杂草对激素类除草剂抗药性研究进展
2021-03-12谷涛李永丰张自常杨霞曹晶晶杨倩
谷涛 李永丰 张自常 杨霞 曹晶晶 杨倩
摘要 :农田杂草严重影响作物的产量和品质,对除草剂的过度依赖和长期使用,使杂草对除草剂的抗性问题日益突出。目前已有262种杂草(152种双子叶和110种单子叶)的512个生物型对23类中的167个除草剂产生抗性。激素类除草剂作为除草剂的重要成员,为禾谷类作物田的杂草防除提供了保障,然而在使用了几十年后,44种杂草对此类除草剂产生了抗药性。本文对激素类除草剂的分类应用、除草机理、抗性现状、抗性机理等进行了综述,以期为激素类除草剂的应用和抗激素类除草剂杂草的防除提供参考。
关键词 :杂草; 激素类除草剂; 抗药性; 抗性机理
中图分类号:
S 481, S 482.4文獻标识码: A
DOI: 10.16688/j.zwbh.2019595
Research progress in weed resistance to auxin herbicides
GU Tao, LI Yongfeng*, ZHANG Zichang, YANG Xia, CAO Jingjing, YANG Qian
(Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China)
Abstract :Weeds in farmlands seriously affect the yield and quality of crops, and the weed resistance has become a serious problem due to prolonged use and overreliance on limited herbicides. At present, 512 biotypes of 262 weeds (152 dicots and 110 monocots) have evolved resistance to 167 herbicides with 23 different sites of action. Auxin herbicides, as one of the important herbicides, provide a critical tool for weed control in cereal crops. However, 44 weed species have developed resistance to them after decades of continuous use. In this paper, the classification and application, weed control mechanism, resistance status and resistance mechanism on auxin herbicides were systematically summarized, in order to provide a reference for the application of auxin herbicides and control of resistant weeds.
Key words :weeds; auxin herbicides; herbicide resistance; resistance mechanism
杂草是农田生态系统中非有意识栽培的植物。全球有杂草8 000多种,对农业生产造成危害的有2 500余种[1]。我国幅员辽阔,气候多样,杂草种类庞杂。迄今为止,我国已发现农田杂草1 400多种,其中641种列入《中国主要农作物杂草名录》,分属89科374属;这些杂草中,对我国农业生产造成严重危害的杂草有130余种,恶性杂草37种[2]。多数杂草生长迅速、根系发达、繁殖能力突出,在农田生态系统中具有较强的竞争能力,它们与作物竞争水分、养分和光照,严重影响农作物的产量和品质[3]。人类在栽培作物的过程中,与杂草进行了长期的斗争,经历了从“徒手拔草”的人工除草时代到今天以化学除草为主,生态、物理方法为辅的综合治理阶段。我国使用化学除草剂始于20世纪70年代,此后大量的化学除草剂从国外引进,国内化学除草剂的品种日益丰富。在这些除草剂中,激素类除草剂的作用不容忽视,此类除草剂的结构与生长素吲哚3乙酸(IAA)类似,低浓度时对植物有促生作用,高浓度时杀死植物[4]。它的出现为禾谷类作物田阔叶杂草的防除提供了有力的保障。
单一除草剂大量使用,抗性问题日益凸显,促使化学除草剂从单剂向复配剂的转变。复配剂的出现,一定程度上遏制了抗性杂草的蔓延,保证了粮食生产安全,但交互抗性和多抗性杂草的出现,又缩短了复配剂的使用寿命,增加了农业生产者的除草压力,尤其是作用方式独特的激素类除草剂也相继出现抗性问题,给杂草的有效治理带来了前所未有的挑战。全球学者在杂草生物学与生态学、杂草群落演替规律、杂草化学与生物防治技术,以及综合防治技术等方面进行了大量的研究工作[5],取得了一系列可喜的成绩,但也发现了一些亟待解决的问题。本文总结了杂草对激素类除草剂的抗性现状,归纳了杂草对激素类除草剂抗性机理方面的研究进展,展望了今后的研究趋势,为激素类除草剂的研究和应用提供参考。
1 激素类除草剂的种类及其作用方式
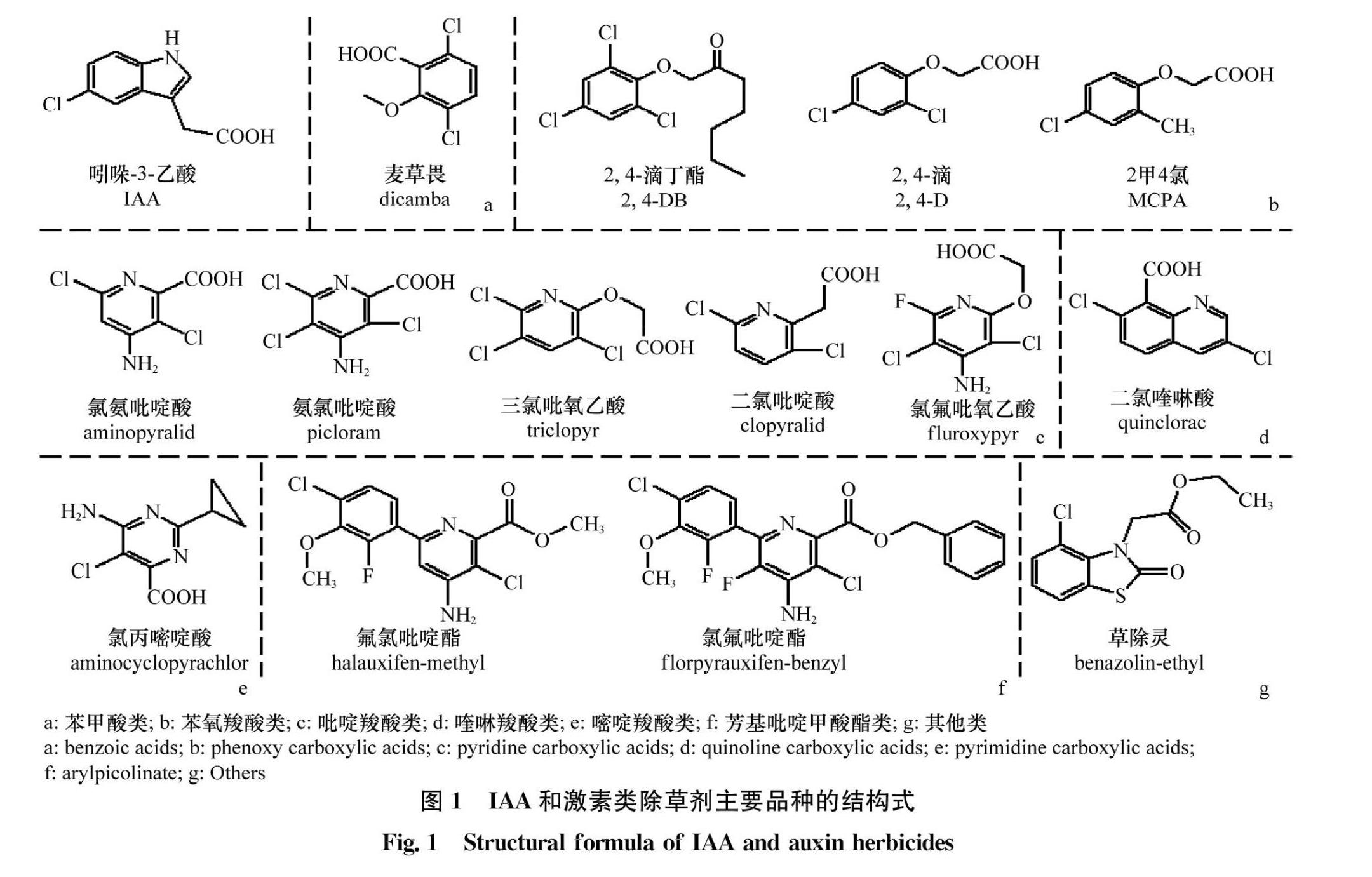
自1942年发现2,4滴的除草活性以后,许多激素类除草剂被相继开发利用。根据分子结构中的芳基、杂环或羧酸位置的不同,激素类除草剂可分为苯甲酸(benzoic acids)、苯氧羧酸(phenoxy carboxylic acids)、吡啶羧酸(pyridine carboxylic acids)、喹啉羧酸(quinoline carboxylic acids)、嘧啶羧酸(pyrimidine carboxylic acids)、芳基吡啶甲酸酯(arylpicolinate)和其他,共7类。如常见除草剂麦草畏属于苯甲酸类(图1a);2,4滴、2,4滴丁酯、2甲4氯等属于苯氧羧酸类(图1b);氯氨吡啶酸、氨氯吡啶酸、三氯吡氧乙酸、二氯吡啶酸、氯氟吡氧乙酸等属于吡啶羧酸类(图1c);二氯喹啉酸属于喹啉羧酸类(图1d);氯丙嘧啶酸属于嘧啶羧酸类(图1e);氯氟吡啶酯、氟氯吡啶酯等属于芳基吡啶甲酸酯类(图1f);草除灵不属于上述任何一类,归为其他类(图1g)。
激素类除草剂可影响植物体内的激素平衡,对植物生长和发育有广泛的影响。植物在激素类除草剂的作用下“过度”生长,从而死亡[4]。Grossmann等将杂草对激素类除草剂的反应分为三个阶段:第一阶段是刺激阶段,这一过程发生在除草剂作用后的数小时内,1氨基环丙烷1羧酸合成酶(1aminocyclopropane1carboxylic acid (ACC) synthase,ACS EC 4.4.1.14)被大量诱导合成,乙烯含量增加,植物畸形生长(叶偏上性生长,组织膨大,茎卷曲),脱落酸开始累积。第二阶段是抑制阶段,发生在24 h之内,此时根茎生长受阻,气孔关闭,蒸腾作用减弱,碳同化和淀粉合成变少,活性氧ROS增加。第三个阶段是组织衰败阶段,叶绿体损伤,进而失绿,膜和维管系统崩塌,最终植物萎蔫、坏死[4]。除对植物的生理生化有影响以外,最新的研究发现,激素类除草剂主要通过受体蛋白发挥效应。受体蛋白在感知除草剂后,调控下游基因表达,对植物造成影响。受体蛋白的差异是激素类除草剂在作物和杂草之间产生选择性的主要原因[67]。
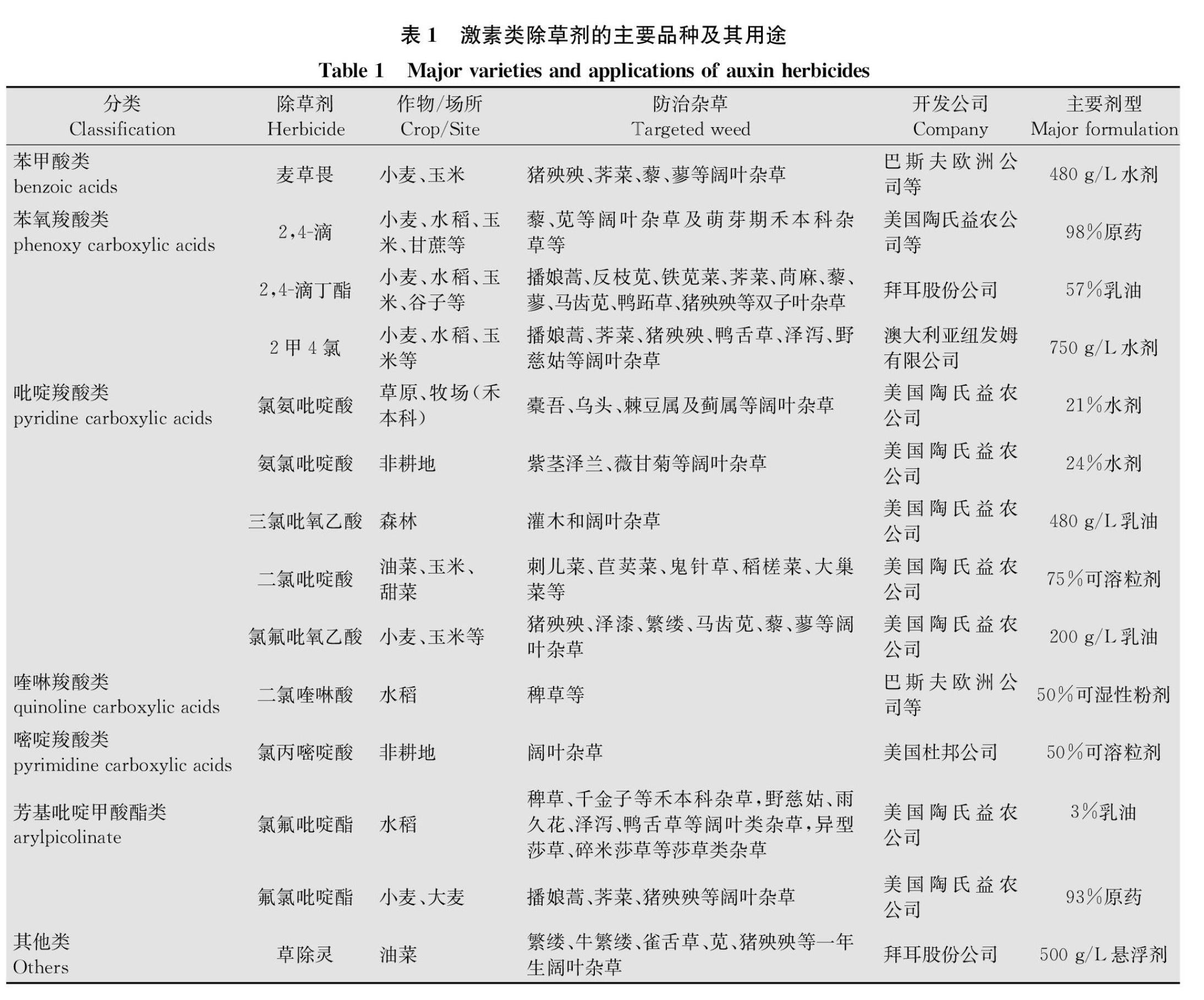
激素类除草剂在农业生产中具有重要的作用,水稻、小麦、玉米、油菜等大宗作物田,均有激素类除草剂的使用(表1)。大多数激素类除草剂用来防除阔叶杂草,对禾本科杂草无效,但是也有个别除草剂可以用来防除禾本科杂草,如二氯喹啉酸、氯氟吡啶酯等。二氯喹啉酸是巴斯夫公司于1984年开发的除草剂,其经济、高效、安全,对“臭名昭著”的稻田杂草稗草Echinochloa spp.有特效。氯氟吡啶酯(灵斯科)是陶氏益农新开发的激素类除草剂,登记作物为水稻,可用来防除稗草、千金子Leptochloa chinensis等禾本科杂草,野慈姑Sagittaria trifolia、雨久花Monochoria korsakowii等阔叶类杂草,异型莎草Cyperus difformis、碎米莎草C.iria等莎草,杀草谱广,应用前景广阔。二氯吡啶酸、草除灵分别为陶氏益农和拜耳股份公司开发的除草剂,可用作油菜田阔叶杂草的防除。激素类除草剂除了在作物田中大显身手外,在非耕地如草原、牧场、森林等领域也起到了重要的作用,这些除草剂包括氯氨吡啶酸、氨氯吡啶酸、氯丙嘧啶酸等(表1)。
2 杂草对激素类除草剂的抗药性现状
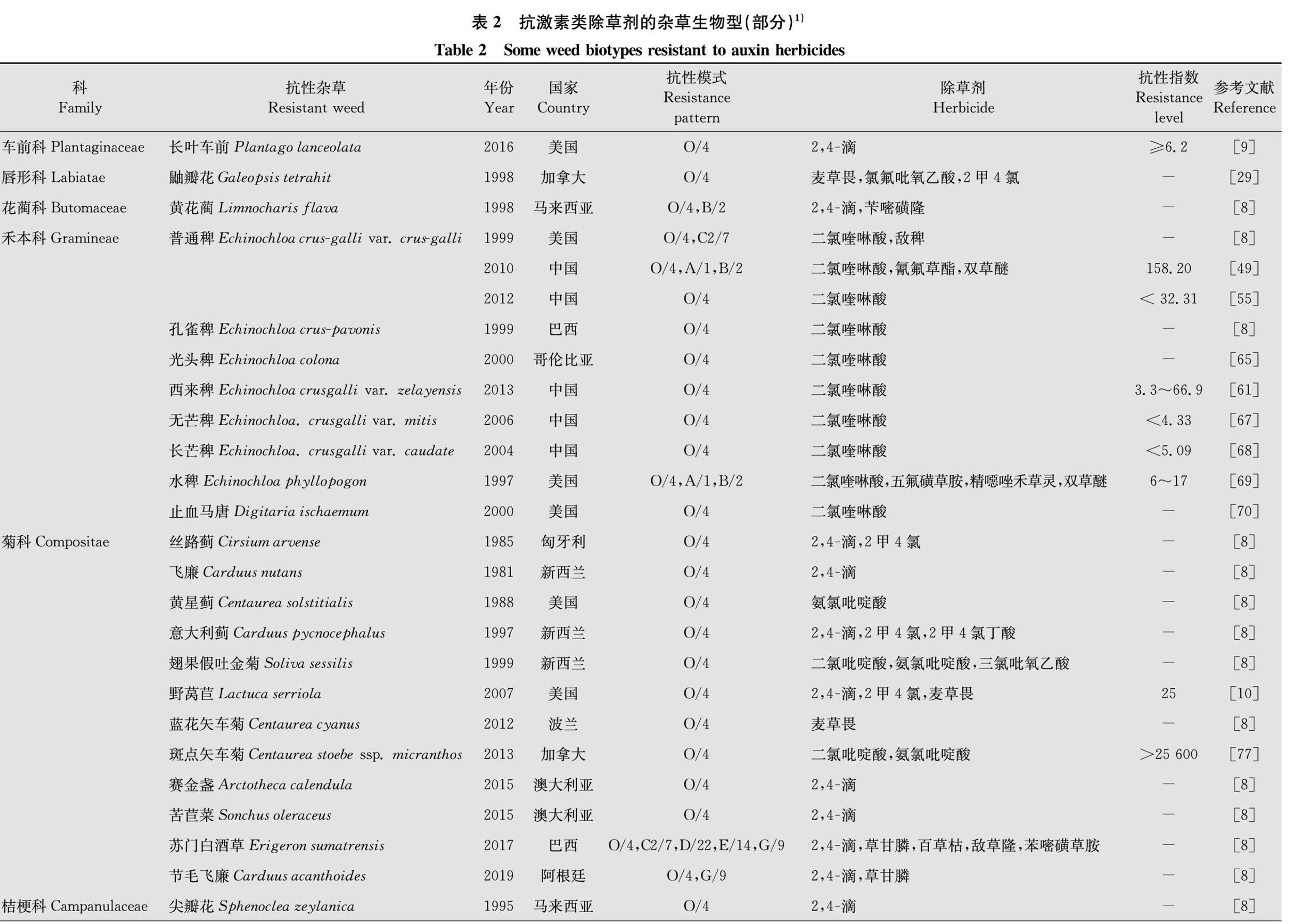
从20世纪50年代发现鸭跖草Commelina communis和野胡萝卜Daucus carota对2,4滴的抗药性开始,激素类除草剂的抗性问题引起了人们的广泛关注。20世纪70-80年代,发现抗激素类除草剂杂草生物型仅有8例;21世纪初(2000年-2019年),发现抗性杂草生物型已达47例(图2)。对激素类除草剂产生抗药性的杂草主要为阔叶类杂草,禾本科杂草仅有止血马唐Digitaria ischaemum和一些稗属杂草。目前,已经有17科44种杂草对激素类除草剂产生了抗药性[8](表2)。阔叶杂草中菊科、十字花科、苋科占比最大,它们对2,4滴、麦草畏、氨氯吡啶酸、二氯吡啶酸、2甲4氯、三氯吡氧乙酸等除草剂产生了抗药性。禾本科杂草中止血马唐、普通稗Echinochloa crusgalli var. crusgalli、孔雀稗E.cruspavonis、西来稗E.crusgalli var.zelayensis、光头稗E.colona、无芒稗E.crusgalli var.mitis、长芒稗E.crusgalli var. caudate和水稗E.phyllopogon对二氯喹啉酸产生了抗性(表2)。
激素类除草剂中,2,4滴已经使用了70多年,抗性杂草出现最早,相关报道最多。目前对2,4滴产生抗药性的杂草主要有长叶车前Plantago lanceolata[9]、黄花蔺Limnocharis flava[8]、丝路蓟Cirsium arvense[8]、飞廉Carduus nutans[8]、意大利蓟Carduus pycnocephalus[8]、野莴苣Lactuca serriola[10]、赛金盏Arctotheca calendula[8]、苦苣菜Sonchus oleraceus[8]、苏门白酒草Conyza sumatrensis[8](种名已订正为Erigeron sumatrensis)、节毛飞廉Carduus acanthoides[8]、尖瓣花Sphenoclea zeylanica[8]、猪殃殃Galium aparine[8]、野胡萝卜[11]、水虱草Fimbristylis miliacea[8]、野芥Sinapis arvensis[8,1213]、野萝卜Raphanus raphanistrum[1416]、东方大蒜芥Sisymbrium orientale[8,1718]、芜菁Brassica rapa[8]、短果芥Hirschfeldia incana[8]、直立石龙尾Limnophila erecta[8]、糙果苋Amaranthus tuberculatus[1921]、长芒苋Amaranthus palmeri[8]、绿穗苋Amaranthus hybridus[22]、竹节菜[8]、虞美人Papaver rhoeas[8,2325]、地肤Kochia scoparia[2628]等(表2)。1957年,在美国夏威夷发现了抗2,4滴的鸭跖草。同年,在加拿大安大略首次发现了抗2,4滴的野胡萝卜[8]。2007年,Burke等在美国华盛顿州发现的野莴苣生物型对2,4滴、2甲4氯和麦草畏均产生了抗药性,其中对2,4滴抗性达到了25倍[10]。2009年,Bernards等在美国内布拉斯加州发现了抗2,4滴的糙果苋,其相对抗性指数为9.12[19]。1999年到2013年间,在澳大利亚发现了多个抗2,4滴的野萝卜生物型,其中2010年发现的抗性生物型对激素类(O/4)、ALS抑制剂类(B/2)、类胡萝卜素合成抑制剂类(F1/12)三种作用方式不同的除草剂产生了抗药性[1416]。2016年,Dellaferrera等在阿根廷发现了对2,4滴、麦草畏、草甘膦产生了抗药性的绿穗苋[22]。同年,Patton等首次发现了抗2,4滴的长叶车前种群,該种群对2,4滴抗性指数达到了6.2倍以上[9]。2017年,Christoffoleti等发现了对O/4、光系统Ⅱ抑制剂类(C2/7)、光系统Ⅰ抑制剂类(D/22)、PPO抑制剂类(E/14)、EPSP合酶抑制剂类(G/9)等5种作用方式不同的除草剂均产生抗药性的苏门白酒草[8]。
麦草畏作为激素类除草剂中的重要一员,属于苯甲酸类激素类除草剂,被广泛应用于小麦、玉米田的杂草防除。对其产生抗性的杂草主要有鼬瓣花Galeopsis tetrahit[29]、野莴苣[10]、蓝花矢车菊Centaurea cyanus[8]、地肤[2628,3039]、藜Chenopodium album[4042]、野芥[8,43]、绿穗苋[22]、虞美人[23]等。其中地肤、藜等为农田常见杂草,在我国各地均有分布,部分田块发生量大,危害严重。早在1994年,Cranston等和Goss等就发现了对麦草畏和氯氟吡氧乙酸产生抗药性的地肤[30,32],此后大量的抗性地肤生物型被发现[2628,31,3339]。如2009年,Crespo等在美国内布拉斯加州采集了67个地肤种群,对其抗性指数进行测定,结果最不敏感种群和最敏感种群的相对抗性指数达到了11.3[31]。2015年,Ghanizadeh等发现了两个抗麦草畏的藜种群(种群L和M),抗性指数分别为7和19[40];2017年发现这两个抗性种群对氯氨吡啶酸、氨氯吡啶酸、二氯吡啶酸有交互抗性[41]。
二氯喹啉酸是在二氯吡啶酸结构基础上开发的激素类除草剂,作为主要除稗剂,已经使用了近30年。目前稗属杂草中的普通稗[4460]、西来稗[6164]、孔雀稗[8]、光头稗[6566]、无芒稗[67]、长芒稗[68]和水稗[69]对其产生了抗药性。1992年,LopezMartinez等在西班牙南部单季稻区发现了两种抗二氯喹啉酸的稗草生物型I、R,抗性指数分别达到6和26,发现的另一个生物型X对二氯喹啉酸和莠去津均产生了抗药性[44]。2000年,我国湖南省安乡县发现了抗二氯喹啉酸的普通稗草,其相对抗性指数为28.7[45]。2007年,吴声敢等发现采自浙江陶堰和塘下的稗草种群对二氯喹啉酸的相对抗性指数非常高,达695.8和718.5[46]。2010年,Xu等从我国江苏、安徽和上海等多个稻区采集西来稗种群,其中部分种群对二氯喹啉酸产生了抗性,抗性指数处于3.3~66.9之间[61]。除了稗属杂草以外,马唐属的止血马唐[70]和拉拉藤屬锯锯藤Galium spurium[7173]也对二氯喹啉酸产生了抗性,关于二氯喹啉酸抗性报道已屡见不鲜[8,4473],其抗性问题非常普遍,抗性水平呈上升态势。
杂草除了对以上几种激素类除草剂产生抗性以外,对其他激素类除草剂诸如氯氨吡啶酸、氨氯吡啶酸、二氯吡啶酸、氯氟吡氧乙酸等也产生了抗性。其中对氯氨吡啶酸产生抗性的杂草主要有糙果苋[20]、虞美人[23]、藜[41],对氨氯吡啶酸产生抗性的杂草主要有翅果假吐金菊Soliva sessilis[8]、糙果苋[20]、藜[41]、黄星蓟Centaurea solstitialis[8,7476]、斑点矢车菊Centaurea stoebe ssp. micranthos[77]、野芥[8,78],对二氯吡啶酸产生抗性的杂草主要有翅果假吐金菊[8]、藜[41]、斑点矢车菊[77]、黄星蓟[74,76,79],对氯氟吡氧乙酸产生抗性的杂草主要有繁缕Stellaria media[8]、鼬瓣花[29]、地肤[2627,32,3637,39]。2017年,Crespo等对前期采集的糙果苋材料进行抗性水平分析,发现糙果苋种群FS对2,4滴、氯氨吡啶酸、氨氯吡啶酸三种激素类除草剂产生了交互抗性,抗性指数分别为52、3.9、3.6[20]。2016年,Mangin等首次报道了斑点矢车菊对二氯吡啶酸和氨氯吡啶酸的抗药性,其中对氨氯吡啶酸的抗性指数高达25 600[77]。激素类除草剂的抗性问题已不容忽视,解决杂草对激素类除草剂的抗性问题已刻不容缓。
3 杂草对激素类除草剂的抗性机理
虽然已经发现44种杂草对激素类除草剂产生了抗药性,但是由于激素类除草剂的作用机理复杂,对其抗性机制的研究一直是难点。梳理目前已获得的研究结果,激素类除草剂的抗性机理可以分为两类:即由于抗性相关基因位点突变而产生抗药性和非位点突变产生抗药性。
3.1 抗性相关基因位点突变
激素类除草剂抗性机理研究比较多的是关于二氯喹啉酸的抗性研究。现有证据表明,对二氯喹啉酸的抗药性与植物体内乙烯生物合成有关(图3)[4,6063,69,73,8184]。植物接触二氯喹啉酸后,激发ACS和ACC氧化酶(ACC oxidase,ACO EC 1.14.17.4)的活性,体内乙烯含量增加,副产物—氰化物(cyanide)大量增加,植物受到氰化物的毒害,生长受到抑制进而死亡[82]。ACS和ACO是乙烯生物合成途径中的关键酶,β氰丙氨酸合成酶(βcyanoalanine synthase,βCAS EC 4.4.1.9)是植物体内降解氰化物的关键酶,这3个酶被认为与二氯喹啉酸的抗药性紧密相关(图3)。如2015年,董明超等从二氯喹啉酸抗性和敏感生物型稗草材料中克隆ACO基因(EcACO),并通过异源表达对其功能进行验证,结果发现抗性和敏感生物型材料EcACO之间存在5个差异位点,其中3个位点处于保守区域内,敏感材料的EcACO催化乙烯生成速率是抗性材料的2.15倍,预示着ACO位点突变引起的酶活性变化可能是该型稗草产生抗性的原因[85]。
用孟德尔规律对杂草的抗性特征进行遗传分析,发现对二氯喹啉酸产生抗性的猪殃殃[72]和对二氯吡啶酸产生抗性的黄星蓟[76]的抗性性状属单基因隐性遗传。类似研究发现,抗2,4滴和氨氯吡啶酸地肤的抗性性状受显性基因控制,回交试验表明抗2,4滴、氨氯吡啶酸和麦草畏的基因可能位于相邻的连锁区域[86]。2009年,Preston等也发现抗麦草畏地肤生物型的抗性性状受单基因显性控制[26]。上述这些研究表明激素类除草剂的抗药性可能与靶标位点突变相关。目前,在拟南芥中已经发现了TIR1、AFB1、AFB2、AFB3、AFB4、AFB5等6个激素受体,这些受体发生突变或缺失后,拟南芥表现为对激素不敏感或者发育缺陷[8790],进一步研究发现蛋白AUX/IAA与ARF(生长素响应因子)参与了这一过程(图3)[6,91]。当激素或者激素类除草剂不施加作用时,AUX/IAA与ARF结合,阻遏了下游基因的表达;当激素或激素类除草剂施加作用时,SCFs复合物中的激素受体TIR/AFB识别AUX/IAA,并将其泛素化,最后泛素化的AUX/IAA被26S蛋白酶体降解,原来被阻遏的基因在ARF的作用下转录表达。激素或者激素类除草剂在TIR/AFB识别AUX/IAA的过程中起到了一个“分子胶”的作用[4](图3)。不同的TIR/AFB蛋白负责识别不同激素或激素类除草剂,TIR/AFB和AUX/IAA的突变可能导致杂草对某种激素类除草剂产生抗药性[87]。如Leclere等对敏感地肤生物型和兼抗2,4滴、氯氟吡氧乙酸、麦草畏的地肤生物型进行转录组测序,提取抗性相关基因进行比对,发现抗性和敏感生物型的IAA16存在位点差异,基因IAA16的突变导致抗药性产生[27]。
[7] PRIGGE M, GREENHAM K, ZHANG Y, et al. The Arabidopsis auxin receptor Fbox proteins AFB4 and AFB5 are required for response to the synthetic auxin picloram[J]. G3: Genes, Genomes, Genetics, 2016, 6(5): 13831390.
[8] HEAP I. International survey of herbicide resistant weeds [DB/OL]. http:∥www.weedscience.com. 20191009.
[9] PATTON A J, WEISENBERGER D V, SCHORTGEN G P. 2, 4Dresistant buckhorn plantain (Planta golanceolata) in managed turf [J]. Weed Technology, 2018, 32(2): 182189.
[10]BURKE I C, YENISH J P, PITTMANN D, et al. Resistance of a prickly lettuce (Lactuca serriola) biotype to 2, 4D [J]. Weed Technology, 2009, 23(4): 586591.
[11]STACHLER J M, KELLS J J, PENNER D. Resistance of wild carrot (Daucus carota) to 2, 4D in Michigan [J]. Weed Technology, 2000, 14(4): 734739.
[12]HALL J C, WEBB S R, DESHPANDE S. An overview of auxinic herbicide resistance: wild mustard (Sinapis arvensis L.) as a case study [J]. ACS Symposium Series, 1996, 645: 2843.
[13]YAJIMA W, HALL J C, KAV N N V. Proteomelevel differences between auxinicherbicidesusceptible andresistant wild mustard (Sinapis arvensis L.) [J]. Journal of Agricultural & Food Chemistry, 2004, 52(16): 50635070.
[14]OWEN M J, MARTINEZ N J, POWLES S B. Multiple herbicideresistant wild radish (Raphanus raphanistrum) populations dominate Western Australian cropping fields [J]. Crop and Pasture Science, 2015, 66(10): 10791085.
[15]WALSH M J, POWLES S B, BEARD B R, et al. Multipleherbicide resistance across four modes of action in wild radish (Raphanus raphanistrum) [J]. Weed Science, 2004, 52(1): 813.
[16]WALSH M J, OWEN M J, POWLES S B. Frequency and distribution of herbicide resistance in Raphanus raphanistrum populations randomly collected across the Western Australian wheatbelt [J]. Weed Research, 2007, 47(6): 542550.
[17]PRESTON C, DOLMAN F C, BOUTSALIS P. Multiple resistance to acetohydroxyacid synthaseinhibiting and auxinic herbicides in a population of oriental mustard (Sisymbrium orientale) [J]. Weed Science, 2013, 61(2): 185192.
[18]DANG H T, MALONE J M, BOUTSALIS P, et al. Reduced translocation in 2,4Dresistant oriental mustard populations (Sisymbrium orientale L.) from Australia [J]. Pest Management Science, 2017, 74(6): 15241532.
[19]BERNARDS M L, CRESPO R J, KRUGER G R, et al. A waterhemp (Amaranthus tuberculatus) population resistant to 2, 4D [J]. Weed Science, 2012, 60(3): 379384.
[20]CRESPO R J, WINGEYER A B, KRUGER G R, et al. Multipleherbicide resistance in a 2,4Dresistant waterhemp population from Nebraska [J]. Weed Science, 2017, 65(6): 743754.
[21]SHERGILL L S, BARLOW B R, BISH M D, et al. Investigations of 2,4D and multiple herbicide resistance in a Missouri waterhemp (Amaranthus tuberculatus) population [J]. Weed Science, 2018, 66(3): 386389.
[22]DELLAFERRERA I, CORTS E, PANIGO E, et al. First report of Amaranthus hybridus with multiple resistance to 2, 4D, dicamba, and glyphosate [J/OL]. Agronomy, 2018, 8(8): 140.DOI:10.3390/agronomy8080140.
[23]REYCABALLERO J, MENNDEZ J, GINBORDONABA J, et al. Unravelling the resistance mechanisms to 2,4D (2,4dichlorophenoxyacetic acid) in corn poppy (Papaver rhoeas) [J]. Pesticide Biochemistry and Physiology, 2016, 133: 6772.
[24]JOEL T, ROJANODELGADO A M, JORDI R C, et al. Enhanced 2,4D metabolism in two resistant Papaver rhoeas populations from Spain [J/OL]. Frontiers in Plant Science, 2017, 8:1584.DOI:10.3389/fpls.2017.01584.
[25]KATI V, SCARABEL L, THIERYLANFRANCHI D, et al. Multiple resistance of Papaver rhoeas L. to 2,4D and acetolactate synthase inhibitors in four European countries [J]. Weed Research, 2019, 59(5): 367376.
[26]PRESTON C, BELLES D S, WESTRA P H, et al. Inheritance of resistance to the auxinic herbicide dicamba in kochia (Kochia scoparia) [J]. Weed Science, 2009, 57(1): 4347.
[27]LECLERE S, WU Chenxi, WESTRA P, et al. Crossresistance to dicamba, 2, 4D, and fluroxypyr in Kochia scoparia is endowed by a mutation in an AUX/IAA gene [J]. Proceedings of the National Academy of Sciences, 2018, 115(13): 29112920.
[28]NANDULA V K, MANTHEY F A. Response of kochia (Kochia scoparia) inbreds to 2,4D and dicamba [J]. Weed Technology, 2002, 16(1): 5054.
[29]WEINBERG T, STEPHENSON G R, MCLEAN M D, et al. MCPA(4chloro2ethylphenoxy acetate)resistance in hempnettle (Galeopsis terahit L.) [J]. Journal of Agricultural and Food Chemistry,2006, 54(24): 91269134.
[30]CRANSTON H J, KERN A J, HACKETT J L, et al. Dicamba resistance in kochia [J]. Weed Science, 2001, 49(2): 164170.
[31]CRESPO R J, BERNARDS M L, SBATELLA G M, et al. Response of Nebraska kochia (Kochia scoparia) accessions to dicamba [J]. Weed Technology, 2014, 28(1): 151162.
[32]GOSS G A, DYER W E. Physiological characterization of auxinic herbicideresistant biotypes of kochia (Kochia scoparia) [J]. Weed Science, 2003, 51(6): 839844.
[33]KERN A J, CHAVERRA M E, CRANSTON H J, et al. Dicambaresponsive genes in herbicideresistant and susceptible biotypes of kochia (Kochia scoparia) [J]. Weed Science, 2005, 53(2): 139145.
[34]HOWATT K A, PHILIP W, NISSEN S J. Ethylene effect on kochia (Kochia scoparia) and emission following dicamba application [J]. Weed Science, 2006, 54(1): 3137.
[35]VARANASI V K, GODAR A S, CURRIE R S, et al. Fieldevolved resistance to four modes of action of herbicides in a single kochia (Kochia scoparia L. Schrad.) population [J]. Pest Management Science, 2015, 71(9): 12071212.
[36]JHA P, KUMAR V, LIM C A. Variable response of kochia [Kochia scoparia (L.) Schrad.] to auxinic herbicides dicamba and fluroxypyr in Montana [J]. Canadian Journal of Plant Science, 2015, 95(5): 965972.
[37]KUMAR V, JHA P. Differences in germination, growth, and fecundity characteristics of dicambafluroxypyrresistant and susceptible Kochia scoparia [J/OL]. PLoS ONE, 2016, 11(8): e0161533.DOI:10.1371/journal.pone.016133.
[38]PETTINGA D J, OU J, PATTERSON E L, et al. Increased chalcone synthase (CHS) expression is associated with dicamba resistance in Kochia scoparia [J]. Pest Management Science, 2018, 74(10): 23062315.
[39]KUMAR V, CURRIE R S, JHA P, et al. First report of kochia (Bassia scoparia) with crossresistance to dicamba and furoxypyr in Western Kansas [J]. Weed Technology, 2019, 33(2): 335341.
[40]GHANIZADEH H, HARRINGTON K C, JAMES T K, et al. A quick test using seeds for detecting dicamba resistance in fathen (Chenopodium album) [J]. Australian Journal of Crop Science, 2015, 9(4): 337343.
[41]GHANIZADEH H, HARRINGTON K C. Crossresistance to auxinic herbicides in dicambaresistant Chenopodium album [J]. New Zealand Journal of Agricultural Research, 2017, 60(1): 4553.
[42]GHANIZADEH H, HARRINGTON K C, JAMES T K. A comparison of dicamba absorption, translocation and metabolism in Chenopodium album populations resistant and susceptible to dicamba [J]. Crop Protection, 2018, 110: 112116.
[43]MEIKLE A, FINCH R P, MCROBERTS N, et al. A molecular genetic assessment of herbicideresistant Sinapis arvensis [J]. Weed Research, 1999, 39(2): 149158.
[44]LOPEZMARTINEZ N, MARSHALL G, DEPRADO R. Resistance of barnyardgrass(Echinochloa crusgalli) to atrazine and quinclorac [J]. Pesticide Science, 1997, 51(2): 171175.
[45]李拥兵, 黃华枝, 黄炳球, 等. 我国中部和南方稻区稗草对二氯喹啉酸的抗药性研究[J].华南农业大学学报, 2002, 23(2): 3336.
[46]吴声敢, 赵学平, 吴长兴, 等. 我国长江中下游稻区稗草对二氯喹啉酸的抗药性研究[J]. 杂草科学, 2007, 27(3): 2526.
[47]MALIK M S, BURGOS N R, TALBERT R E. Confirmation and control of propanilresistant and quincloracresistant barnyardgrass (Echinochloa crusgalli) in rice [J]. Weed Technology, 2010, 24(3): 226233.
[48]RAHMAN M M, SAHID I B, JURAIMI A S. Study on resistant biotypes of Echinochloa crusgalli in Malaysia [J]. Australian Journal of Crop Science, 2010, 4(2): 106114.
[49]俞欣妍, 葛林利, 劉丽萍, 等. 直播稻田稗草对二氯喹啉酸, 氰氟草酯与双草醚除草剂复合抗性的初步研究[J]. 江苏农业学报, 2010, 26(6): 14381440.
[50]杨彩,冯莉, 杨红梅, 等. 稻田稗草对丁草胺和二氯喹啉酸抗药性的测定[J]. 农药, 2011, 50(8): 606607.
[51]常向前, 张舒, 余柳青, 等. 湖北省稻田稗草对二氯喹啉酸的抗性及生物学特性观察[J]. 湖北农业科学, 2011(24): 109111.
[52]马国兰, 余柳青, 刘都才, 等. 湖南稻区稗草对二氯喹啉酸的抗性研究[J]. 杂草科学, 2012, 30(1): 2225.
[53]YANG Xia, YU Xinyan, LI Yongfeng, et al. De novo assembly and characterization of the barnyardgrass (Echinochloa crusgalli) transcriptome using nextgeneration pyrosequencing [J/OL]. PLoS ONE, 2013, 8(7): e69168.DOI:10.1371/journal.pone.0069168.
[54]LI Gang, WU Shenggan, CAI Leiming, et al. Identification and mRNA expression profile of glutamate receptorlike gene in quincloracresistant and susceptible Echinochloa crusgalli [J]. Gene, 2013, 531(2): 489495.
[55]马国兰, 柏连阳, 刘都才, 等. 我国长江中下游稻区稗草对二氯喹啉酸的抗药性研究[J]. 中国水稻科学, 2013, 27(2): 184190.
[56]MATZENBACHER F O, BORTOLY E D, KALSING A, et al. Distribution and analysis of the mechanisms of resistance of barnyardgrass (Echinochloa crusgalli) to imidazolinone and quinclorac herbicides [J]. Journal of Agricultural Science, 2015, 153(6): 115.
[57]张纪利, 吴尚, 李保同, 等. 江西省稻田稗草对丁草胺和二氯喹啉酸的抗药性测定[J]. 杂草科学, 2015(3): 2933.
[58]WU Lamei, FANG Yong, YANG Haona, et al. Effects of droughtstress on seed germination and growth physiology of quincloracresistant Echinochloa crusgalli [J/OL]. PLoS ONE, 2019, 14(4): e0214480.DOI:10.1371/journal.pone.0214480.
[59]MARCHESI C, SALDAIN N E. First report of herbicideresistant Echinochloa crusgalli in Uruguayan rice fields [J]. Agronomy, 2019, 9(12): 790.
[60]PENG Qiong, HAN Heping, YANG Xia. Quinclorac resistance in Echinochloa crusgallifrom China [J]. Rice Science, 2019, 26(5): 300308.
[61]XU Jiangyan, LYU Bo, WANG Qiong, et al. A resistance mechanism dependent upon the inhibition of ethylene biosynthesis [J]. Pest Management Science, 2013, 69(12): 14071414.
[62]GAO Yuan, PAN Lang, SUN Yu, et al. Resistance to quinclorac caused by the enhanced ability to detoxify cyanide and its molecular mechanism in Echinochloa crusgalli var. zelayensis [J]. Pesticide Biochemistry and Physiology, 2017, 143: 231238.
[63]GAO Yuan, LI Jun, PAN Xukun. Quinclorac resistance induced by the suppression of the expression of 1aminocyclopropane1carboxylic acid(ACC) synthase and ACC oxidase genes in Echinochloa crusgalli var. zelayensis [J]. Pesticide Biochemistry and Physiology, 2018, 146: 2532.
[78]MITHILA J, HALL J C. Production of an auxinic herbicideresistant microsporederived doubled haploid wild mustard (Sinapis arvensis L.) plant [J]. Crop Protection, 2007, 26(3): 357362.
[79]VALENZUELAVALENZUELA J M, LOWNDS N K, STERLING T M. Clopyralid uptake, translocation, metabolism, and ethylene induction in picloramresistant yellow starthistle (Centaurea solstitialis L.) [J]. Pesticide Biochemistry and Physiology, 2001, 71(1): 1119.
[80]LUSK C S, HURRELL G A, HARRINGTON K C, et al. Resistance of Ranunculus acris to flumetsulam, thifensulfuronmethyl and MCPA in New Zealand dairy pastures [J]. New Zealand Journal of Agricultural Research, 2015, 58(3): 271280.
[81]GROSSMANN K, SCHELTRUP F. Selective induction of 1aminocyclopropane1carboxylic acid (ACC) synthase activity is involved in the selectivity of the auxin herbicide quinclorac between barnyard grass and rice [J]. Pesticide Biochemistry and Physiology, 1997, 58(2):145153.
[82]GROSSMANN K, KWIATKOWSKI J. The mechanism of quinclorac selectivity in grasses [J]. Pesticide Biochemistry and Physiology, 2000, 66(2): 8391.
[83]GROSSMANN K. Mode of action of auxin herbicides: A new ending to a long, drawn out story [J]. Trends in Plant Science, 2001, 5(12): 506508.
[84]GROSSMANN K. Auxin herbicide action: lifting the veil step by step [J]. Plant Signaling & Behavior, 2007, 2(5): 421423.
[85]董明超, 楊霞, 张自常, 等. 抗性稗草1氨基环丙烷1羧酸氧化酶基因的克隆与表达分析[J]. 中国农业科学, 2015, 48(20): 40774085.
[86]JUGULAM M, MCLEAN M D, HALL J C. Inheritance of picloram and 2, 4D resistance in wild mustard (Brassica kaber) [J]. Weed Science, 2005, 53(4): 417423.
[87]MOCKAITIS K, ESTELLE M. Auxin receptors and plant development: a new signaling paradigm [J]. Annual Review of Cell and Developmental Biology, 2008, 24(1): 5580.
[88]DHARMASIRI N, DHARMASIRI S, ESTELLE M. The Fbox protein TIR1 is an auxin receptor [J]. Nature, 2005, 435(7041): 441445.
[89]KEPINSKI S, LEYSER O. The Arabidopsis Fbox protein TIR1 is an auxin receptor [J]. Nature, 2005, 435(7041): 446451.
[90]DHARMASIRI N, DHARMASIRI S, WEIJERS D, et al. Plant development is regulated by a family of auxin receptor F box proteins [J]. Developmental Cell, 2005, 9(1): 109119.
[91]XU Tan, CALDERONVILLALOBOS L I A, SHARON M, et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase [J]. Nature, 2007, 446(7136): 640645.
[92]RIAR D S, BURKE I C, YENISH J P, et al. Inheritance and physiological basis for 2,4D resistance in prickly lettuce (Lactuca serriola L.) [J]. Journal of Agricultural and food Chemistry, 2011, 59(17): 94179423.
[93]GOGGIN D E, CAWTHRAY G R, POWLES S B. 2,4D resistance in wild radish: reduced herbicide translocation via inhibition of cellular transport [J]. Journal of Experimental Botany, 2016, 67(11): 32233235.
[94]LOVELACE M L, TALBERT R E, HOAGLAND R E, et al. Quinclorac absorption and translocation characteristics in quincloracand propanilresistant and susceptible barnyardgrass (Echinochloa crusgalli) biotypes [J]. Weed Technology, 2007, 21(3): 683687.
[95]CHAYAPAKDEE P, SUNOHARA Y, ENDO M, et al. Quinclorac resistance in Echinochloa phyllopogon is associated with reduced ethylene synthesis rather than enhanced cyanide detoxification by βcyanoalanine synthase [J]. Pest Management Science, 2020, 76(4): 11951204.
[96]LI Gang, XU Mingfeng, CHEN Liping, et al. A novel EcGH3 gene with a different expression pattern in quincloracresistant and susceptible barnyardgrass (Echinochloa crusgalli) [J]. Plant Gene, 2016, 5: 6570.
[97]WALSH M J, MAGUIRE N, POWLES S B. Combined effects of wheat competition and 2,4D amine on phenoxy herbicide resistant Raphanus raphanistrum populations [J]. Weed Research, 2009, 49(3): 316325.
[98]WALSH T A, NEAL R, MERLO A O, et al. Mutations in an auxin receptor homolog AFB5 and in SGT1b confer resistance to synthetic picolinate auxins and not to 2, 4dichlorophenoxyacetic acid or indole3acetic acid in Arabidopsis [J]. Plant Physiology, 2006, 142(2): 542552.
(責任编辑:田 喆)
