蛋白激发子PevD1诱导本生烟植保素辣椒醇的产生和积累
2021-03-12李泽梁颖博段佳琪曾洪梅李广悦赵国存杨秀芬
李泽 梁颖博 段佳琪 曾洪梅 李广悦 赵国存 杨秀芬
摘要 :诱导抗病性是实现植物病害绿色防控的重要途径,大丽轮枝菌蛋白激发子PevD1能激活植物免疫系统,提高本生烟对烟草花叶病毒(TMV)和烟草野火病病原菌Pseudomonas syringae pv. tabaci、棉花对大丽轮枝菌Verticillium dahliae的抗病性,但分子机制不清晰。前期转录组测序(RNASeq)分析结果显示,本生烟响应PevD1诱导的差异表达基因显著富集在倍半萜烯和三萜烯的合成途径中。本文进一步分析了这些差异表达基因的功能,并通过测定倍半萜植保素辣椒醇合成关键基因EAS 和 EAH的转录表达水平和辣椒醇积累量,证明PevD1能诱导本生烟产生植保素辣椒醇,明确了PevD1诱导植保素辣椒醇的产生是提高植物抗病性的重要机制之一。
关键词 :蛋白激发子PevD1; 转录组测序; 植保素; 辣椒醇
中图分类号: S 432.2
文献标识码: A
DOI: 10.16688/j.zwbh.2019605
Elicitor PevD1 induces phytoalexin capsidiol production and accumulation in Nicotiana benthamiana
LI Ze1, LIANG Yingbo1, DUAN Jiaqi1, ZENG Hongmei1, LI Guangyue1, ZHAO Guocun2, YANG Xiufen1*
(1. State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection,
Chinese Academy of Agricultural Sciences, Beijing 100193, China; 2. Hebei Provincial
Pesticide Testing and Monitoring Station, Shijiazhuang 050031, China)
Abstract :Induced disease resistance is an important approach for plant disease management. The Verticillium dahliae protein elicitor PevD1 could trigger plant immune response and improved the disease resistance of tobacco to Tobacco mosaic virus (TMV) and Pseudomonas syringae pv. tabaci, and the resistance of cotton to V.dahliae. However, the molecular mechanism is still unclear. In previous research, the differential expression genes (DEGs) in response to PevD1 in Nicotiana benthamiana were obtained by RNAseq and a lot of DEGs were significantly enriched in the sesquiterpene and triterpene synthesis pathway. In this study, we analyzed the functions of these DEGs in sesquiterpene and triterpene synthesis, and confirmed the transcriptional expression level of the key genes in the sesquiterpene synthesis pathway after PevD1 inducement. The results showed that the expression level of the key genes, EAS and EAH in capsidiol synthesis were significantly elevated. The capsidiol content also increased after PevD1 inducement. Our results indicated that PevD1induced capsidiol production is one of the important mechanism of plant disease resistance improvement.
Key words :protein elicitor PevD1; RNASeq; phytoalexin; capsidiol
在长期进化过程中,植物为了抵御有害生物侵袭已经形成了精细而复杂的免疫防御系统,包括病原物/微生物相关分子模式(pathogen/microbeassociated molecular pattern, P/MAMP)诱导的PTI(PAMPtriggered immunity)和效应子诱导的ETI(effectortriggered immunity)[12]。当植物受到病原菌侵染或病原菌产生的激发子诱导后可以启动多重免疫防御反应,主要包括活性氧(ROS)暴发、NO积累、细胞膜通透性改变、病程相关蛋白表达、植物激素积累以及植保素的产生等,使植物产生局部抗性和系统抗性。利用植物诱导抗性提高植物自身免疫力、减轻病害发生已经成为现代植物保护的新技术。植保素是植物受到外界因素诱导产生的小分子植物次生代谢产物,能抑制或杀死多种植物病原微生物,常常被用作植物抗病性的分子标志[3],在植物免疫防御系统中发挥着重要作用,其化学合成速度及积累量与植物抗病性密切正相关,被誉为植物的“化学武器”。不同植物产生的植保素种类和产物组成不同,其中萜类化合物是植物在代谢过程中产量最高的植保素。倍半萜和三萜次生代谢产物是马铃薯、烟草和辣椒等茄科植物重要的植保素,如日齐素(rishitin)、辣椒醇(capsidiol)和二烯酮/香根酮(solavetivone)[45]。香根酮和日齐素是马铃薯响应花生四烯酸和欧文氏杆菌Erwinia carotovora 侵染产生的植保素[67],这些植保素能抑制马铃薯致病疫霉Phytophthora infestans和欧文氏杆菌的生长。烟草中倍半萜烯植保素主要有东莨菪内酯/东莨菪素(scopoletin)[89]和辣椒醇[1011]。辣椒醇能抑制多種真菌菌丝生长和孢子形成,包括辣椒疫霉Phytophthora capsici和灰葡萄孢Botrytis cinerea[1213]。
大量研究已经证明,烟草受到病原菌侵染或激发子诱导后,植株体内产生并积累植保素辣椒醇,从而减轻自身受到的侵染和危害。例如烟草Nicotiana attenuata被链格孢Alternaria alternata侵染后积累大量辣椒醇[14];被辣椒疫霉菌侵染的辣椒产生的细胞坏死区域有高浓度辣椒醇积累[1516];隐地蛋白(cryptogein)、麦角甾醇(ergosterols)、致病疫霉P.infestans激发素INF1或花生四烯酸等PAMP因子均能诱导烟草辣椒醇的产生和积累[1719]。PevD1是作者实验室从大丽轮枝菌Verticillium dahliae胞外分离的蛋白激发子,能诱导烟草产生细胞坏死反应,产生NO和H2O2,胼胝质、酚类化合物及木质素的积累,提高烟草对TMV、烟草野火病以及棉花对大丽轮枝菌的抗性[2022],但是PevD1诱导植物抗病性的分子机制尚不清楚。前期通过RNASeq技术已经获得了PevD1诱导本生烟前后大量差异表达的基因[23],本文将进一步分析倍半萜和三萜合成通路上的差异表达基因的功能,测定倍半萜植保素辣椒醇的积累及其合成关键基因的转录水平,以期明确植保素辣椒醇是PevD1诱导本生烟提高植物抗病性的重要作用机制。
1 材料与方法
1.1 蛋白激发子PevD1的表达与纯化
构建PevD1真核表达载体pPICZαAPevD1,转化毕赤酵母细胞后通过甲醇诱导表达,采用NiNTA纯化介质(ProteinIsoNiNTA Resin,北京全式金)亲和纯化PevD1His重组蛋白,利用SDSPAGE与Western blot技术验证PevD1His重组蛋白的正确性,具体方法参照文献[24]。
1.2 PevD1处理本生烟
本生烟种子由本实验室保存,将种子播种在装有营养土的培养钵中,在25℃下培养。选取4周龄的本生烟,用去掉针头的1 mL注射器在本生烟叶片背部注射20 μL PevD1蛋白液(蛋白浓度10 μmol/L),以注射20 μL TrisHCl(浓度20 mmol/L,pH 8.0)为对照,每个处理10片叶。处理后分别在6、12、24 h对全叶片进行取样,液氮速冻后于-80℃保存用于转录组测序。
1.3 转录组测序
用EasyPure Plant RNA Kit试剂盒提取植株叶片总RNA,质检合格后通过华大BGISEQ500平台建库测序。
1.4 差异表达基因的筛选
通过DEGseq 进行差异表达基因的检测,DEGseq方法基于泊松分布[25]。将差异倍数为2倍以上(Fold Change≥2)并且Qvalue≤0.001的基因确定为差异显著的差异表达基因。
1.5 差异表达基因KEGG富集分析
根据KEGG pathway注释来分类,通过R软件中的phyper函数进行富集分析,通常情况下FDR≤0.01的功能被认为是显著富集。
1.6 差异表达基因的qRTPCR检测
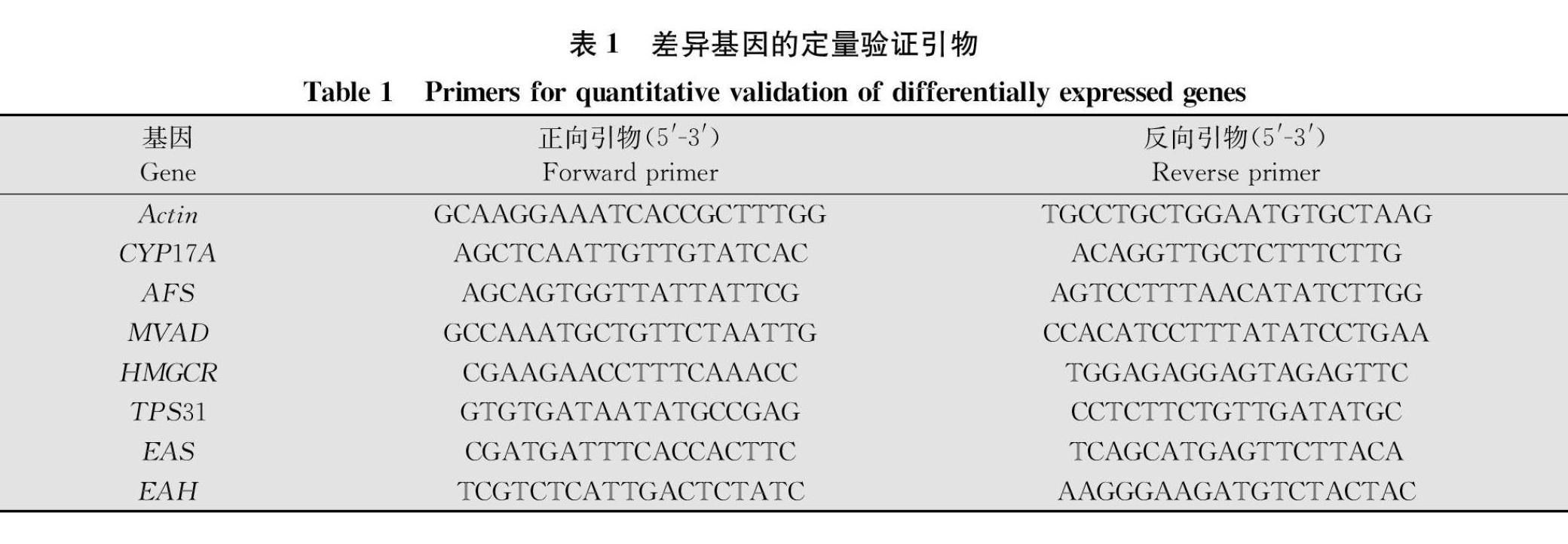
参考茄科数据库(https:∥solgenomics.net/)的目的基因序列,通过Beacon Designer 8.0软件设计荧光定量PCR的特异性引物(表1)。以上述样品提取的总RNA反转录后的cDNA为荧光定量PCR的模板,以Actin基因为内参基因,通过荧光定量PCR试剂盒(北京全式金)来特异性扩增每个目的基因。最后参照2-ΔΔCt计算方法,对每个mRNA进行相对定量分析[26]。
1.7 植保素辣椒醇的检测
取PevD1渗入本生烟叶片处理后9、12 h和16 h的叶片200 mg,液氮研磨后用1 mL 二氯甲烷提取,振荡10 min后离心(3 000 g, 5 min)收集上清液,沉淀物进行第二次提取,合并提取液旋转蒸发去除有机溶剂,用1 mL甲醇溶解后用HPLC(Agilent 1260, USA)检测,具体检测条件参照文献[14]。辣椒醇标准品由中国科学院昆明植物所吴劲松研究员馈赠,将标准品稀释6个浓度(6.25、12.5、25、50、100、200 μg/mL),以标准品浓度为横坐标,HPLC产生的峰面积为纵坐标,制作辣椒醇标准曲线,根据标准曲线方程计算样品中辣椒醇浓度。
2 结果与分析
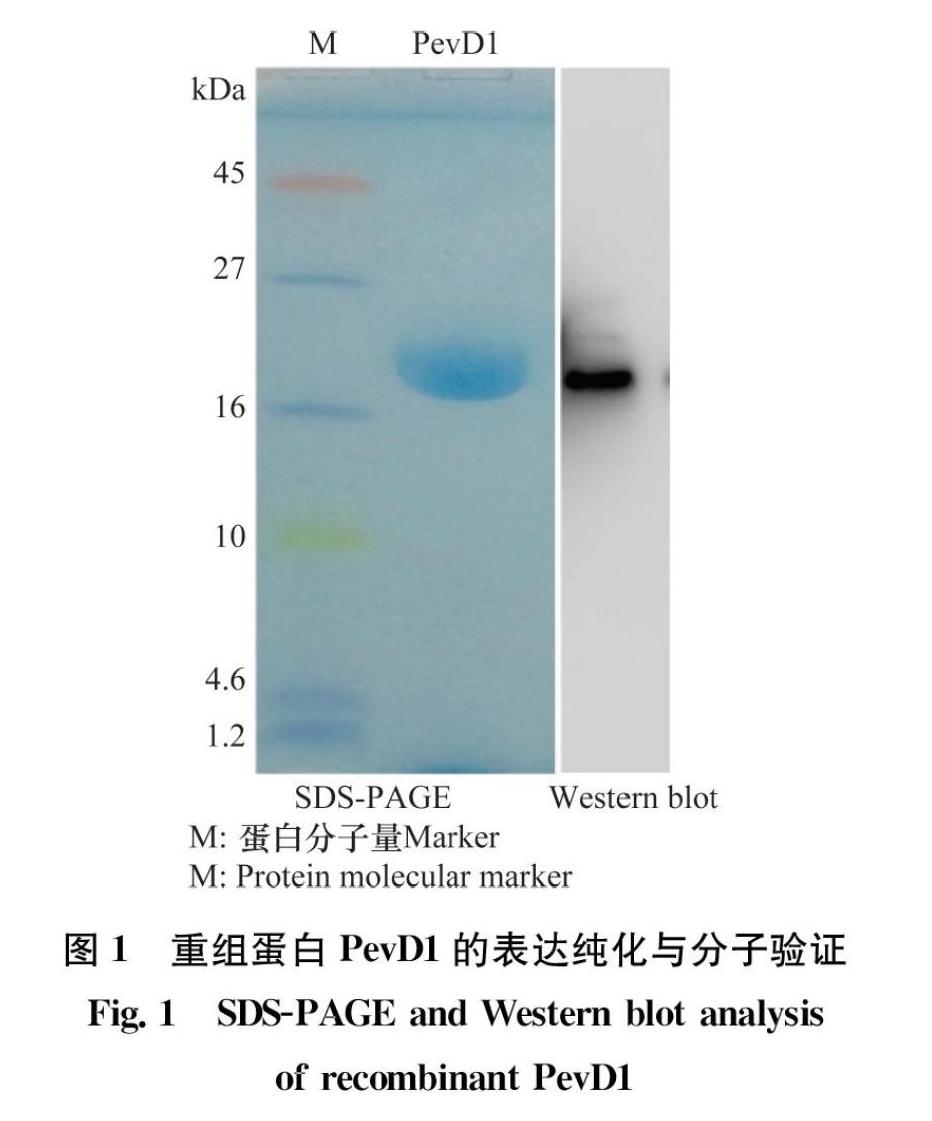
2.1 PevD1蛋白的表达纯化及验证
将获得的酵母表达菌株用无菌甲醇诱导培养3~4 d后收集发酵上清液。用亲和柱层析过滤发酵液,收集表达的PevD1重组蛋白。蛋白稀释后进行SDSPAGE凝胶电泳并染色,在分子量17 kDa左右处可见一条蛋白条带,并且与PevD1His重组蛋白的理论分子量一致(图1)。为了进一步确定表达的蛋白是PevD1His重组蛋白,进行了Western blot检测,以抗His标签的鼠单克隆抗体为一抗,以辣根过氧化物酶HRP标记的羊抗鼠IgG为二抗。根据结果显示只有单一条带能够与抗His标签的抗体结合并发生显色反应,并且条带大小与SDSPAGE显示的大小一致,说明成功表达了PevD1His重组蛋白(图1)。
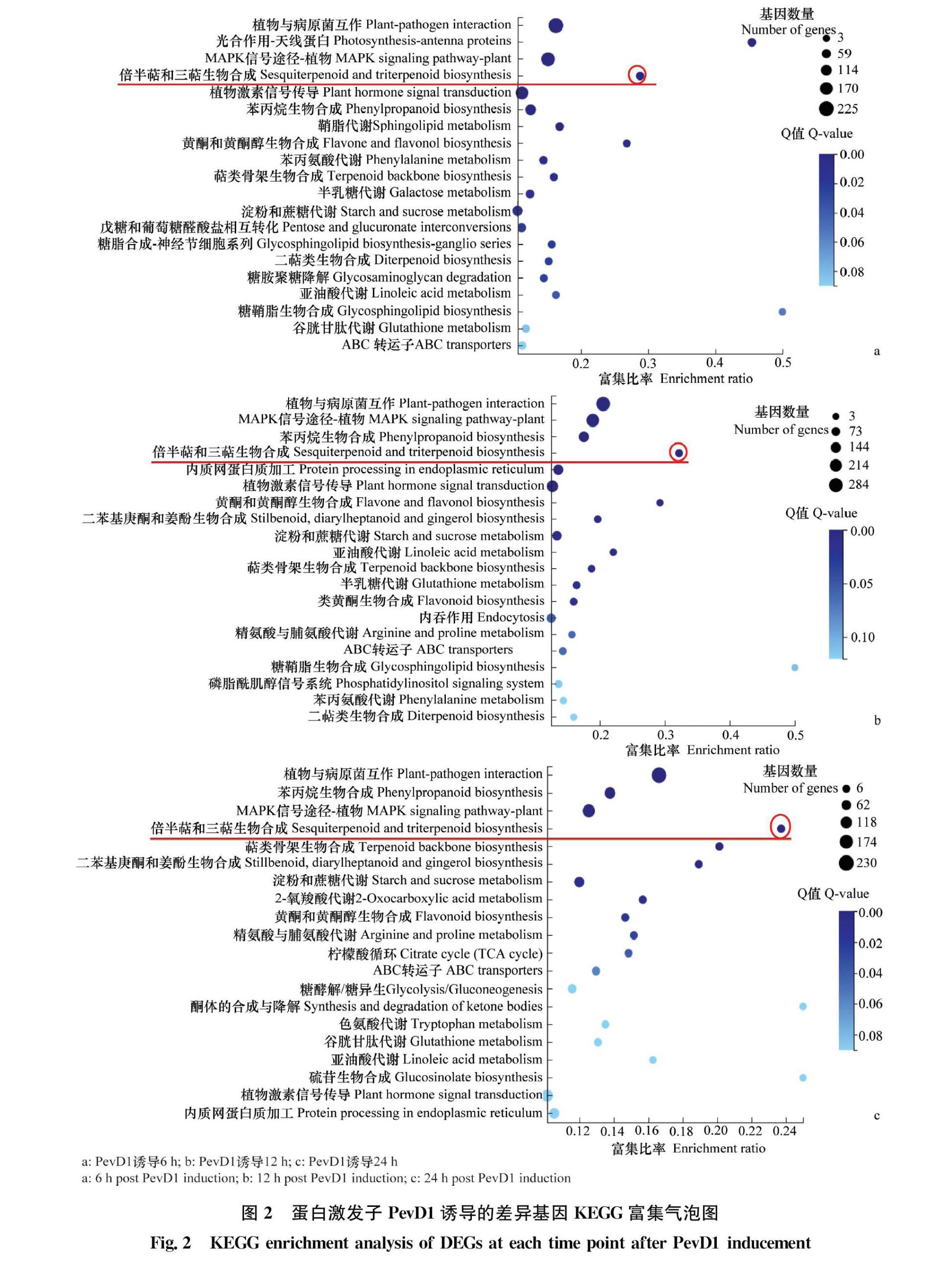
2.2 PevD1诱导的大量差异表达基因富集于倍半萜和三萜次生代谢产物合成通路
PevD1诱导本生烟6、12 h和24 h差异表达基因KEGG pathway富集分析显示,排在前4位的有植物与病原菌互作、MAPK信号通路、苯丙烷合成途径和倍半萜和三萜次生代谢产物合成通路(图2a, b,c)。为了进一步明确PevD1诱导后不同时间富集在倍半萜和三萜次生代谢产物合成通路上的差异表达基因情况,根据差异基因KEGG富集气泡图,统计了诱导不同时间后的差异基因数量。结果显示,PevD1处理6 h共有34个差异表达基因,其中32个上调;PevD1处理12 h时,共有38个差异表达基因,其中33个基因上调;PevD1处理24 h时,共筛选到28个差异表达基因,全部为上调表达基因(图3),说明PevD1蛋白激发子诱导了本生烟倍半萜和三萜次生代谢产物合成通路上的大量差异表达基因上调表达。提取部分差异表达上调基因的序列,并在NCBI的Nr数据库进行BLAST序列比对及注释(表2),发现多数基因与辣椒醇植保素合成代谢相关。如5马兜铃烯合成酶(5epiaristolochene synthase,EAS)催化反式法尼基焦磷酸(Farnesyl pyrophosphate,FPP)的环化,形成双环的中间体5马兜铃烯,是转化成倍半萜类植保素辣椒醇的初始步骤[27]。螺二烯加氧酶(premnaspirodiene oxygenase,CYP71)是一種细胞色素P450单加氧酶,不仅能够使三萜类底物羟基化从而增强三萜类化合物的活性,而且在5马兜铃烯羟基化形成辣椒素的过程中也起到一定的作用。
2.3 PevD1诱导倍半萜和三萜次生代谢合成关键基因转录上调表达
本研究选取倍半萜和三萜次生代谢产物合成途径中的6个关键基因进行qPCR检测(表3,图4)。结果表明,PevD1诱导后这些基因的表达模式与转录组数据基本吻合,PevD1诱导后的基因表达水平明显高于未诱导的对照,说明倍半萜和三萜次生代谢产物合成途径参与了PevD1诱导的本生烟抗病性。转录组分析PevD1诱导了大量的EAS基因上调表达,诱导6 h时32个上调表达基因中有14个与EAS有关,诱导12 h时33个上调表达基因中有16个与EAS基因有关,诱导24 h时上调的28个上调表达基因中有11个与EAS有关,说明富集在倍半萜和三萜次生代谢合成途径上的差异表达基因中有近40%~50% 的基因与辣椒醇合成关键基因EAS有关,qPCR检测EAS基因上调表达近70倍,暗示了PevD1可能诱导了植保素辣椒醇的产生和积累。
2.4 PevD1诱导植保素辣椒醇合成关键基因上调表达和产物积累
EAS和EAH是植保素辣椒醇合成通路上的两个关键酶,沉默NbEAS或NbEAH的本生烟植株接种致病疫霉后3 d均比未沉默植株的辣椒醇含量降低,而且对致病疫霉的抗性也降低[14]。在辣椒醇合成过程中,EAS先将法尼烯焦磷酸(FPP)环化成马兜铃烯,经马兜铃烯二羟基化酶(5epiaristolochene dihydroxylase,EAH) 催化2个羟基化反应,形成终产物辣椒醇[2829]。本研究分析发现,PevD1诱导6~12 h富集在倍半萜和三萜次生代谢产物合成通路上的基因中有50%以上的差异表达基因与EAS相关,意味着PevD1可能诱导辣椒醇的产生。为了验证该推测,用PevD1分别诱导本生烟叶片9 h和12 h后取样,用HPLC检测辣椒醇的含量。结果表明,PevD1诱导的叶片均能检测到辣椒醇的积累,而未诱导的对照检测不到辣椒醇,同时PevD1诱导的叶片EAS和EAH基因转录水平也大幅度提高(图5),说明PevD1确实诱导了植保素辣椒醇的积累。
3 讨论
植保素是植物防御病原物的主要生化壁垒,在病原菌侵染点周围产生并积累, 杀死病原物或抑制其侵染,是植物抗性的重要机制。萜类化合物是植物代谢过程中产生最多的一类化合物[30],根据其结构可分为半萜、单萜、倍半萜、二萜、三萜以及多聚萜等。异戊二烯(IPP)是萜类化合物的基本结构单位,萜类化合物的合成途径非常复杂,目前已经克隆了萜类化合物合成路径中的一些关键基因并进行了功能分析。5马兜铃烯合成酶(EAS)是最早被研究的倍半萜合成酶,能够催化法尼基焦磷酸(FPP)合成倍半萜类植保素辣椒醇的前体物质5马兜铃烯[31],是辣椒醇合成的关键酶之一,萜烯合成酶(terpenoid synthase,TPS)是异戊二烯、单萜、倍半萜烯和二萜等低分子量萜类形成的关键酶,TPS转录水平的提高伴随着萜类物质的大量积累[32]。α法尼烯是挥发性倍半萜类次生代谢物,苹果中的α法尼烯的代谢水平直接影响到苹果虎皮病害的发展[3334]。α法尼烯合成酶(AFS)是合成α法尼烯的限速酶,主要功能是催化法尼烯基焦磷酸(FPP)合成α法尼烯。3羟基3甲基戊二酸单酰辅酶A还原酶(HMGCR/HMGR)、法尼基焦磷酸合成酶(FPS)以及马兜铃烯合成酶(EAS)是倍半萜生物合成途径中的关键酶[35]。当烟草中的HMGCR表达受到抑制后,植株体内的倍半萜化合物的含量会明显下降。FPS催化IPP与其他化合物生成FPP,FPP不仅是多萜醇、甾体和泛醌等重要初生代谢物的合成前体,它还能在倍半萜环化酶的作用下生成倍半萜类植保素等物质[28]。马兜铃烯二羟基化酶EAH是5马兜铃烯羟基化形成辣椒醇的另一个重要酶, 法尼烯二磷酸盐经过EAH和EAS两步催化形成辣椒醇[34]。
辣椒醇属于倍半萜植保素,其产生受多个途径的调控。渐狭叶烟草N.attenuata被链格孢侵染后可以诱导产生辣椒醇植保素,用VIGS技术沉默EAS或EAH基因的表达则辣椒醇积累显著减少,同时降低了对链格孢病原菌侵染的抗性,而且辣椒醇的产生不依赖于茉莉酸(JA)和乙烯(ET)信号途径,其合成受到转录因子ERF2的正调控[14]。用纤维素酶/花生四烯酸处理或灰葡萄孢侵染野生烟草N.plumbaginifolia ABA合成缺陷型烟草突变体,其辣椒醇含量比野生型高2倍以上[9],说明ABA负调控辣椒醇植保素的合成。此外,大量研究表明,植保素产生依赖于活性氧和HR[3637]。前期研究表明,PevD1能诱导MAPK激活和大量ERF转录因子转录上调,MAPK和ERF转录因子是否参与PevD1诱导辣椒素的合成,从而提高烟草抗病性有待于进一步研究。另外,PevD1转化拟南芥能显著提高对灰葡萄孢菌和丁香假单胞菌的抗性,而且JA含量明显提高,ABA响应通路中的3个重要负调控因子基因WRKY40, PP2CA 和 HAI2顯著上调表达,意味着PevD1抑制了ABA响应通路[38]。JA和ABA通路是否参与PevD1诱导辣椒醇的积累有待于深入探讨。
参考文献
[1] DODDS P N, RATHJEN J P. Plant immunity: towards an integrated view of plantpathogen interactions [J]. Nature Reviews Genetics, 2010, 11(8): 539548.
[2] ZIPFEL C. Early molecular events in PAMPtriggered immunity [J]. Current Opinion in Plant Biology, 2009, 12(4): 414420.
[3] AHUJA I, KISSEN R, BONES A M. Phytoalexins in defense against pathogens [J]. Trends in Plant Science, 2012, 17: 7390.
[4] DESJARDINS A E, MCCORMICK S P, CORSINI D L. Diversity of sesquiterpenes in 46 potato cultivars and breeding selections [J]. Journal of Agricultural and Food Chemistry, 1995, 43(8):22672272.
[5] ENGSTRM K, WIDMARK A K, BRISHAMMAR S, et al. Antifungal activity to Phytophthora infestans of sesquiterpenoids from infected potato tubers [J]. Potato Research,1999, 42:4350.
[6] LYON G D, BAYLISS C E. The effect of rishitin on Erwinia carotovora var. atroseptica and other bacteria [J]. Physiological and Molecular Plant Pathology, 1975, 6(2):177186.
[7] EL OIRDI M, TRAPANI A, BOUARAB K. The nature of tobacco resistance against Botrytis cinerea depends on the infection structures of the pathogen [J]. Environmental Microbiology, 2010, 12(1): 239253.
[8] SUN Huanhuan, SONG Na, MA Lan, et al. Ethylene signalling is essential for the resistance of Nicotiana attenuata against Alternaria alternata and phytoalexin scopoletin biosynthesis [J]. Plant Pathology, 2017, 66(2): 277284.
[9] MIALOUNDAMA A S, HEINTZ D, DEBAYLE D, et al. Abscisic acid negatively regulates elicitorinduced synthesis of capsidiol in wild tobacco [J]. Plant Physiology, 2009, 150:15561566.
[10]SHIBATA Y, KAWAKITA K, TAKEMOTO D. Agerelated resistance of Nicotiana benthamiana against hemibiotrophic pathogen Phytophthora infestans requires both ethyleneand salicylic acidmediated signaling pathways [J]. Molecular Plant Microbe Interactions, 2010, 23(9): 11301142.
[11]GROSSKINSKY D K, NASEEM M, ABDELMOHSEN U R, et al. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling [J]. Plant Physiology, 2011, 157(2): 815830.
[12]STOESSL A, UNWIN C H, WARD E W B. Postinfectional inhibitors from plants I. capsidiol, an antifungal compound from capsicum frutescens [J]. Phytopathology, 1972, 74(2): 141152.
[13]WARD E W B, UNWIN C H, STOESSL A. Postinfectional inhibitors from plants. XIII. Fungitoxicity of the phytoalexin, capsidiol, and related sesquiterpenes [J]. Canadian Journal of Botany, 1974, 52(12): 24812488.
[14]SONG Na, MA Lan, WANG Weiguang, et al. An ERF2like transcription factor regulates production of the defense sesquiterpene capsidiol upon Alternaria alternata infection [J]. Journal of Experimental Botany, 2019, 70(20): 58955908.
[15]MOLOT P M, MAS P, CONUS M, et al. Relations between capsidiol concentration, speed of fungal invasion and level of induced resistance in cultivars of pepper (Capsicum annuum) susceptible or resistant to Phytophthora capsici [J]. Physiological Plant Pathology, 1981, 18(3): 379389.
[16]EGEA C, ALCAZAR M D, CANDELA M E. Capsidiol: its role in the resistance of Capsicum annuum to Phytophthora capsici [J]. Physiologia Plantarum, 1996, 98(4): 737742.
[17]HOSHINO T, CHIDA M, YAMAURA T, et al. Phytoalexin induction in green pepper cell cultures treated with arachidonic acid [J]. Phytochemistry, 1994, 36(6): 14171419.
[18]KELLER H, CZERNIC P, PONCHET M, et al. Sesquiterpene cyclase is not a determining factor for elicitorand pathogeninduced capsidiol accumulation in tobacco [J]. Planta, 1998, 205: 467476.
[19]TUGIZIMANA F, STEENKAMP P A, PIATER L A, et al. Ergosterolinduced sesquiterpenoid synthesis in tobacco cells [J]. Molecules, 2012, 17(2): 16981715.
[20]WANG Bingnan, YANG Xiufen, ZENG Hongmei, et al. The purification and characterization of a novel hypersensitivelike responseinducing elicitor from Verticillium dahliae that induces resistance responses in tobacco [J]. Applied Microbiology & Biotechnology 2012, 93(1):191201.
[21]BU Bingwu, QIU Dewen, ZENG Hongmei, et al. A fungal protein elicitor PevD1 induces Verticillium wilt resistance in cotton [J]. Plant Cell Reports, 2014, 33(3): 461470.
[22]王炳楠, 楊秀芬, 曾洪梅, 等. 大丽轮枝菌分泌蛋白激发子的分离纯化及生物功能研究[J]. 生物技术通报, 2011(11): 166171.
[23]梁颖博,李泽,邱德文,等.本生烟响应蛋白激发子PEVD1的差异表达基因鉴定与分析[J].中国农业科学, 2019, 52(21):37943805.
[24]ZHANG Yi, GAO Yuhan, LIANG Yingbo, et al. Verticillium dahliae PevD1, an Alt a 1like protein, targets cotton PR5like protein and promotes fungal infection [J]. Journal of Experimental Botany. 2019,70(2):613626.
[25]WANG Likun, FENG Zhixing, WANG Xi, et al. DEGseq: an R package for identifying differentially expressed genes from RNAseq data [J]. Bioinformatics, 2010,26(1):136138.
[26]SCHMITTGEN T D, LEE E J, JIANG Jinmai. Highthroughput realtime PCR [J]. Methods in Molecular Biology, 2008, 429: 8998.
[27]STARKS C M, BACK K, CHAPPELL J, et al. Structural basis for cyclic terpene biosynthesis by tobacco 5epiaristolochene synthase [J]. Science, 1997, 277(5333): 18151820.
[28]FACCHINI P J, CHAPPELL J. Gene family for an elicitorinduced sesquiterpene cyclase in tobacco [J]. Proceedings of the National Academy of Sciences of the United States of America, 1992, 89(22): 1108811092.
[29]RALSTON L, KWON S T, SCHOENBECK M, et al. Cloning, heterologous expression, and functional characterization of 5epiaristolochene1,3dihydroxylase from tobacco (Nicotiana tabacum) [J]. Archives of Biochemistry and Biophysics, 2001, 393(2): 222235.
[30]DIXON R A. Natural products and plant disease resistance [J]. Nature, 2001, 411(6839): 843847.
[31]WHITEHEAD L M, THRELFAL D R, EWING D F. 5epiaristolochene is a common precursor of the sesquiterpenoid phytoalexins capsidiol and debneyol [J]. Phytochemistry,1989, 28(3): 775779.
[32]GAO Yang, HONZATKO R, PETERS R. Terpenoid synthase structures: a so far incomplete view of complex catalysis [J]. Natural product reports, 2012, 29:11531175.
[33]PECHOUS S W, WHITAKER B D. Cloning and functional expression of an (E, E)alphafarnesene synthase cDNA from peel tissue of apple fruit [J]. Planta, 2004, 219(1): 8494.
[34]ROWAN D D, HUNT M B, FIELDER S, et al. Conjugated triene oxidation products of alphafarnesene induce symptoms of superficial scald on stored apples [J]. Journal of Agricultural & Food Chemistry, 2001, 49(6): 27802787.
[35]CHOI D, WARD B L, BOSTOCK R M. Differential induction and suppression of potato 3hydroxy3methylglutaryl coenzyme A reductase genes in response to Phytophthora infestans and to its elicitor arachidonic acid [J]. Plant Cell, 1992, 4(10): 13331344.
[36]PERRONE S T, MCDONALD K L, SUTHERLAND M W, et al. Superoxide release is necessary for phytoalexin accumulation in Nicotiana tabacum cells during the expression of cultivarrace and nonhost resistance towards Phytophthora spp. [J]. Physiological and Molecular Plant Pathology, 2003, 62(3): 127135.
[37]ARACELI A C, ELDA C M, EDMUNDO L G, et al. Capsidiol production in pepper fruits (Capsicum annuum L.) induced by arachidonic acid is dependent of an oxidative burst [J]. Physiological and Molecular Plant Pathology, 2007, 70(13): 6976.
[38]LIU Mengjie, KHAN N U, WANG Ningbo, et al. The Protein elicitor PevD1 enhances resistance to pathogens and promotes growth in Arabidopsis [J]. International journal of biological sciences, 2016, 12(8): 931943.
(責任编辑:田 喆)
