初花期淹水胁迫对大豆叶片AsA-GSH循环的损伤及烯效唑的缓解效应
2021-03-08王诗雅郑殿峰项洪涛冯乃杰刘雅刘美玲靳丹牟保民
王诗雅,郑殿峰,项洪涛,冯乃杰,刘雅,刘美玲,靳丹,牟保民
初花期淹水胁迫对大豆叶片AsA-GSH循环的损伤及烯效唑的缓解效应

1广东海洋大学滨海农业学院,广东湛江 524088;2黑龙江八一农垦大学农学院,黑龙江大庆 163319;3黑龙江省农业科学院耕作栽培研究所,哈尔滨 150086

大豆;淹水胁迫;AsA-GSH循环;叶片;烯效唑
0 引言
【研究意义】大豆()是世界上最重要的豆科作物之一[1],在黑龙江种植较为广泛[2]。黑龙江省属典型的雨养农业种植地区,是气候变化敏感区和生态脆弱区,异常降雨频率增加,农田涝渍灾害多发,特别是7—8月正值大豆生殖生长期,对大豆生长发育构成严重威胁[3-4]。初花期(R1)是大豆的水分敏感期,也是最易受涝灾损伤的时期,研究R1期大豆涝灾损伤机制并寻求解决方案,是一个重要的现实和科学问题,意义重大。涝渍胁迫会使作物处于缺氧状态,导致作物体内活性氧的快速积累及膜脂过氧化程度加剧,抑制作物的株高、茎粗和生物量积累[5]。植物生长调节剂是一类人工合成并与植物激素相类似的活性物质,可直接调控作物本身,使作物生长发育得到定向控制,在增强作物的抗逆能力方面效果显著[6-8]。因此,研究淹水胁迫下喷施调节剂,对提高大豆的耐涝性具有重要意义。【前人研究进展】正常情况下,植物体内ROS水平在抗氧化酶的调节下维持在恒定水平,但在严重的逆境胁迫下,ROS的产生和清除机制将失去平衡,导致植物细胞受损[9-10]。抗坏血酸-谷胱甘肽(AsA-GSH)循环是植物体内存在的一个重要抗氧化保护系统,AsA和GSH是2种重要的抗氧化剂,参与循环中的反应,并与循环中的关键酶(APX、GR、MDHAR、DHAR)共同作用,可有效地清除逆境胁迫所产生的活性氧(ROS)和过氧化氢(H2O2)[11-12]。AsA-GSH循环作为重要的抗氧化代谢途径之一,在许多逆境胁迫下,如镉胁迫[13]、干旱胁迫[14]、盐胁迫[15]等中得到验证。但随胁迫时间的延长,作物体内的ROS清除剂能力下降,导致ROS代谢失调,ROS的过量积累导致脂质过氧化和蛋白质氧化,致使细胞完整性丧失,影响叶片的正常生长[16]。目前,植物生长调节剂的理论研究及其在生产上的应用越来越多,已成为作物优质、高产、高效栽培中的一项重要技术措施[17]。在逆境胁迫下,喷施植物生长调节剂可有效缓解逆境对作物所造成的伤害,前人已对大豆[18]、绿豆[19]、芸豆[20]和水稻[21]等作物进行了相关报道。植物生长调节剂包括促进型植物生长调节剂和延缓型植物生长调节剂等[22],其中,延缓型植物生长调节剂烯效唑(uniconazole,S3307)被广泛应用[23],S3307为三唑类植物生长调节剂,可提高植物在逆境胁迫下的抵抗能力,包括低氧[24]、高温[25]、盐胁迫[26]和干旱[27]等。项洪涛等[28]研究指出,叶面喷施S3307可通过降低MDA含量,提高保护酶活性等生理过程,抵御低温胁迫对红小豆造成的伤害。AHMED等[29]研究表明,S3307可提高植株内SOD、POD等相关保护酶活性,减少MDA的生成量和积累量提高细胞膜透性,进而增强细胞膜对抗逆境的应激防御能力。另有研究指出,S3307处理能够有效缓解盐胁迫对大豆叶片细胞膜的伤害[30]。目前,研究表明S3307可提高淹水胁迫下大豆体内抗氧化酶SOD和POD活性,降低体内膜脂过氧化物MDA含量,减少淹水胁迫对大豆造成的生理伤害[5],但关于S3307缓解淹水胁迫下AsA-GSH循环损伤的研究尚未见报道。【本研究切入点】目前,淹水胁迫对作物AsA-GSH循环的影响研究较少,且关于S3307缓解大豆淹水伤害的研究更是鲜见。【拟解决的关键问题】通过探究R1淹水胁迫对耐涝性不同的2个大豆品种叶片内活性氧和AsA-GSH循环中关键酶和抗氧化剂含量的影响,以及喷施S3307后对其调控效应,旨在揭示S3307增强大豆耐涝性的作用机理,以期为大豆的耐涝栽培提供理论依据。
1 材料与方法
1.1 试验材料
选用大豆品种垦丰14和垦丰16为试验材料,其中垦丰14为耐涝品种,垦丰16为涝渍敏感品种[5,31]。
供试生长调节剂选用烯效唑(S3307),由黑龙江八一农垦大学植物生长调节剂工程技术研究中心提供。
1.2 试验设计
于2019年在黑龙江八一农垦大学国家杂粮工程技术研究中心盆栽场进行盆栽试验。选用带有隔水层和排水口的树脂花盆(上口径×底径×高= 32 cm×23 cm×31 cm),试验用土按栽培土﹕腐殖土﹕沙子=7﹕2﹕1体积比例混合组成,每盆装土18 kg。5月19日进行播种,每个品种设正常水分管理(CK)、淹水胁迫处理(W)和淹水胁迫+S3307处理(W+S),采用完全随机区组设计。每盆播种10粒,子叶期定苗5株,具体设计见表1。待植株生长至R1期(播种后53 d),进行叶面喷施S3307(浓度为50 mg·L-1,喷施量为225 L·hm-2),并于喷药后第5天(播种后58 d,淹水胁迫处理第0天,记作R1+5)进行淹水处理(套盆淹水,水淹没土表面,高于土面2—3 cm为准),淹水持续5 d(淹水第5天为播种后第63天,记作R1+10),淹水胁迫处理5 d后(播种后68 d,记作R1+15)放水恢复正常水分管理。处理期间,分别对R1+5、R1+10、R1+15 3个时间点进行取样,各处理取样后立即放入液氮中,而后置于-80℃冰箱中保存,供生理指标测定。

表1 试验设计方案
1.3 测定项目与方法

超氧阴离子产生速率(superoxide anion production rates,)采用羟胺氧化法[33-34]进行测定。取0.5 ml提取液,加入0.5 ml PBS(0.05 mol·L-1,pH 7.8),1 ml 10 mmol·L-1盐酸羟胺溶液后摇匀,同时对照管以PBS代替样品提取液;在25℃下保温1 h,保温后需加入2 ml乙醚萃取叶绿素;依次加入1ml 17 mmol·L-1对氨基苯磺酸,1ml 7 mmol·L-1a-萘胺,混合后涡旋,在25℃下保温20 min后3 000×离心3 min,在530 nm下测定OD值。
过氧化氢(hydrogen peroxide,H2O2)含量采用碘化钾法[35]测定。取1 g植物样品,加入0.5 mL的0.1% TCA,在液氮下研磨,研磨后所得匀浆以19 000×离心20 min。取上清液0.5 mL,加2 ml 1 mol·L-1KI溶液和0.5 ml 100 mmol·L-1硫酸钾的缓冲液,暗反应1 h;反应结束后,以0.1%的TCA为参比,在390 nm下测定OD值。
1.3.2 AsA和DHA含量的测定 还原型抗坏血酸(ascorbic acid,AsA)和氧化型抗坏血酸(dehydroascorbate,DHA)采用Zhang等[36]方法测定。称取1 g样品置于研钵中,加入5 mL的5%的偏磷酸,在4℃下研磨,所得匀浆于8 000×离心15 min,收集上清液用于测定(AsA+DHA)和AsA的含量。取0.3 mL上清液,加入0.75 mL含5 mmol.L-1EDTA的磷酸缓冲液(150 mmol·L-1,pH 7.4)和0.15 mL 10 mmol·L-1的二硫苏糖醇溶液(DTT)。室温下放置10 min后,加入0.15 mL 0.5% N-乙基马来酰亚胺以消除多余的DTT。加入0.6 mL的10%三氯乙酸(TCA)、0.6 mL的44%正磷酸溶液、0.6 mL的0.5% BP-乙醇溶液和0.15 mL的0.3%(W/V)FeCl3溶液。混匀后40℃水浴40 min,测525 nm处AsA+DHA的OD值。AsA的测定过程中以0.3 mL水代替DTT和N-乙基马来酰亚胺,其余操作步骤如上所述。DHA为AsA+DHA与AsA的差值。
1.3.3 GSH和GSSG含量的测定 还原型谷胱甘肽(glutathione,GSH)和氧化型谷胱甘肽(glutathiol,GSSG)含量测定采用二硫代硝基苯甲酸法[34,37]测定。取植物叶片1.0 g,加5 ml的5%偏磷酸溶液,在4℃下研磨,8 000 r/min离心25 min,上清液供测定,5℃保存。取样品母液0.2 ml,加入150 mmol·L-1磷酸二氢钠溶液(pH 7.7)2.6 ml,混匀后,加入0.2 ml DTNB溶液,在30℃保温10 min,测定412 nm 下的OD值。GSSG的测定需要在反应体系中加入0.1 mmol·L-12-乙烯吡啶,在27℃反应1 h,计算GSSG的含量。
1.3.4 APX、GR、MDHAR和DHAR活性的测定 过氧化氢酶(aseorbateperoxidase,APX)参考高俊凤[34]的测定方法。称取0.5 g样品,分3次加入10 ml 50 mmol·L-1预冷的磷酸缓冲液(pH 7.8),在冰浴上研磨成匀浆,转入离心管中,在4℃、12 000×下离心20 min,上清夜即为APX粗提液。以3 ml体系测定,取0.10 ml酶液,加入2.60 ml含0.1 mmol·L-1乙二胺四乙酸二钠的PBS(0.05 mol·L-1,pH 7.0),再加入0.15 ml 5 mmol·L-1的AsA,最后加入0.15 ml 20 mmol·L-1H2O2,立即在20℃下测定290 nm下每30 sOD值的变化。
谷胱甘肽还原酶(glutathione reductase,GR)根据ZHU等[38]和Wheeler等[39]方法测定,称取0.2 g样品,分3次加入1.6 ml(0.6、0.5、0.5 ml)50 mmol·L-1预冷的磷酸缓冲液(pH 7.8),在冰浴上研磨成匀浆,转入离心管中,在4℃、12 000×下离心20 min,上清液即为GR粗提液。取样品上清液0.1 ml,放入试管中,加入Hepes 1.7 ml,NADPH 0.1 μl及GSSG 0.1 μl,在340 nm下测定OD值。
(3)矿山企业规模越大,工业总产值越高。2017年河北省持证矿山企业大型、中型、小型及小矿工业总产值依次为379.23亿元、130.34亿元、36.37亿元及3.62亿元。数据显示矿山企业规模越大、工业总产值越高。
脱氢抗坏血酸还原酶(dehydroascorbatereductase,DHAR)根据Murshed等[40]的方法测定。称取0.8 g植株样品,在50 mmol·L-1,pH7.5的磷酸缓冲液中研磨成匀浆,定容至8 ml,4℃、12 000×下离心20 min,提取上清液。3 ml的反应体系含有100 mmol·L-1Hepes-KOH(pH 7.0)、2.5 mmol·L-1H2O2、0.5 mmol·L-1AsA及0.05 ml酶提取液,测定290 nm处OD值的变化。
APX、GR、DHAR酶活性(U·g-1·min-1)=(△340×0)/(0.01×1××FW)。其中,△340为时间内吸光值变化的代数和,0表示酶提取液体积,1表示测定时酶液体积,表示为测定变化时间,FW表示样品鲜重。以每分钟OD值变化0.01为1个酶活性单位(U)。
单脱氢抗坏血酸还原酶(monodehydroascorbate reductase,MDHAR)采用试剂盒法(Solarbio公司)。
1.4 数据处理
采用Microsoft Excel 2013进行数据录入及整理、用SPSS 25和Origin 2018进行数据统计分析及制图。
2 结果
2.1 烯效唑对R1期淹水胁迫下大豆叶片膜脂过氧化程度的影响
R1+5 时,W+S处理有效降低了垦丰14和垦丰16叶片中MDA含量,但与CK相比未达显著差异水平。R1+10 时,W处理的2个大豆品种叶片MDA含量与CK相比显著增加,其中,垦丰14增加60.48%,垦丰16增加70.09%;与W处理相比,W+S处理显著降低了2个大豆品种叶片内MDA含量,其中垦丰14降低4.57%,垦丰16降低32.79%。恢复正常水分后(R1+15),2个品种W处理的MDA含量有所降低,但仍显著高于CK;W+S处理后2个品种大豆叶片恢复能力快于W处理,其中垦丰16 W+S处理恢复至CK水平,说明叶面喷施S3307能够缓解活性氧对膜脂过氧化程度的损伤,在淹水胁迫下效果显著(图1)。
2.2 烯效唑对R1期淹水胁迫下大豆叶片产生速率和H2O2含量的影响
R1+5时,S3307喷施抑制了2个大豆品种叶片内产生速率和H2O2含量的积累,其中,垦丰14分别较CK显著降低10.21%和4.35%,垦丰16较CK显著降低9.36%和6.04%(图2)。R1+10 时,2个大豆品种叶片中产生速率和H2O2含量上升趋势一致,淹水胁迫显著增加了2个大豆品种叶片内产生速率和H2O2含量;S3307喷施可有效抑制2个大豆品种叶片内产生速率和H2O2含量的积累,其中,垦丰14较W处理显著降低6.48%和3.65%,垦丰16较W处理显著降低18.78%和2.46%。恢复正常水分后(R1+15),2个品种W处理的产生速率和H2O2含量明显降低,但仍显著高于CK,S3307可进一步缓解产生速率和H2O2含量的积累,且垦丰14的降幅大于垦丰16,说明耐涝品种受淹水胁迫造成的伤害小于涝渍敏感品种,S3307对维持淹水胁迫条件下ROS代谢平衡具有积极作用(图2)。

不同小写字母表示处理间在0.05水平差异显著。下同Different small letters indicate significantly different at 0.05 probability level. The same as below
2.3 烯效唑对R1期淹水胁迫下大豆叶片AsA和DHA含量的影响
R1+5时,垦丰14和垦丰16 W+S处理与各自对照CK相比,提高了2个大豆品种叶片内AsA、DHA和AsA+DHA含量。R1+10时,垦丰14和垦丰16各处理叶片的AsA、DHA和AsA+DHA含量均表现为W+S>W>CK,且差异达到显著水平。恢复正常水分后(R1+15),垦丰14和垦丰16各处理与R1+10时相比呈相反变化趋势,但垦丰14和垦丰16 AsA、DHA和AsA+DHA含量高低顺序与R1+10时相同,且各处理之间达差异显著水平。说明S3307可促进淹水胁迫下叶片内AsA、DHA和AsA+DHA含量的增加,并在恢复正常水分处理后延缓其含量的降低,以缓解淹水造成的伤害(图3)。
2.4 烯效唑对R1期淹水胁迫下大豆叶片GSH和GSSG含量的影响
R1+5时,垦丰14 W+S处理叶片内的GSH、GSSG和GSH+GSSG含量显著高于CK,分别较CK增加6.23%、7.80%和6.70%;垦丰16 W+S处理分别较CK增加8.65%、1.24%和6.05%,差异显著。R1+10时,垦丰14和垦丰16叶片内GSH、GSSG和GSH+GSSG含量的趋势表现为W+S>W>CK,方差分析表明各处理之间差异显著,说明S3307在淹水胁迫下可显著提高叶片内GSH、GSSG和GSH+GSSG含量。恢复正常水分后(R1+15),垦丰14和垦丰16 W和W+S处理的GSH、GSSG和GSH+GSSG含量迅速降低,但2个处理上述指标含量高低与R1+10时趋势相同,说明叶面喷施S3307具有延缓淹水胁迫下大豆叶片内GSH、GSSG和GSH+GSSG含量减少的作用(图4)。
2.5 烯效唑对R1期淹水胁迫下大豆叶片关键酶活性的影响

图2 烯效唑对R1期淹水胁迫下大豆叶片产生速率和H2O2含量的影响

图3 烯效唑对R1期淹水胁迫下大豆叶片AsA、DHA和AsA+DHA含量的影响

图4 烯效唑对R1期淹水胁迫下大豆叶片GSH、GSSG和GSH+GSSG含量的影响
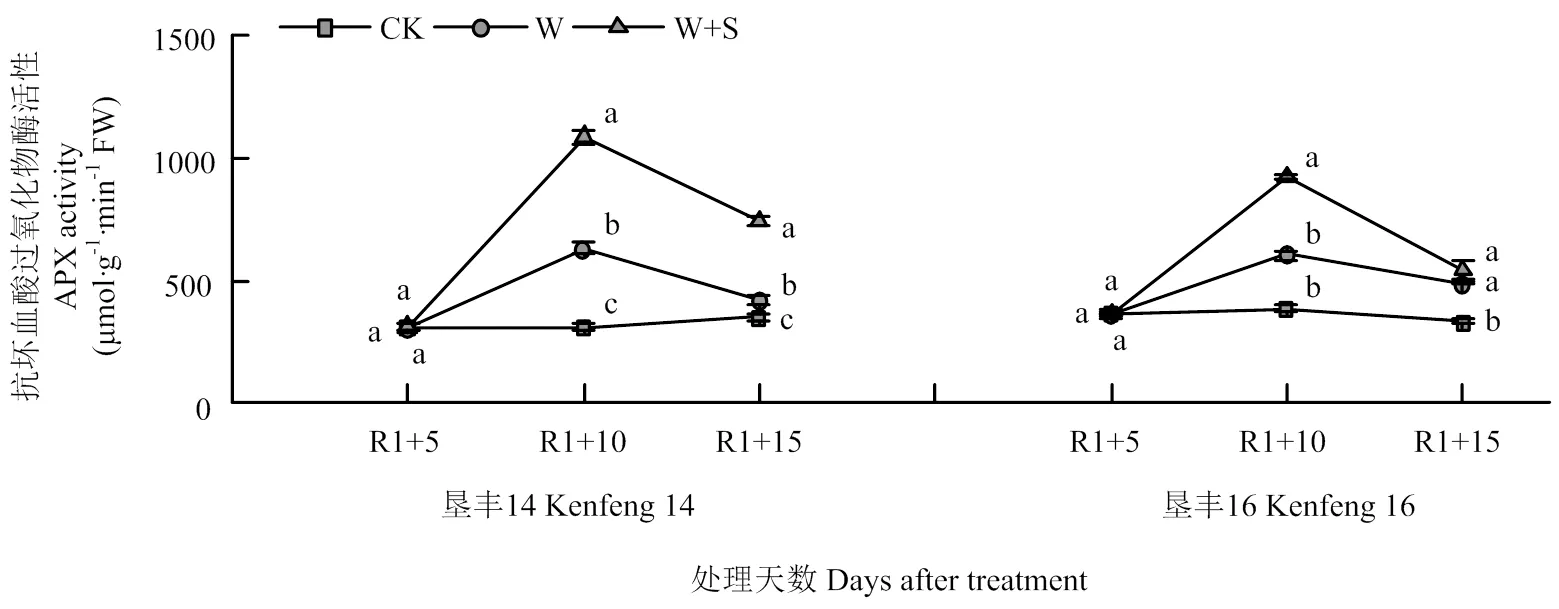
图5 烯效唑对R1期淹水胁迫下大豆叶片APX活性的影响
2.5.2 烯效唑对R1期淹水胁迫下大豆叶片GR活性的影响 R1+5时,W+S处理可提高垦丰14和垦丰16叶片内GR活性,与2个品种CK相比分别增加5.39%和1.43%,但均未达显著差异水平。R1+10时,垦丰14和垦丰16各处理之间趋势表现为W+S>W>CK,淹水胁迫提高了2个品种叶片内GR活性,叶面喷施S3307后可进一步促进2个品种叶片内GR活性的提高,缓解大豆在淹水胁迫下所受到的伤害。R1+15时,垦丰14和垦丰16各处理之间高低趋势与R1+10时相同,说明淹水胁迫下喷施S3307具有延缓大豆叶片内GR活性降低的作用(图6)。
2.5.3 烯效唑对R1期淹水胁迫下大豆叶片MDHAR活性的影响 R1+5时,W+S处理略提高垦丰14和垦丰16叶片内MDHAR活性,但与2个品种CK相比未达显著差异水平。R1+10时,垦丰14和垦丰16各处理之间MDHAR活性高低趋势均表现为W+S>W>CK,说明淹水胁迫提高了大豆叶片内MDHAR活性,喷施S3307后进一步提高了MDHAR活性。恢复正常水分后(R1+15),2个品种W处理MDHAR活性均呈降低趋势,但仍显著高于CK,W+S处理的2个品种MDHAR活性虽有降低,但仍维持较高水平,说明S3307可延缓MDHAR活性的降低,减缓淹水胁迫对大豆所造成的伤害(图7)。
2.5.4 烯效唑对R1期淹水胁迫下大豆叶片DHAR活性的影响 R1+5时,S3307喷施可提高垦丰14和垦丰16叶片内DHAR活性,但与CK相比无显著性差异。R1+10时,垦丰14和垦丰16叶片内DHAR活性高低趋势均表现W+S>W>CK,说明淹水胁迫提高了大豆叶片体内DHAR活性,喷施S3307后可进一步提高DHAR活性。恢复正常水分后(R1+15),垦丰14和垦丰16 W处理和W+S处理叶片内DHAR活性虽有降低,但高低趋势与R1+10时相同,W+S处理虽有降低,但显著高于W处理和CK,说明S3307具有延缓DHAR活性降低的作用,有利于增强清除活性氧的能力(图8)。

图6 烯效唑对R1期淹水胁迫下大豆叶片GR活性的影响

图7 烯效唑对R1期淹水胁迫下大豆叶片MDHAR活性的影响

图8 烯效唑对R1期淹水胁迫下大豆叶片DHAR活性的影响
2.6 R1期淹水胁迫对大豆叶片AsA-GSH的损伤及S3307的缓解效应
淹水胁迫导致产生速率的增加,随后被歧化为H2O2,和H2O2含量的增加会改变膜的流动性,形成MDA等膜脂过氧化物。AsA-GSH可有效清除淹水胁迫积累的ROS。AsA可被APX催化与H2O2反应生成MDAHAR,MDHAR可协同APX清除H2O2,使H2O2分解为H2O和O2。DHAR是存在于AsA和DHA之间的关键酶,在GSH和NADH存在的条件下催化DHA转化为AsA。GSH具有清除的作用,可被DHAR氧化生成GSSG。淹水胁迫增加了AsA、GSH、DHA、GSSG含量,并提高了关键酶(APX、GR、DHAR、MDHAR)的活性,S3307处理后可进一步增加上述指标的活性,并有效抑制了ROS和积累和MDA含量的增加,进而起到提高大豆耐涝性的作用(图9)。
3 讨论
淹水胁迫会对植物生长发育造成严重影响,且表现为淹水时间越长伤害作用越高的趋势。初花期(R1)是大豆营养生长和生殖生长并进的时期,在R1期,大豆受到淹水胁迫,会严重抑制植株的生长发育,导致产量降低[41]。烯效唑是重要的外源控长调节剂,通过浸种[42]、叶喷等方式[21],均可提高作物的抗逆性。研究表明,烯效唑可通过提高抗氧化酶含量,清除过多活性氧,保持较高的抗氧化剂含量,增强作物抗氧化能力,减轻膜脂过氧化程度,缓解逆境胁迫对植物造成的伤害[43-45]。

红色箭头表示R1期淹水胁迫对各指标的影响(增加或减少);绿色箭头表示R1期淹水胁迫下S3307处理对各指标的影响(增加或减少)
3.1 R1期淹水胁迫及S3307处理对大豆膜脂过氧化程度的影响
MDA是植物细胞膜脂过氧化产物,其含量是衡量植物细胞膜脂过氧化程度和质膜损伤的重要指标[46]。叶片内MDA含量的增加,会导致表面细胞膜受害程度加大,MDA可与膜上酶和蛋白质等结合,使细胞失活,从而破坏生物膜的结构和功能,影响细胞的物质代谢[47]。本试验结果表明,淹水胁迫下垦丰14和垦丰16 2个品种大豆叶片MDA含量增加,加剧膜脂过氧化和细胞膜破坏程度,且涝渍敏感品种垦丰16受到破坏程度大于耐涝品种垦丰14,说明耐涝品种承受淹水胁迫能力更强,这与张洪鹏等[48]研究结果一致。于奇等[49]研究表明,在淹水胁迫下应用S3307处理可降低R1期绿豆叶片MDA含量,缓解淹水胁迫对绿豆叶片细胞膜的伤害。项洪涛等[28]研究指出,S3307能够影响植物体内自由基和△-二氢吡咯-5-羧酸合成酶的合成,自由基作用于膜脂过氧化合成,最终产物是MDA,S3307处理可抑制自由基的合成,并降低MDA含量,提高作物的耐寒性。本试验研究结果表明,淹水胁迫条件下,S3307处理后,降低了2个大豆品种叶片内MDA含量,减轻了淹水胁迫下膜脂过氧化程度对大豆叶片造成的伤害,保证大豆在淹水胁迫下正常生长发育。
3.2 R1期淹水胁迫及S3307处理对大豆活性氧代谢的影响
一般来说,植物在正常条件下生长,可通过自身产生的酶促和非酶促氧化防御体系来抵抗过量的ROS积累,促进植物正常生长,而淹水胁迫会抑制作物的正常生长,使植物体内相对平稳的活性氧遭到淹水胁迫的破坏,进而导致超氧阴离子()和过氧化氢(H2O2)的增生。过多的活性氧自由基不能被及时清除,会加快膜脂过氧化进程,降低膜系统的完整性,损伤蛋白质,最终导致植物体内生理生化代谢紊乱,抑制植物生长发育[50]。本研究得出,R1期淹水胁迫显著增加2个大豆品种叶片中产生速率和H2O2含量,与各自CK相比,R1期淹水胁迫5 d时(R1+10),垦丰14增加了1.60倍和1.30倍,垦丰16增加了1.28倍和1.16倍。研究指出,外施S3307可提高作物在逆境胁迫下的耐受性,原因可能是S3307可帮助降低由ROS过度积累引起的膜损伤[5,28-29]。本试验研究表明,S3307可有效减少淹水胁迫下2个大豆品种叶片内产生速率和H2O2含量的积累,说明S3307对维持ROS平衡具有积极作用。
3.3 R1期淹水胁迫及S3307处理对大豆AsA-GSH循环的影响
AsA-GSH循环参与植物胁迫反应,其中,AsA和GSH是循环中重要的抗氧化物质,是清除自由氧系统的重要组成物质,可有效清除H2O2和ROS[50]。APX、GR、MDHAR、DHAR是AsA-GSH循环中的关键酶,和其他抗氧化物质共同作用清除ROS,使ROS保持平衡,维持细胞膜的稳定性[51]。吴秀等[52]研究表明,APX和DHAR是AsA-GSH循环中活性最强的2个关键酶,其活性会直接影响AsA和DHA含量。APX在清除H2O2时,先将H2O2还原成H2O,从而清除H2O2,并伴随MDHAR的产生,MDHAR会氧化成DHA,当MDHAR和DHAR共同存在时,DHAR将利用GSH还原DHA,并有MDHAR和DHA重新生成AsA,AsA可直接参与清除[53],而GSH在反应过程中被氧化成GSSG,再通过GR氧化NADPH重新还原产生[54-55]。前人研究指出,GSH在植物胁迫抵抗中的主要作用是保持较高的含量,促进膜蛋白结构稳定,因此,GSH和GR活性水平被认为是机体抗氧化状态的重要标志[56],同时GR活性可直接影响GSH含量,GR活性上升是造成GSH含量增加的一个因素之一[57]。顾帆等[58]在对高温和干旱胁迫对黄薇抗氧化系统的影响中研究表明,APX是循环中主要的清除酶类,其余的酶则维持循环内的平衡,保证活性氧的清除能力。
本试验结果表明,垦丰14和垦丰16 APX、GR、MDHAR、DHAR活性在淹水胁迫第5天(R1+10)时显著增加,表明在淹水胁迫下,抗氧化酶活性急剧增加,这可能是大豆为适应淹水胁迫所产生的应激反应,叶面喷施S3307后可进一步促进APX、GR、MDHAR、DHAR活性的提高,且耐涝品种的酶活性高于涝渍敏感品种,这说明S3307能快速激活AsA-GSH中关键酶活性,保持AsA和GSH含量在较高水平,及时清除胁迫产生的H2O2和,促进AsA-GSH循环的运转。恢复正常水分后(R1+15),淹水胁迫解除,抗氧化酶活性降低,耐涝品种较涝渍敏感品种恢复能力快,复水后可逐渐恢复至正常水平。另外,AsA、DHA、GSH和GSSG含量变化是可反映淹水胁迫下植物变化特征的重要指标。郭欣欣等[50]指出,淹水胁迫下,植物体内较高含量的抗氧化剂含量(AsA和GSH)和氧化还原力(DHA和GSSG)可保护细胞膜免受伤害,确保植物具有较强的抗逆能力,与本试验结果一致。淹水胁迫5 d(R1+10)时促进了垦丰14和垦丰16 W抗氧化剂含量(AsA和GSH)和氧化还原力(DHA和GSSG)的增加,且S3307处理可进一步促进上述指标含量的增加,说明S3307可清除淹水胁迫下大豆植株体内堆积的大量膜脂过氧化物,加强植株抵御逆境胁迫的性能和自身修复机制,恢复正常水分后(R1+15),S3307处理可保持较高的抗氧化剂含量,有助于快速恢复至正常生理状态。上述指标耐涝品种的含量均高于涝渍敏感品种,表明耐涝性强品种受到的伤害要小于涝渍敏感品种,S3307可有效防止大豆在淹水胁迫下细胞膜受损,提高耐涝性。
4 结论
淹水胁迫导致大豆叶片内ROS代谢紊乱,膜脂过氧化程度提高,喷施S3307可使淹水胁迫下ASA和GSH维持较高含量,提高关键酶活性,保证ASA-GSH循环的正常运行,并维持恢复正常水分管理后关键酶和抗氧化剂含量,提高大豆抗氧化能力,减轻膜脂过氧化程度,进而增加大豆的耐涝性。耐涝品种垦丰14受淹水胁迫造成的伤害程度低于涝渍敏感品种垦丰16。
[1] SAKAMOTO K, OGIWARA N , KAJI T,SUGIMOTO Y, UENO M, SONODA M, MATSUL, ISHIDA J, TANAKA M, TOTOKI Y, SHINOZAKI K, SEKI M. Transcriptome analysis of soybean () root genes differentially expressed in rhizobial, arbuscular mycorrhizal, and dual symbiosis. Journal of Plant Research, 2019, 132(4): 1-28.
[2] 王红蕾. 黑龙江省大豆产业振兴发展路径分析. 黑龙江农业科学, 2019(10): 103-106.
WANG H L. Analysis on the development path of soybean industry revitalization in Heilongjiang province. Heilongjiang Agricultural Sciences,2019(10): 103-106. (in Chinese)
[3] 姜丽霞, 朱海霞, 闫敏慧, 闫平, 王晾晾, 韩俊杰, 高明, 吕佳佳, 纪仰慧, 王萍. 黑龙江省主汛期异常降水变化及其与洪涝的关系研究. 灾害学, 2019, 34(2): 1-6.
JIANG L X, ZHU H X, YAN M H, YAN P, WANG L L, HAN J J, GAO M, Lü J J, JI Y H, WANG PChanges of abnormal rainfall and relationship between precipitation and flood during main flood seasons of Heilongjiang provinceJournal of Catastrophology, 2019, 34(2): 1-6. (in Chinese)
[4] 李秀芬, 郭昭滨, 朱海霞, 王萍, 宫丽娟, 姜丽霞, 赵慧颖. 黑龙江省大豆生长季旱涝时序特征及其对产量的影响. 应用生态学报, 2020, 31(4): 1223-1232.
LI X F, GUO Z B, ZHU H X, WANG P, GONG L J, JIANG L X, ZHAO H Y.Time series characteristics of drought and flood in soybean growing season in Heilongjiang province and its effect on yield. Chinese Journal of Applied Ecology,2020, 31(4): 1223-1232. (in Chinese)
[5] 张洪鹏. 不同时期淹水胁迫对大豆生长和产量的影响及烯效唑调控效应[D]. 大庆: 黑龙江八一农垦大学, 2017.
Zhang H P. Effect of waterlogging stress on growth and yield in different of periods and the regulation effect of uniconazole[D]. Daqing: HeiLongjiang Bayi Agricultural University, 2017. (in Chinese)
[6] Shaw R E, Meyer W S, Mcneill A, Tyerman S D. Waterlogging in Australian agricultural landscapes: a review of plant responses and crop models. Crop and Pasture Science, 2013, 64(6): 549.
[7] 吴奇峰, 何桂红, 董志新, 李广华. 植物生长调节剂在我国大豆种植上的研究与应用. 作物杂志, 2005(1): 12-15.
WU Q F, HE G H, DONG Z X, LI G H. Research and application of plant growth regulator in soybean planting in China. Crops, 2005(1): 12-15. (in Chinese)
[8] ANTONIO D S J C, NANNI M R, SHAKIR M, teodoro p e, oliveira-junior j f, cezar e, gois g d, lima m g, wojciechowski j, shiratsuchi l. Soybean varieties discrimination using non-imaging hyperspectral sensor.Infrared Physics & Technology, 2018(89): 338-350.
[9] GECHEV T S, HILLE J. Hydrogen peroxide as a signal controlling plant programmed cell death. The Journal of Cell Biology, 2005, 168(1): 17-20.
[10] Gill S S, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology & Biochemistry, 2010, 48(12): 909-930.
[11] 山溪, 秦文斌, 张振超, 姚悦梅, 肖燕, 戴忠良. 低温胁迫对不同品系甘蓝幼叶AsA-GSH循环代谢的影响. 南方农业学报, 2018, 49(11): 2230-2235.
SHAN X, QIN W B, ZHANG Z C, YAO Y M, XIAO Y, DAI Z L. Effects of low temperature stress on leaf AsA-GSH cycle metabolism in different varietiesL. Journal of Southern Agriculture, 2018, 49(11): 2230-2235. (in Chinese)
[12] Hasanuzzaman M, Nahar K, Anee T, FUJITA M. Exogenous silicon attenuates cadmium induced oxidative stress inL. by modulating AsA-GSH pathway and glyoxalase system. Frontiers in Plant Science, 2017, 8: 1061.
[13] Khan N A, Umar S, Anjum N A, Iqbal M. Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbate-glutathione cycle metabolism. Russian Journal of Plant Physiology, 2011, 58(1): 92-99.
[14] ZHANG S L, OU X Q, SHAN C J. The roles of H2S and H2O2in regulating AsA-GSH cycle in the leaves of wheat seedlings under drought stress. Protoplasma, 2018, 255(4): 1257-1262.
[15] HUANG G Y, SHAN C J. Lanthanum improves the antioxidant capacity in chloroplast of tomato seedlings through ascorbate- glutathione cycle under salt stress. Scientia Horticulturae, 2018, 232: 264-268.
[16] 郑春芳, 刘伟成, 魏龙, 陈继浓, 张呈念, 仇建标, 丁文勇, 郑青松. 外施褪黑素对低温胁迫下红树植物秋茄光合作用和抗坏血酸-谷胱甘肽循环的调控. 植物生理学报, 2019, 55(8): 1211-1221.
ZHANG C F, LIU W C, WEI L, CHEN J N, ZHANG C N, QIU J B, DING W Y, ZHENG Q S. Regulation of photosynthesis and ascorbic acid-glutathione circulation in mangrove plants under low temperature stress by external application of melatonin. Plant physiology journal, 2019, 55(8): 1211-1221. (in Chinese)
[17] Ramburan S , Greenfield P L. The effects of chlormequat chloride and ethephon on agronomic and quality characteristics of south African irrigated wheat. South African Journal of Plant and Soil, 2007, 24(2): 106-113.
[18] 刘春娟, 宋双伟, 冯乃杰, 郑殿峰, 宫香伟, 孙秋霞, 邢豹, 高杰, 吕金莹. 干旱胁迫及复水条件下烯效唑对大豆幼苗形态和生理特性的影响. 干旱地区农业研究, 2016, 34(6): 222-227, 256.
LIU C J, SONG S W, FENG N J, ZHENG D F, GONG X W, SUN Q X, XING B, GAO J, Lü J Y. Effects of plant growth regulator S3307 on morphological and physiological characteristics of soybean seedling under drought stress and rewater treatment. Agricultural Research in the Arid Areas, 2016, 34(6): 222-227, 256. (in Chinese)
[19] 张伟, 刘冰, 李茂盛, 白子裕, 张治安. 烯效唑对绿豆产量及叶片某些生理指标的影响. 吉林农业, 2017(22): 60-61.
ZHANG W, LIU B, LI M S, BAI Z Y, ZHANG Z A. Effects of alfenidazole on yield of mung bean and some physiological indexes of leaf. Jilin Agriculture,2017(22): 60-61. (in Chinese)
[20] 王景伟, 黄玉兰, 金喜军, 张玉先. 干旱胁迫下烯效唑拌种对芸豆保护酶活性及渗透调节物质的影响. 江苏农业科学, 2017, 45(12): 59-61.
WANG J W, HUANG Y L, JIN X J, ZHANG Y X. Effects of aldoxazole seed mixing on the activity of protective enzymes and osmotic regulators in kidney beans under drought stress. Jiangsu Agricultural Sciences,2017, 45(12): 59-61. (in Chinese)
[21] 张巫军, 段秀建, 姚雄, 唐永群, 杨小艳, 刘强明, 肖人鹏, 张现伟, 李经勇. 烯效唑对遮阴下重穗型水稻渝香203茎秆形态结构和抗倒伏性的影响. 四川农业大学学报, 2019, 37(6): 755-761.
ZHANG W J, DUAN X J, YAO X, TANG Y Q, YANG X Y, LIU Q M, XIAO R P, ZHANG X W, LI J Y. Effects of enfuzole on stem morphology and lodging resistance of heavy panicle rice yuxiang 203 under shade. Journal of Sichuan Agricultural University,2019, 37(6): 755-761. (in Chinese)
[22] 崔洪秋, 冯乃杰, 孙福东, 刘春娟, 何天明, 赵晶晶, 刘洋, 龚屾, 师臣, 郑殿峰. DTA-6和S3307对大豆存留荚和脱落荚生理调控的效应. 中国农业科学, 2016, 49(15): 2921-2931.
CUI H Q, FENG N J, SUN F D, LIU C J, HE T M, ZHAO J J, LIU Y, GONG S, SHI C, ZHENG D F. Effects of DTA-6 and S3307 on physiological regulation in normal and abscission pods of soybean. Scientia Agricultura Sinica,2016, 49(15): 2921-2931. (in Chinese)
[23] 陈文杰, 汤复跃, 韦清源, 郭小红, 梁江, 陈渊. 不同浓度烯效唑拌种对套作夏大豆农艺性状及产量的影响. 南方农业学报, 2019, 50(09): 1960-1966.
CHEN W J, TANG F Y, WEI Q Y, GUO X H, LIANG J, CHEN Y. Effects of seeds dressing with uniconazole in different concentrations before sowing on the intercropping summer soybean agronomic characters and yield. Journal of Southern Agriculture,2019, 50(9): 1960-1966. (in Chinese)
[24] Llewellyn D J, Christianson J A, Wilson I W, Dennis E S. The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of arabidopsis seeds following low-oxygen treatment. Plant physiology, 2009, 149(4): 1724-1738.
[25] Booker H M, Gillespie T J, Hofstra G, FLTCHER R A. Uniconazole-induced thermotolerance in wheat seedlings is mediated by transpirational cooling. Physiologia Plantarum, 1991, 81(3): 335-342.
[26] Bakheta M A, Hussein M M. Uniconazole effect on endogenous hormones, proteins and proline contents of barley plants () under salinity stress (NaCl). Nusantara Bioscience, 2014, 6(1): 39-44.
[27] Zhang M C, He Z P, Li Z H, Tian X L, Wang B M, Duan L S, Li J M. Uniconazole-induced tolerance of soybean to water deficit stress in relation to changes in photosynthesis, hormones and antioxidant system. journal of plant physiology, 2007, 164(6): 709-717.
[28] 项洪涛, 李琬, 何宁, 王雪扬, 郑殿峰, 王彤彤, 梁晓艳, 唐晓东, 李一丹. 苗期低温胁迫下烯效唑对红小豆根系抗寒生理及产量的影响. 草业学报, 2019, 28(7): 92-102.
XIANG H T, LI W, HE N, WANG X Y, ZHENG D F, WANG T T, LIANG X Y, TANG X D, LI Y D. Effects of S3307 on physiology of chilling resistance in root and on yield of adzuki bean under low temperature stress during seedling stage. Acta Prataculturae Sinica, 2019, 28(7): 92-102. (in Chinese)
[29] Ahmed S, Nawata E, Hosokawa M, DOMASE Y, SAKURATANI T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Science, 2002, 163(1): 117-123.
[30] 孟娜, 魏胜华. 喷施烯效唑调控大豆根部解剖结构缓解盐逆境伤害. 生态学杂志, 2018, 37(12): 3605-3609.
MENG N, WEI S H. Uniconazole spraying ameliorates salt injury to soybean seedlings by regulating anatomical structure in roots.Chinese Journal of Ecology, 2018, 37(12): 3605-3609. (in Chinese)
[31] 宋晓慧, 张智杰, 李春光, 张代平, 韩英鹏, 李冬梅, 李文滨.淹水时间对不同耐涝性大豆品种苗期根部形态和叶部生理指标的影响. 大豆科学, 2014, 33(1): 70-72, 102.
SONG X H, ZHANG Z J, LI C G, ZHANG D P, HAN Y P, LI D M, LI W B. Effect of waterlogging time on root morphology and foliar physiological indexes of soybean varieties.Soybean Science, 2014, 33(1): 70-72, 102. (in Chinese)
[32] 唐章程. 现代植物生理学实验指南. 北京: 科学出版社, 1999: 127.
TANG Z C. Guide to experiments in modern plant physiology. Beijing: science press, 1999: 127. (in Chinese)
[33] 王爱国, 罗广华. 植物的超氧物自由基与羟胺的反应. 植物生理学通讯, 1990(6): 55-57.
WANG A G, LUO G H. Reaction of plant superoxide radical with hydroxylamine. Plant Physiology Communications, 1990(6): 55-57. (in Chinese)
[34] 高俊凤. 植物生理学实验指导. 北京: 高等教育出版社, 2006: 221-223.
GAO J F. Experimental Instruction in Plant Physiology. Beijing: Higher Education Press, 2006: 221-224. (in Chinese)
[35] Chakrabarty D, Datta S K. Micropropagation of gerbera: Lipid peroxidation and antioxidant enzyme activities during acclimatization process. Physiology Plant, 2008(30): 325-331.
[36] Zhang J, Kirkham M B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytologist, 1996(132): 361-373.
[37] 李玲. 植物生理学模块实验指导. 北京: 科学出版社, 2009.
LI L. Plant physiology module experimental guidance. Beijing: science press, 2009. (in Chinese)
[38] Zhu H, Cao Z x, Zhang L, Trush M A, Li Y b. Glutathione and glutathione-linked enzymes in normal human aortic smooth muscle cells: chemical inducibility and protection against reactive oxygen and nitrogen species-induced injury. Molecular and Cellular Biochemistry, 2007(301): 47-59.
[39] Wheeler C R, Salzman J A, Elsayed N M, Omaye S T, Korte D W. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Analytical Biochemistry, 1990(184): 193-199.
[40] Murshed R, Lopez-Lauri F, Sallanon H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (, cv. Micro-tom). Physiology and Molecular Biology of Plants, 2013, 19(3): 363-378.
[41] 邢兴华, 徐泽俊, 齐玉军, 孙东雷, 卞能飞, 王晓军, 王幸. 外源二乙基二硫代氨基甲酸钠对花期淹水大豆碳代谢的影响. 中国油料作物学报, 2019, 41(1): 64-74.
XING X H, XU Z J, QI Y J, SUN D L, BIAN N F, WANG X J, WANG X. Effect of exogenous sodium diethyldithiocarbamate on carbon metabolism of soybean under waterlogging at flowering stage. Chinese Journal of Oil Crop Sciences, 2019, 41(1): 64-74. (in Chinese)
[42] 李青苗, 杨文钰, 韩惠芳, 关华. 烯效唑浸种对玉米幼苗生长和内源激素含量的影响. 植物生理学报, 2005, 41(6): 752-754.
LI Q M, YANG W Y, HAN H F, GUAN H. Effects of seed soaking with uniconazole on endogenous hormone content and growth of maize (L.) seedling, Plant Physiology Journal,2005, 41(6): 752-754. (in Chinese)
[43] 黄玉兰, 赵蕊, 向君亮, 蔡森, 曾伟光. 外源烯效唑对低温胁迫下薏苡幼苗的缓解效应的研究. 中国中药杂志, 2019, 44(11): 2213-2218.
HUANG Y L, ZHAO R, XIANG J L, CAI S, ZENG W G. Study of exogenous uniconazole on alleviating low-temperature stress of coix seedlings. China Journal of Chinese Materia Medica, 2019, 44(11): 2213-2218. (in Chinese)
[44] 孟娜, 徐航, 魏明, 魏胜华. 叶面喷施烯效唑对盐胁迫下大豆幼苗生理及解剖结构的影响. 西北植物学报, 2017, 37(10): 1988-1995.
MENG N, XU H, WEI M, WEI S H. Effect of foliar uniconazole spraying under salt stress on physiological and anatomical characteristics in. Acta Botanica Boreali-Occidentalia Sinica, 2017, 37(10): 1988-1995. (in Chinese)
[45] 刘丽琴, 张永清, 李鑫, 王姣. 烯效唑浸种对干旱胁迫下红小豆生长及其根系生理特性的影响. 西北植物学报, 2017, 37(1): 144-153.
LI L Q, ZHANG Y Q, LI X, WANG J. Influence of seed soaking with uniconazole on growth and root physiological characteristics of adzuki bean under drought stress. Acta Botanica Boreali-Occidentalia Sinica, 2017, 37(1): 144-153. (in Chinese)
[46] 刘会芳, 何晓玲, 马展, 徐巍, 刘苗苗, 刘慧英. 外源GSH对NaCl胁迫下番茄幼苗生长及AsA-GSH循环的影响.石河子大学学报(自然科学版), 2014, 32(3): 265-271.
LIU H F, HE X L, MA Z, XU W, LIU M M, LIU H Y. Effects of exogenous GSH on the growth and AsA-GSH cycle in tamato seedlings under NaCl stress. Journal of Shihezi University (Natural Science), 2014, 32(3): 265-271. (in Chinese)
[47] 韩亮亮. 淹水胁迫对大豆生长和生理特性的影响[D]. 南京: 南京农业大学, 2011.
HAN L L. Effects of waterlogging stress on soybean growth and physiological characteristics[D]. Nanjing: Nanjing Agricultural University, 2011. (in Chinese)
[48] 张洪鹏, 张盼盼, 李冰, 李东, 刘文彬, 冯乃杰, 郑殿峰. 烯效唑对淹水胁迫下大豆农艺性状及生理生化指标的影响. 中国油料作物学报, 2017, 39(5): 655-663.
ZHANG H P, ZHANG P P, LI B, LI D, LIU W B, FENG N J, ZHANG D F. Effects of uniconazole on alleviation of waterlogging stress in soybean. Chinese Journal of Oil Crop Sciences, 2017, 39(5): 655-663. (in Chinese)
[49] 于奇, 冯乃杰, 王诗雅, 左官强, 郑殿峰. S3307对始花期和始粒期淹水绿豆光合作用及产量的影响. 作物学报, 2019, 45(7): 1080-1089.
YU Q, FENG N J, WANG S Y, ZUO G Q, ZHENG D F. Effects of S3307 on the photosynthesis and yield of mung bean at R1 and R5 stages under waterlogging stress. Acta Agronomica Sinica, 2019, 45(7): 1080-1089. (in Chinese)
[50] 郭欣欣, 李晓锋, 朱红芳, 朱玉英, 刘金平. 淹水胁迫对不结球白菜抗坏血酸-谷胱甘肽循环的影响. 植物生理学报, 2015, 51(12): 2181-2187.
GUO X X, LI X F, ZHU H F, ZHU Y Y, LIU J P. Effects of waterlogging stress on ascorbate-glutathione cycle inssp.. Plant physiology journal, 2015,51(12):2181-2187. (in Chinese)
[51] 孙军利, 赵宝龙, 郁松林. SA对高温胁迫下葡萄幼苗AsA-GSH循环的影响. 核农学报, 2015, 29(4): 799-804.
SUN J L, ZHAO B L, YU S L. Effects of exogenous salicylic acid (SA) on ascorbate glutathione cycle (AsA-GSH) circulation metabolism in grape seedlings under high temperature stress. Journal of Nuclear Agricultural Sciences,2015, 29(4): 799-804. (in Chinese)
[52] 吴秀, 陆晓民. 油菜素内酯对亚适宜温光盐环境下黄瓜幼苗根系生长及其AsA-GSH循环的影响. 生态学杂志, 2015, 34(8): 2149-2154.
WU X, LU X M. Effects of brassinolide on the growth and ascorbate-glutathione cycle of cucumber seedling roots under suboptimal temperature, light and salt environment. Chinese Journal of Ecology,2015, 34(8): 2149-2154. (in Chinese)
[53] 郁敏, 任亚萍, 米银法, 崔瑞红. 根际低氧对不同抗性牡丹植株AsA-GSH循环代谢的影响. 北方园艺, 2016(16): 69-75.
YU M, REN Y P, MI Y F, CUI R H. Effect of root zone hypoxia stress on AsA-GSH between two peony varieties.Northern Horticulture, 2016(16): 69-75. (in Chinese)
[54] Du Y j, Hao Z q, Te J, Zhou Y b, Tao D l, Liu H l, Jin Y h. Environmental stresses and redox status of ascorbate. Acta Botanica Sinica, 2003, 45(7): 795-801.
[55] Aravind P, Prasad M N VModulation of cadmium- induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate- glutathione cycle and glutathione metabolismPlant Physiology and Biochemistry, 2005, 43(2): 107-116.
[56] 杨庆贺, 郑成淑. 低温弱光胁迫下外源ASA与CaCl2对菊花叶片AsA-GSH循环的影响. 山东农业大学学报(自然科学版), 2018, 49(3): 495-499.
YANG Q H, ZHENG C S. Effects of exogenous acetylsalicylic acid and calcium chloride on AsA-GSH cycle in chrysanthemum leaves under stress of low temperature and poor light. Journal of Shandong Agricultural University(Natural Science Edition), 2018, 49(3): 495-499. (in Chinese)
[57] Uchida A , Jagendorf A T , Hibino T , TAKABE t, takabe T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Science, 2002, 163(3): 515-523.
[58] 顾帆, 季梦成, 顾翠花, 郑钢, 郑绍宇. 高温干旱胁迫对黄薇抗氧化防御系统的影响. 浙江农林大学学报, 2019, 36(5): 894-901.
GU F, JI M C, GU C H, ZHENG G, ZHENG S Y. Heat and drought stress with an antioxidant defense system in. Journal of Zhejiang A&F University, 2019, 36(5): 894-901. (in Chinese)
Damage of AsA-GSH Cycle of Soybean Leaves Under Waterlogging Stress at Initial Flowing Stage and the Mitigation effect of Uniconazole

1College of Coastal Agricultural Sciences, Guangdong Ocean University, Zhanjiang 524088, Guangdong;2College of Agriculture, Heilongjiang Bayi Agricultural University, Daqing 163319, Heilongjiang;3Institute of Crop Cultivation and Tillage, Heilongjiang Academy of Agricultural Sciences, Harbin 150086
【】The aim of this study was to investigate effects of waterlogging stress on the ascorbate-glutathione (AsA-GSH) cycle of soybean leaves and the regulating effect of uniconazole (S3307) during initial flowering stage (R1), so as to provide a theoretical basis for improving soybean waterlogging resistance and the application of uniconazole.【】This study was conducted in the pot plant of the National Coarse Grain Engineering Technology Research Center of Bayi Agricultural University in Heilongjiang in 2019. The water-tolerant variety KenFeng 14 and the waterlogging-sensitive variety KenFeng 16 were used as test materials for pot planting experiments. The leaves were sprayed with S3307 (concentration 50 mg·L-1, appropriate spray amount 225 L·hm-2). After spraying S3307 for 5 days, the waterlogging stress treatment was started after 0 d (R1+5) and 5 d (R1+10) and normal water treatment for 5 days (R1+15) after sampling, respectively, and the various physiological indicators were measured by using spectrophotometer to study the degree of membrane lipid peroxidation (MDA) of soybean leaves under waterlogging stress. The effects of reactive oxygen species (ROS) and non-enzymatic antioxidants (AsA, DHA, GSH, and GSSG) and key enzymes (APX, GR, MDHAR, and DHAR) in the AsA-GSH circulatory system and the mitigating effects of S3307 were analyzed. 【】In the R1 stage after R1+5 of waterlogging stress, compared with CK, the S3307 treatment reduced the MDA,production rate and H2O2content, and also increased the content of non-enzyme antioxidants and key enzymes in the AsA-GSH cycle to maintain ROS balance, thus promoted the growth and development of two soybean varieties. At R1+10, W treatment significantly increased the MDA content in the leaves of the two soybean varieties, which were significantly increased by 40.02% and 37.53% higher than that of CK, respectively, and accelerated ROS (and H2O2); theproduction rate of the two varieties under waterlogging stress was significantly increased by 60.29% and 27.77%, compared with CK, respectively; The H2O2content was significantly increased by 49.45% and 43.40% compared with CK, respectively; The waterlogging sensitive variety KenFeng 16 suffered more damage than the waterlogging resistant variety KenFeng 14, and at the same time, under the waterlogging stress, the antioxidant substances and key enzyme activities in the leaves of both soybean varieties were increased to adapt to the waterlogging stress response from stress. After S3307 treatment, W+S treatment could further increase antioxidant substances (AsA, GSH), redox substances (DHA, GSSG), total ascorbic acid (AsA + DHA) and total glutathione (GSH +GSSG) content under waterlogging stress.content, and increase the activity of antioxidant enzymes (APX, GR, MDHAR, DHAR), reduce the MDA content of the leaves, inhibit the accumulation of ROS, and reduce the damage caused by waterlogging stress to the membrane system. After returning to normal water treatment for 5d (R1 + 15), the above indexes of two soybean varieties W treatment were reduced, but W+S treatment could maintain the two soybean leaves. The higher antioxidant enzyme activity and antioxidant substance content accelerated the removal of excessive accumulation of MDA and ROS, promoted the AsA-GSH cycle operation in soybean leaves under waterlogging stress, and then promoted the return of the two soybean varieties to normal state.【】Waterlogging stress had different degrees of influence on membrane lipid peroxidation, ROS accumulation, and key enzyme activities and non-enzyme antioxidants in the AsA-GSH cycle of soybean leaves. Spraying S3307 could improve key enzyme activity at some extent to promote oxidation ability, so as to reduce the harm caused by waterlogging stress.
soybean; waterlogging stress; AsA-GSH cycle; leaves; uniconazole

10.3864/j.issn.0578-1752.2021.02.004
2020-03-14;
2020-05-12
国家自然科学基金面上项目(31871576)、国家“十三五”重点研发计划(2019YFD1002205)
王诗雅,E-mail:wsy1106ok@126.com。通信作者郑殿峰,E-mail:zdffnj@163.com。通信作者冯乃杰,E-mail:byndfnj@126.com
(责任编辑 杨鑫浩)
