Geographical range evolution of the genus Polypedates (Anura:Rhacophoridae) from the Oligocene to present
2021-03-01Li-MeiYuan,Xi-LingDeng,De-ChunJiang等
DEAR EDITOR,
Currently,the genusPolypedatescomprises 26 species distributed in South,Southeast,and East Asia.Because of their relatively low dispersal capability and intolerance to seawater,this genus is ideal for the study of terrestrial range evolution that extends into the island archipelagos of southeastern Asia.In this study,based on data compiled forPolypedatesfrom previous studies and partial mitochondrial and nuclear genes collected in this study,we performed systematic biogeographical analysis. We confirmed a Sundaland origin for the extant genus and showed northward dispersal into mainland Southeast Asia and Asia,which coincided with the timing of paleoclimatic change from the Oligocene to Middle Miocene.Climate fluctuations had a profound impact on species diversification within the genusPolypedates.Furthermore,the Red River did not mediate species exchange between Southeast Asia and mainland Asia until the end of the Miocene,with the sudden onset of northward dispersal in several clades independently at that time.Alternatively,the lineage of widespread insularP.leucomystaxstrongly supports the hypothesis of terrestrial connection between island archipelagos of Southeast Asia during the Mid-Pleistocene paleoclimate fluctuations.Our biogeographical analysis also supports the recent introduction ofP.leucomystaxto the Philippines and Ryukyus,as previously suggested.
Biogeographical studies aim to understand when and how dispersal events resulted in current patterns of species distribution (Jiang et al.,2019; Klaus et al.,2016).South,Southeast,and East Asia harbor extraordinarily high levels of species diversity and share important zoogeographic fauna in the Oriental Region (Holt et al.,2013).A number of so called"transition zones" between zoogeographic regions have been described for these areas.For example,the Isthmus of Kra represents a transition zone between mainland Southeast Asia and Sundaic faunal elements in mammals and birds(Hughes et al.,2003; Woodruff & Turner,2009),and the Ailao Shan-Red River (ASRR) shear zone plays an important role in shaping the present tectonic framework between mainland Southeast Asia and southern China (Anczkiewicz et al.,2007;Searle,2006).A complex geological and climatic history is behind the high species richness in these regions (Klaus et al.,2016).However,studies with additional groups of animals are required to develop a more comprehensive understanding of these biogeographical processes.Among them,it is of particular importance to unravel the evolutionary processes of geographical range in amphibians.
The genusPolypedates(Rhacophoridae) (von Tschudi,1838),i.e.,whipping frogs (Werner,1996),is widespread,covering India,Southeast Asia,South China,and eastward to the Philippines and Japan (Frost,2019).Its current distribution includes several biodiversity hotspots,namely,the Himalayas,Hengduan Mountains, Indo-Burma, Sundaland, part of Wallacea,and the Philippines (Myers et al.,2000).The genusPolypedatesoccurs withChiromantisandRhacophorusand can resist desiccation,with females characteristically laying a foamy egg nest on or near water (Kuraishi et al.,2013).This indicates that,albeit dependent on the presence of forest and rainfall,Polypedatesexhibits good survival skills under unfavorably dry conditions,which has likely facilitated its colonization into various regions.Polypedateshas diversified since the Oligocene (Li et al.,2013).Therefore,it seems that the dispersal processes ofPolypedatescan be attributed to a series of paleotectonic and paleoclimatic events,including the Indian-Eurasian collision,uplift of Himalayas,and Pleistocene climate oscillations with their associated sea-level fluctuations.However,it is still necessary to present convincing evidence to clarify how these geological events have affected the evolution of these frogs.
In recent decades,most molecular studies on the genusPolypedateshave focused on systematic and taxonomic problems and have used a phylogenetic rather than a phylogeographic approach. These studies attempted to understand the molecular phylogenetic relationships amongPolypedatesspecies and, particularly, thePolypedates leucomystaxspecies complex,whose members are difficult to classify (Narins et al.,1998; Pan et al.,2013; Sutthiwisesa et al.,2020; Trépanier et al.,1999; Zhang et al.,2005).TheP.leucomystaxcomplex was once regarded as a highly dispersed group,distributed from India to eastern Indonesia(Dutta & Manamendra-Arachchi,1996; Inger,1999; Taylor,1962).Nevertheless,after considerable taxonomic confusion,the reclassification of theP.leucomystaxcomplex led to the exclusion ofP.mutus,P.impresus,P.braueri,andP.megacephalusin South China (Pan et al.,2013).Kuraishi et al.(2013) conducted a comprehensive phylogenetic study of theP.leucomystaxcomplex based on both mtDNA and nDNA and foundP.macrotisto be sister to theP.leucomystaxcomplex.Although Kuraishi et al.(2013) estimated divergence time within the complex,comprehensive biogeographic pattern analyses were not included.
Several studies have explored the diffusion biogeographical mechanism of theP.leucomystaxcomplex in Southeast Asia.For example,Brown et al.(2010) reported on the recent population expansion of theP.leucomystaxcomplex in the Philippines and Sulawesi,and suggested human-mediated dispersal between oceanic islands.This is supported by Kuraishi et al.(2009),who indicated thatP.leucomystaxfrom Ryukyus arrived by accidental human transport from Southeast Asia.Blair et al.(2013) studied the evolutionary history of theP.leucomystaxcomplex in South China and the Indochinese Peninsula, which included estimations on divergence times and population genetic analyses,and found a northern origin and southward dispersal.Blair et al.(2013)refuted that the demographic expansion of this species and widespread sympatry of lineages originated from humanmediated dispersal between insular and mainland populations,proposing the Red River as a partial barrier for gene flow.Recently,Buddhachat & Suwannapoom (2018) revealed diversification leading to allopatric and sympatric speciation under climatic pressure in theP.leucomystaxcomplex in Southeast Asia.Although some species ofPolypedates,particularly members of theP.leucomystaxcomplex from the Sunda Islands or mainland Southeast Asia,have been included in molecular phylogenetic studies and evolutionary speculation,few attempts have been made to explore the complete biogeographic history among members ofPolypedates.Hence,the routes and timing of migrations within this genus remain obscure.
In this study,we compiled a large dataset of currently recognized species within the genusPolypedates,constructed phylogenetic relationships,and dated divergence events using relaxed molecular clock approaches.We examined the diversification history ofPolypedatesand its relationship with regional paleoclimatology and paleogeography and reconstructed the paleobiogeography ofPolypedates,including its area of origin and likely causes of subsequent migrations.
Overall,despite some missing data,we generated a wellresolved phylogeny ofPolypedatesand related taxa.Our results support the sister relationship ofPolypedatesandTaruga(Figure 1,Supplementary Figure S2),consistent with previous studies inferring the inter-relationships of Rhacophoridae (Li et al., 2013). Thirteen species (P.otilophus,P. colletti,P. cruciger,P. maculatus,P.pseudocruciger,P. macrotis,P. mutus,P. braueri,P.impresus,P.megacephalus,P.teraiensis,P.leucomystax,andP.discantus) form a monophyletic group (Figure 1,Supplementary Figure S2).The high support for samples 31–119 suggests thatP.macrotisis sister to theP.leucomystaxcomplex.According to our results and previous studies (Buddhachat & Suwannapoom,2018; Grosjean et al.,2015; Kuraishi et al., 2013; Rujirawan et al., 2013;Sutthiwisesa et al.,2020),theP.leucomystaxcomplex is composed of seven groups:P. leucomystax,P.megacephalus,P. mutus,P. braueri,P. discantus,P.impresus,andP.teraiensis.Each highly supported group could be considered a distinct species (Kurniati,2011;Kuraishi et al.,2013; Matsui et al.,1986).Our results also shed light on the phylogenetic relationships ofP.braueriand the most recent common ancestor (MRCA) ofP.impresus,P.megacephalus,P.leucomystax,andP.teraiensis,which have remained unresolved till now.
Amphibians are an excellent model for biogeographic studies on overseas dispersal events (Chen et al.,2018;Gonzalez et al.,2014; Hutter et al.,2018; Lee Grismer et al.,2017; Li et al.,2013; Lv et al.,2018; O’Connell et al.,2018;Pan et al.,2017; Yuan et al.,2019).Several biogeographic studies have suggested that Southeast Asia is a cradle for amphibian speciation (Chen et al.,2018; Lee Grismer et al.,2017; O’Connell et al.,2018; Pan et al.,2017).In the present study,ancestral area reconstruction based on likelihood and Bayesian approaches indicated thatTarugaoccurred in India and the MRCA ofTarugaandPolypedatesoriginated in India.Furthermore,our phylogenetic analyses support the sister relationship ofTarugaandPolypedates.The divergence betweenPolypedatesandTarugalikely occurred between 28 and 22 million years ago (Ma) (Figure 2A),similar to the estimation provided by Li et al.(2013).Notably,this time estimation coincides with the period in which the Indian subcontinent moved further north after collision with Southeast Asia, and distinctly seasonal climates accelerated phylogenetic diversification (Bouilhol et al.,2013).However,our biogeographical analyses confirmed Sundaland as the origin of the genusPolypedates,with subsequent dispersal to mainland Southeast Asia (Figure 2A).Although insufficient specimens and sequences from India were included in this study,it can be inferred that dispersal between India and Southeast Asia did not occur only once.Polypedates cruciger,P.maculatus,andP.pseudocrucigerfirst dispersed from Sundaland to India (19–11 Ma),after whichP.teraiensisdispersed in sympatric regions in mainland Southeast Asia and India (Figure 2A).Thus,if true,two routes may have facilitated the dispersal ofPolypedatesinto India,i.e.,via a terrestrial bridge or the Himalayan foothills.However,the collision between the Indian subcontinent and Southeast Asian islands occurred around 50 Ma and formed a terrestrial corridor during its northward drift,with final collision between the Indian subcontinent and mainland Eurasia about 34 Ma (Li et al.,2013).Given that the first dispersal event occurred between 19 and 11 Ma,the second route is more likely,and would have consisted of a continuous dispersal corridor formed by widespread evergreen rainforests along the Himalayan foothills during the middle Miocene (Wan et al.,2009; Zachos et al.,2008).Our results are consistent with the increasing dispersal events from mainland East Asia to the Indian subcontinent between 21 and 11 Ma (Figure 2A),coinciding with the global Mid-Miocene Climatic Optimum(17–14 Ma) (Klaus et al.,2016; Li et al.,2013).Cryptic species distributed in India may indicate an old dispersal event from India to Southeast Asia,as suggested for other groups (Gower et al.,2002; Klaus et al.,2010; Li et al.,2013; Wilkinson et al.,2002),which could change the original biogeographic history ofPolypedates.Additional sampling from India is required to test these predictions.
From its Sundaland origin,the genusPolypedatescolonized mainland Southeast Asia (14–8 Ma) and then dispersed northward to mainland East Asia during the Pliocene and Pleistocene (Figure 2A).During these periods,the genus underwent range expansions at different times,coinciding with the weaker monsoon system and globally warmer climates during the Mid-Miocene (Clift et al.,2008).Similarly,recent study onLeptobrachella,which is also distributed in Southeast Asia, showed range expansions for this genus from Sundaland to mainland Asia from the Mid-Miocene to Pliocene(Chen et al.,2018).The above evidence supports our belief thatPolypedatesexpanded to mainland Southeast Asia and mainland East Asia from Sundaland,leading to species diversification under the influence of geological conditions and climate change.
The Isthmus of Kra was considered a geographic barrier for many years (Wallace,1876).However,Hughes et al.(2011)stated that the Isthmus of Kra is unlikely to be a significant geographic boundary and suggested the climate zone in mainland Southeast Asia during the Last Glacial Maximum as the explanation for the distinct species distribution.Based on our results suggesting thatPolypedatescolonized mainland Southeast Asia via the Isthmus of Kra in the Mid-Miocene(14–8 Ma) (Figure 2A),this isthmus would have acted as an important dispersal corridor between the Malay Peninsula and mainland Southeast Asia.During the Mid-Miocene,the monsoonal system was probably weaker (Clift et al.,2008)and the global climate became warmer,which may have facilitated dispersal events into mainland Southeast Asia.
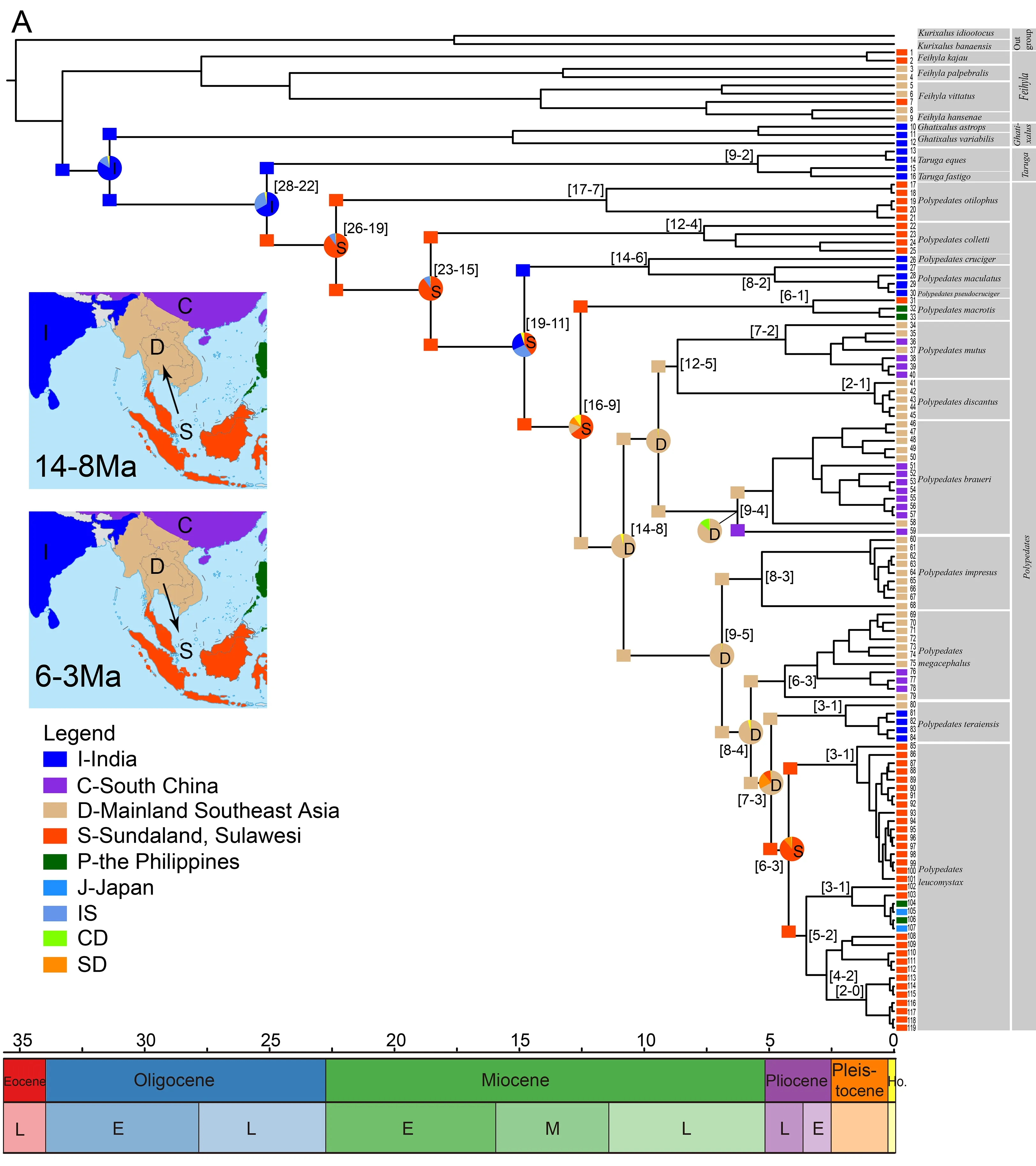
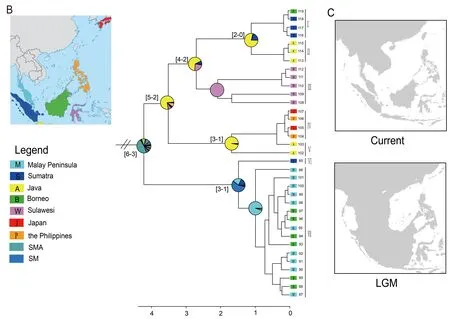
Figure 2 Spatiotemporal reconstruction of Polypedate
The Red River zone has a complex geological history (Hall,1998; Replumaz et al.,2001).The formation of the Red River between southern China and northern Vietnam commenced 27 Ma and ended 21–17 Ma (Cheng et al.,1992; Harrison et al.,1992; Tang et al.,2013),and may have acted as a potential geographic barrier for species dispersal.During this period,no divergence was detected betweenPolypedatesdistributed in mainland Southeast Asia (i.e.,southwest of the Red River) and South China (i.e.,northeast of the Red River).As many taxa only occur on one side of the Red River (e.g.,Sophora davidii,Fan et al.,2013),we hypothesize that any change in the runoff regime could have caused a stronger isolation effect by the Red River at that time,with the caveat that denser population genetic sampling of the respective species will be necessary to draw sound conclusions.Moreover,several transversal movements occurred during the late Miocene in the Red River zone (12–9 Ma,8–6 Ma)(Zhang et al.,2009),which coincide with divergence across the Red River byP.mutus,P.braueri,andP.megacephalus(Figure 2A).Our assertion that the Red River did not constitute a biogeographic barrier from the late Miocene to early Pliocene for the genusPolypedatesis consistent with the results of Blair et al.(2013),although they had a larger dataset.Blair et al.(2013) found that the Red River formed a partial barrier for some lineages and could not explain the overall pattern,most likely due to the indistinct taxonomy ofP.leucomystaxand a circumscribed lineage divide.In view of several studies showing genetic diversification between species on both sides of the Red River (Zhang et al.,2010a,2010b),this zone might function as a diffuse filter barrier for the genusPolypedates.
From the early Pliocene till Holocene,P.leucomystaxdispersed back to Sundaland from mainland Southeast Asia(6–3 Ma) (Figure 2A) and spread to the Malay Peninsula,Greater Sunda Islands,Philippines,and Japan (Figure 2B).The north-to-south dispersal pattern is also consistent with previous phylogenetic and demographic analysis (Blair et al.,2013).Unsurprisingly,P.leucomystaxshowed considerable genetic differentiation across its vast range,in line with earlier research (Brown et al.,2010).In view of the peculiar paleoclimate and tectonics through the continent of Sundaland and nearby areas during the Pleistocene (Miller et al.,2005),species migration between the Borneo and Malay Peninsula populations would be due to the contiguous terrestrial connection between island archipelagos of Southeast Asia during the Mid-Pleistocene paleoclimate fluctuations(Figure 2C).The Pleistocene colonization of Sulawesi from Java,despite the absence of a continuous land connection between Sulawesi and Sundaland (Voris,2000),is not surprising and corresponds to the frequent dispersal across Wallace’s line for freshwater crabs,frogs,reptiles,and mammals (Moss & Wilson,1998).Possibly,the freshwater plume of the South Sunda River System enabled dispersal on driftwood from Java to Sulawesi during an eustatic sea-level lowstand,as hypothesized for freshwater crabs (Klaus et al.,2013).
Polypedates leucomystaxspread to the Philippines from Java and then reached Japan close to the Holocene(Figure 2B).The samples from the Philippines (104 and 106)and Japan (105 and 107) were nested within the Java clade(Figure 2B),indicating that populations ofP.leucomystaxfrom the Philippines originated from Java and subsequently dispersed to Japan.Considering the massive distance and biogeographic barriers for amphibians that make a natural dispersal scenario highly unlikely,we consider it reasonable that transportation of agricultural products between islands facilitated the range expansion ofP.leucomystaxto the Philippines (Brown et al.,2010) and that the accidental transport of a few individuals with military cargo from somewhere around the Philippines established the populations on Ryukyus,as suggested by Kuraishi et al.(2009).We cannot reject the hypothesis that Pleistocene sealevel dynamics may have had effects on the diversification of this species as an explanation for the range expansion ofP.leucomystax.However,further evidence is required to address this possibility.
In conclusion,Polypedatesis an excellent model for biogeographic process studies,and the timing and pattern of its origin and migration fit well with the idea that it mostly originated in insular Southeast Asia during the Oligocene.In the process of northward dispersal,climate change influenced expansion and species diversification from Sundaland to mainland Southeast Asia.The Red River did not act as a diffuse filter barrier for species exchange until the end of the Miocene.From the early Pliocene,P.leucomystaxdispersed all over Sundaland,with Java and Sumatra as source areas.Our findings not only corroborate the hypothesis about climatic and tectonic changes of island archipelagos of southeastern Asia using the genusPolypedates,but also highlight the importance to intergrate tectonics,climate changes and range expansion to reveal the essence of biogeographical evolutionary process involved in diversification.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
All applicable international, national, and/or institutional guidelines for the care and use of animals were strictly followed.All animal sample collection protocols complied with the current laws of China.All animal procedures performed in this research were in accordance with the ethical standards of the institution or organization at which the study was conducted (Experimental Animal Ethics Committee of Chengdu Institute of Biology,Chinese Academy of Sciences;permit No.:20 192 202).
SUPPLEMENTARY DATA
Supplementary data to this paper can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
J.T.L.and K.Y.conceived the study; L.M.Y.and N.L.O.collected experimental samples; L.M.Y and X.L.D.performed data analyses; L.M.Y and X.L.D. prepared the initial manuscript draft; S.K.and D.C.J worked on the approval of the manuscript.All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We would like to thank Dr.Yun-Yun Lv and Mr.Claudio Sabatelli for technical assistance and insightful comments and suggestions; Dr.Jordi López-Pujol for revising an earlier version of this manuscript; and Mr.Jin-Long Ren for photos.
杂志排行
Zoological Research的其它文章
- The forty-year journey of Zoological Research:advancing with the times
- Chromosomal assembly of the Antarctic toothfish(Dissostichus mawsoni) genome using third-generation DNA sequencing and Hi-C technology
- Molecular and morphological evidence for a new species of the genus Typhlomys (Rodentia:Platacanthomyidae)
- Pitfalls of barcodes in the study of worldwide SARSCoV-2 variation and phylodynamics
- Contribution to the taxonomy of the genus Lycodon H.Boie in Fitzinger,1827 (Reptilia:Squamata:Colubridae) in China,with description of two new species and resurrection and elevation of Dinodon septentrionale chapaense Angel,Bourret,1933
- Dynamic evolution of transposable elements,demographic history,and gene content of paleognathous birds
