique properties.The skeleton inherited from organic linkers ordinarily possesses large surface,high conductivity,and porous structure [82]which is conducive to immobilize single atoms and improve the electrochemical performance of derived SACs.The contained heteroatoms make it possible to regulate the coordination environment of active metal centers.These features demonstrate that MOF is an ideal support to anchor single atoms.Recently,SACs supported on 2D MOF have triggered a hot topic and have been widely applied to energy conversion field.For example,Zhou’s group [83]developed a novel surfactant-stabilized coordination strategy (Fig.7a) by directly utilizing PtIItetrakis(4-carboxyphenyl)porphyrin as the linker and Cu2-(COO)4paddlewheel clusters as the metal nodes to synthesize Pt SAC confined in ultrathin 2D MOF nanosheets (denoted as PtSA-MNSs).The loading of the derived PtSA-MNSs could be as high as 12 wt% through this approach.Benefiting from the ultrathin 2D structure which could provide plenty of accessible active sites and accelerate the transportation of photon-generated carriers,the obtained PtSA-MNSs displayed excellent photocatalytic activity in H2evolution reaction(11,320 mmol g-1h-1)viawater splitting under visible-light irradiation (λ >420 nm),which was superior to other contrast photocatalysts (Fig.7b).93% of the original catalytic activity could be preserved after four catalysis cycles,showing its remarkable durability (Fig.7c).It was noteworthy that the MOF nanosheets could be drop-casted onto solid substrates to form thin films while the photocatalytic activity could be retained,which meant that this Pt SAC was suitable to be applied to practical production (Figs.7d and e).DFT calculations indicated that the outstanding performance should be attributed to the positive influence of single Pt active site on H binding optimization and electron transfer (Fig.7f).
Similarly,Leeet al.[84]synthesized a Co SAC supported on 2D conjugated organic framework of C2Nviaa pyrolysis-free wetchemistry strategy.The loading of Co element could be tuned up to 20.5 wt%,which is close to the theoretic limit value of C2N.The prepared Co SAC showed exceptional bifunctional electrocatalytic activities toward OER and ORR.Moreover,the assembled Zn–air flow battery exhibited outstanding rechargeability with a cycling lifetime of 6000 cycles,demonstrating that it was one of the bestperforming Zn–air batteries.Theex situcharacterizations revealed that part of the Co species were leached out and transformed into OER-active CoOOH phase on the surface of C2N which boosted the electrocatalytic activities.In addition,the strategy could be generalized to fabricate other metal SACs (such as Fe and Ni) and bimetallic SACs (such as CoFe and CoMn).
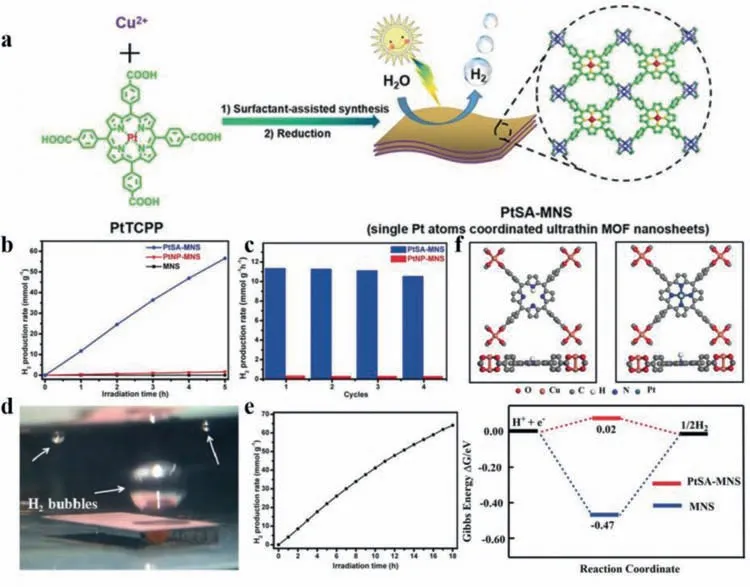
Fig.7.(a) Illustration of the synthetic process of Pt single-atom coordinated ultrathin MOF nanosheets (PtSA-MNSs) by a surfactant-stabilized coordination strategy.(b)Photocatalytic H2 production rates of PtSA-MNSs,PtNP-MNSs and MNSs in similar photocatalytic conditions.(c) The cycling stability of PtSA-MNSs and PtNP-MNSs.(d)Photograph of the film producing H2.(e) The corresponding photocatalytic H2 generation rate of the film on glass.(f) The top and side view for the structure models of H∗adsorbed on MNSs and PtSA-MNSs with ΔGH∗on MNSs and PtSA-MNSs,respectively.Reprinted with permission [83].Copyright 2020,Wiley-VCH Verlag GmbH &Co.KGaA,Weinheim.
4.7.SACs/2D COF
Covalent organic framework,which is metal-free conjugated microporous polymers,has been emerging as a powerful substrate to fabricate SACs due to its unique advantages such as large surface area,flexible structure regulation by changing the choice of monomers,and abundant doped heteroatoms which can stabilize single atoms by coordination.Benefiting from the well-defined structure and synthetic diversity of COF,the coordination environment of active sites could be easily tuned to finally achieve an excellent catalytic performance.Hence,it has attracted enormous attention in the past decades showing great potential.For instance,Zou’s group [85]reported a Ni SAC supported on 2,2′-bipyridinebased COF (Ni-TpBpy) which acted as a synergistic photocatalyst for reduction of CO2to CO under visible-light irradiation condition.During the reduction process,the electrons were transferred from photosensitizer to isolated Ni sites for CO production,bringing about an excellent activity and giving a 4057 μmol/g of CO in a 5 h reaction with a 96% selectivity over H2evolution.In addition,when reducing the CO2partial pressure to 0.1 atm,76% CO selectivity could still be preserved demonstrating its exceptional stability.DFT calculations and experimental results revealed that the outstanding performance should be attributed to synergistic effects of single Ni sites and TpBpy,in which TpBpy not only acted as a host for CO2molecules and isolated Ni sites,but also promoted the activation of CO2and inhibited the competitive evolution of H2.In another work,Nakanishiet al.[86]synthesized a Ru SAC anchored on covalent triazine framework (Ru-CTF) which showed unique selective alcohol oxidation activity without detectable OER process.It was demonstrated that the isolated Ru atoms in the derived Ru-CTF remarkably hampered the OER activity and promoted the benzyl alcohol oxidation process.Additionally,owing to the rigid cross-linked network of covalent bonds in CTF,the Ru-CTF displayed higher stability when compared to other Ru-based organometallic catalysts.This novel selectivity for alcohol oxidation over the OER could also be applied to other hydrocarbons and it was anticipated by the authors that this mechanism could be applicable to other metal centers like cobalt as well.
5.Conclusion and perspective
In the past few years,owing to the distinct structure and exceptional performance,SACs supported on 2D materials have attracted much attention and been extensively applied to various energy conversion reaction.Due to the unique plane spatial structure,almost all active sites can be exposed to the ingredients and participate the actual catalytic process,truly improving the atom utilization compared to 3D supports.Additionally,the interaction between single atoms and the 2D support can bring about unpredicted properties,which means not only the geometric and electronic structure of single active sites can be tuned by the support subsequently enhancing the activity,but the single atoms can also act as auxiliary to stimulate the intrinsic activity of the support to acquire a better performance.Besides,the homogeneity of active sites in SACs/2DM,a bridge between homogeneous and heterogeneous catalytic systems,is beneficial to construct an ideal platform to deeply investigate the essence of heterogeneous catalysis which can profoundly direct the design of next-generation efficient catalysts.
In this review,we have summarized the recent process of SACs supported on diverse 2D materials (including graphene,MXenes,C3N4,LDHs,TMDs,2D MOF and 2D COF) and their applications for some crucial reactions in energy conversion field.However,although great process has been made over the past decades,there are still some challenges which ought to be resolved urgently.First,it is acknowledged that 2D materials ordinarily possess a large specific surface area,but how to elevate the loading amount of single atoms to take full advantages of the surface area is still a gigantic challenge.Some traditional synthetic methods like pyrolysis need to go through a harsh process which may lead to agglomeration issue.Developing more mild and universal synthetic strategies for controllable synthesis of SACs/2DM is highly desired.For example,Li’s group [60]reports a novel Ag SAC supported on mpg-C3N4through a mild wet-chemical method whose loading is as high as 10.21 wt%.In addition,the loading value keeps the same with the further introduction of metal precursor,which indicating the saturated utilization of support.However,there are few similar reports suggesting that it needs more exploration to solve the problem of the insufficient utilization of surface area.Second,the ultimate aim of SACs is to be utilized in industry.The stability of SACs/2DM is one of significant indicator whether the SACs can be applied to practical application.Although there are strong interaction existing between single atoms and the 2D support,it can be possibly weakened by the oxidative addition and reductive elimination cycles which are similar to homogeneous catalysis during the catalytic process.Especially for some non-noble metal such as Fe,Co,and Ni,they are easily converted into oxidative species in ambient environment.The resultant degradation of the activity may make it difficult for SACs to achieve industrial application.Besides,the cost and complexity of synthetic techniques is another factor ought to be taken into consideration.These issues make it a long journey to achieve the real application of SACs/2DM and more efforts need to be devoted to resolve them earlier.Third,how to assure and identify the uniformity of actual active sites which is vital to investigate the catalytic essence is still tricky for researchers.Due to possible harsh synthesis conditions and impurity disturbances in the support,coordination structures of real active sites can be diverse which is disadvantageous to explore the real structureperformance relationship.For instance,SACs prepared by pyrolysis method usually suffer from the issue that the organic precursors generate diverse N species after pyrolysis leading to different coordination environment.In addition,the current characterization techniques can hardly observe the true structures of the active sites in a direct way,which need to be developed further especially for someoperandocharacterization methods.On the basis of perfect homogeneity,the conclusion obtained from theoretic calculations can be more accurate and reliable,which is conducive to guiding the fabrication of novel effective SACs in return.Finally,special for SACs/2DM,regulating the thickness of 2D materials is a crucial approach to modulate the catalytic performance.Once the layer number changes,the electronic states will be tuned and thus the catalytic activity will be distinctly influenced.Additionally,the specific surface area will be increased with the reduction of layers,enhancing the atom utilization of single metal atom.Therefore,precisely fabricating narrow-layer or even single-layer 2D materials is beneficial to boost the activity.In brief,there is a long way to realize the industrialization of SACs/2DM and there are still many issues to be addressed,which means we should devote more efforts to it.
Declaration of competing interest
The authors have no competing interest.
Acknowledgments
This work was supported by Science and Technology Key Project of Guangdong Province of China (No.2020B010188002),the National Key R&D Program of China (2018YFA0702003),and the National Natural Science Foundation of China (Nos.21890383,21871159).
杂志排行
Chinese Chemical Letters的其它文章
- Progress in mechanochromic luminescence of gold(I) complexes
- Spinel-type bimetal sulfides derived from Prussian blue analogues as efficient polysulfides mediators for lithium-sulfur batteries
- Alopecuroidines A-C,three matrine-derived alkaloids from the seeds of Sophora alopecuroides
- Boronic acid-containing diarylpyrimidine derivatives as novel HIV-1 NNRTIs:Design,synthesis and biological evaluation
- Diaminodiacid bridge improves enzymatic and in vivo inhibitory activity of peptide CPI-1 against botulinum toxin serotype A
- Peptide stapling with the retention of double native side-chains
