SARS-CoV-2 may impair pancreatic function via basigin: A single-cell transcriptomic study of the pancreas
2021-03-09QiuRanWang
Qiu-Ran Wang
School of Basic Medical Sciences, Fujian Medical University, Fuzhou China
The coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 infection is a major threat to global public health. SARS-COV-2 not only activates the human immune system but also causes severe damage to multiple organs by binding to specific virus receptors[1].Basigin (BSG) is considered as the second important target for SARS-CoV-2 invasion after angiotensin converting enzyme 2(ACE2)[2], which may mediate a variety of tissue damage, such as in the uterus[3]and brain[4]. Some patients infected with SARSCoV-2 were reported to show pancreatic injuries or worsening of diabetes[5], but the pathogenic mechanism remains uncertain.Given the low level of ACE2 expression in the pancreatic tissue,we hypothesized that this pathogenicity process is mediated by other potential virus-binding receptors except ACE2, such as BSG.Therefore, we attempted to test this hypothesis through the analysis of sRNA-seq data.
The PanglaoDB database (https://panglaodb.se/)[6]was selected to retrieve published scRNA-seq datasets. Each selected dataset contains more than 2500 cells. First, the downloaded R data were converted to Seurat object. Then, the raw gene expression matrix was normalized with default parameters. The ‘FindVariableFeatures’function was used to calculate the set of genes that showed the highest variation between individual cells. Each dataset returned 2000 genes. After scaling the data related to 2000 genes by the ScaleData function, the principal component analysis (PCA) was used to reduce the dimension of scaling. The ‘Find Clusters’ function was used to set different resolution parameters. Next, the data was inputted into t-SNE and visualized in two dimensions. The most matching cluster was selected from multiple clusters generated by different parameters, and marker genes provided by the PanglaoDB database website were used to annotate the cells. Scatter plots,violin plots and the dot-plot were drawn for SARS-CoV-2 receptorrelated genes to show their transcription levels and distributions in different types of cells.In this study, three datasets were selected and combined:pancreatic islets (SRA701877:SRS3279692), pancreatic islets (SRA701877:SRS3279693), and pancreatic islets
(SRA701877:SRS3279695). The samples sequenced from each dataset contained similar cell types. We found low transcript levels of ACE2, transmembrane protease serine 2 (TMPRSS2),and dipeptidyl peptidase-4 (DPP4) in pancreatic cells. In contrast,BSG was highly expressed in most functional cell types of the pancreas (Figure 1), and BSG had the highest transcript level in the pancreatic α-cell group, followed by the PP cell group. Although the mRNA expression of BSG was not high in pancreatic β-cells,the proportional distribution of BSG indicated that most pancreatic β-cells contained BSG transcripts.
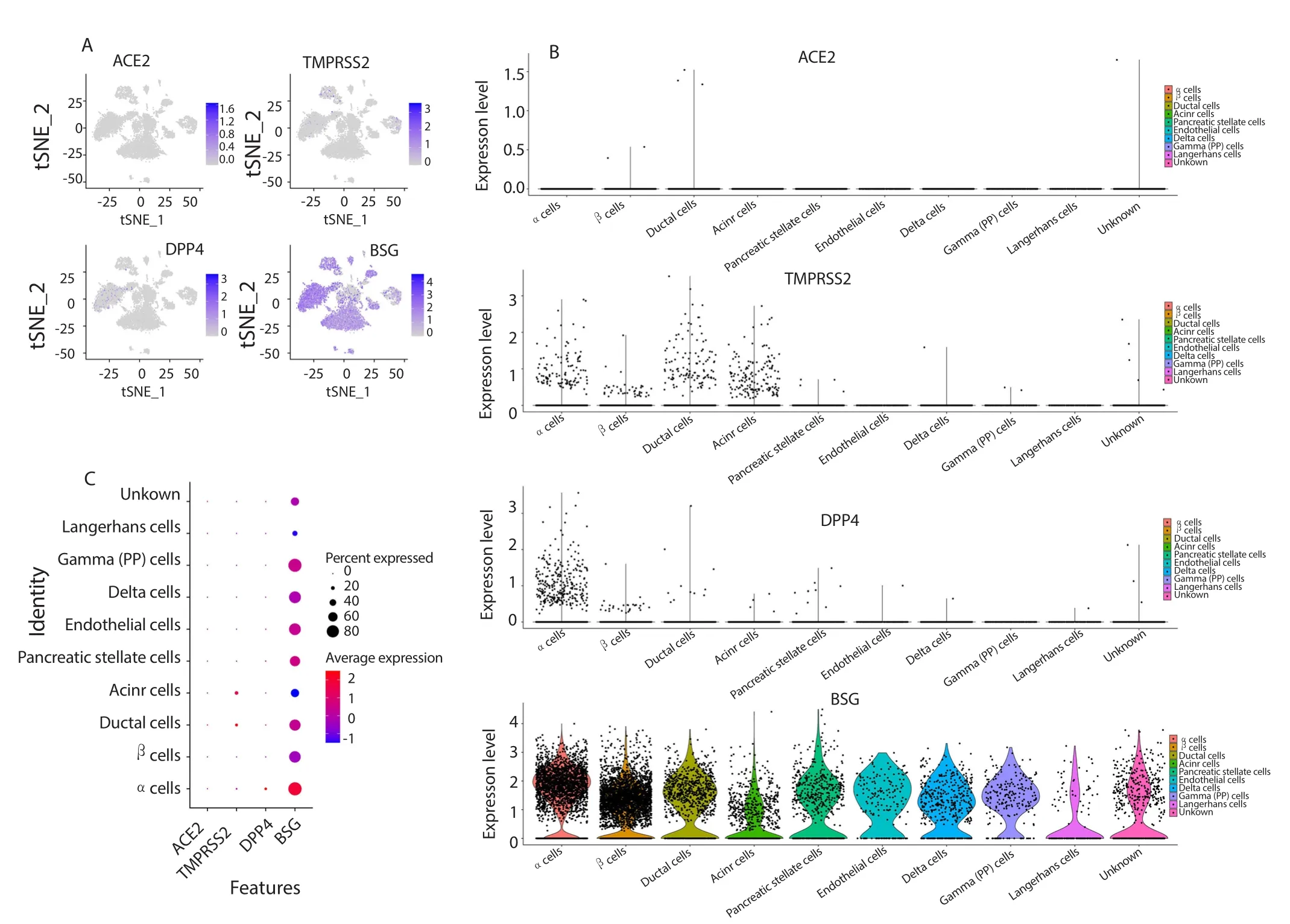
Figure 1. The expression level of ACE2, TMPRSS2, DDP4, and BSG in different cell types of human pancreas islet. (A) Scatter plots are visualized by RStudio software after data merging. The points colored blue are the cells expressing ACE2, TMPRSS2, DDP4 and BSG. (B) Violin plots show the gene expression level of ACE2, TMPRSS2, DDP4 and BSG in different cell types. (C) The size of the dot reflects the percentage of cells expressing a certain gene within a cell type, and color reflects the average expression level.
Compared to the other virus-receptors, BSG is widely distributed in pancreatic cells, which suggests that SARS-CoV-2 may affect pancreatic cells via this receptor. Since diabetes patients are susceptible to SARS-CoV-2 infections, and most pancreatic β-cells contained BSG transcripts, SARS-CoV-2 infection may further lead to the progression of diabetes. Thus, medical staff should pay more attention to diabetes patients who are in higher risk of getting this infection, and treat basic diseases as soon as possible to avoid the overlapping impacts of SARS-CoV-2 infection and diabetes.
Conflict of interest statement
The author declares that there are no conflicts of interest.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Prevalence of cryptosporidiosis in animals in Iran: A systematic review and metaanalysis
- Prevalence and intensity of soil-transmitted helminth infections among school-age children in the Cagayan Valley, the Philippines
- How knowledge of hepatitis B disease and vaccine influences vaccination practices among parents in Ho Chi Minh City, Vietnam
- In vitro anti-plasmodial activity of new synthetic derivatives of 1-(heteroaryl)-2-((5-nitroheteroaryl)methylene) hydrazine
- Burkholderia pseudomallei infection manifests as mediastinal/hilar lymphadenopathy:A case report
