Inhibitory Effects of Sixteen Fungicides on Mycelial Growth and Spore Germination of Monilinia fructicola
2021-02-26SongHuawenXuNanaGaoDeliangHuZunjiZhuangZhiguoLiuYuWuXibaoZhuangZhanxing
Song Huawen,Xu Nana,Gao Deliang,Hu Zunji,Zhuang Zhiguo,Liu Yu,Wu Xibao,Zhuang Zhanxing
Shandong Academy of Pesticides Sciences,Key Laboratory for Chemical Pesticide of Shandong Province,Ji'nan 250033,China
Abstract [Objective]The paper was to compare the indoor toxicities of sixteen fungicides on mycelial growth and spore germination of Monilinia fructicola,to screen out effective fungicides and to discuss use characteristics of various types of fungicides.[Method]The inhibitory activities of 16 fungicides on mycelial growth and spore germination were determined by mycelial growth rate method and spore germination method.[Result]The EC50values of 16 fungicides against mycelial growth ranged from 0.018 4 to 61.530 5 mg/L.Prochloraz,tetramycin,fenbuconazole and fludioxonil had strong inhibitory activities on mycelial growth,and their EC50values were 0.018 4,0.045 6,0.053 1 and 0.081 4 mg/L,respectively,significantly lower than those of other 12 fungicides.The EC50values of 16 fungicides against spore germination ranged from 0.008 4 to 189.393 8 mg/L.Tetramycin and chlorothalonil had strong inhibitory activities on mycelial growth,and their EC50values were 0.008 4 and 0.037 8 mg/L,respectively,significantly lower than those of other 14 fungicides.[Conclusion]The 16 fungicides had great value in preventing and controlling peach brown rot.Benzimidazoles,diformimides and ergosterol inhibitors had good inhibitory activities on mycelial growth.Strobilurins,succinate dehydrogenase inhibitors and multiple-site protective fungicides had good inhibitory activities on spore germination.The agricultural antibiotics tetramycin,phenazine-1-carboxylic acid and pyrrole fungicide fludioxonil had good inhibitory activities on mycelial growth and spore germination.
Keywords Monilinia fructicola;Mycelial growth;Spore germination;Fungicides;Toxicity;Inhibitory activities
Peach brown rot is an important disease caused by Monilinia spp.,and occurs in peach producing areas all over the world[1].The damage caused by peach brown rot in flowering period leads to flower rot,stiff fruit and branch ulcer,and the harm in mature period leads to rotten fruit before and after picking and in storage period[2],which seriously threatens healthy development of peach industry.Peach brown rot was caused by three types of pathogenic bacteria,namely M.fructicola,M.fructigena and M.laxa.M.fructicola is the major pathogen in most areas of China[1].Monilinia spp.harms not only peach but also apple,pear,plum,cherry,hawthorn and other kernel and drupe fruits,as well as Rosaceae crops[3].
Chemical control is still amain method to control peach brown rot.As the largest peach producing country in the world[4],there are only two pesticides registered against peach brown rot,fenbuconazole and berberine hydrochloride.Therefore,it is of great significance for scientific prevention and control of peach brown rot by screening fungicides with high efficiency and low toxicity for mixed and alternative use in production.
The indoor toxicity of fungicides against M.fructicola has been tested many times[5-18],but all the fungicides involved are not systematic and comprehensive.Fang et al.[6]used mycelial growth rate method and spore germination method to determine indoor toxicity,while others all used mycelial rate method,and no systematic determination by spore germination method has been reported.Moreover,there is neither comparative study on measured data of the two methods,nor comprehensive and systematic evaluation on action characteristics of various fungicides.The indoor toxicities of 16 fungicides against M.fructicola in Feicheng,Shandong Province were determined by mycelial growth rate method and spore germination method,respectively,in order to provide the theoretical basis for chemical control of peach brown rot.
1 Materials and Methods
1.1 Materials On August 1,2019,M.fructicola was collected from peach orchard in Beitai Village,Taoyuan Town,Feicheng District,Shandong Province.Peach had been planted for a long history in the area,and peach brown rot occurred severely in previous years.Benzimidazole and protective fungicides were used frequently in the peach orchard.Diseased fruits infected by peach brown rot were brought back to the laboratory for single spore isolation.After purification and culture in PDA medium,the pathogen was morphologically identified as Monilinia fructicola.The general situation of tested agents is shown in Table 1.

Table 1 General situation of tested agents
1.2 Methods
1.2.1 Preparation of drug-containing PDA medium.According to the results of preliminary test,16 fungicides were diluted into 5-8 gradient concentrations with sterile water;3 mL of diluent was added into the conical flask containing 25 mL of 50℃sterilized PDA medium,and then set to a constant volume of 30 mL.After shaken evenly,the solution was poured into a petri dish (diameter 9 cm),and drug-containing PDA medium was pre-pared after solidification.Those added with sterile water was used as blank control.
1.2.2 Preparation of drug-containing WA medium.According to the results of preliminary test,16 fungicides were diluted into 5-8 gradient concentrations with sterile water;3 mL of diluent was added into the conical flask containing 25 mL of sterilized WA medium,and then set to a constant volume of 30 mL.After shaken evenly,the solution was poured into a petri dish(diameter 9 cm),and drug-containing WA medium was developed after solidification.Those added with sterile water was used as blank control.
1.2.3 Mycelial growth rate method.Under aseptic conditions,fungal cake was picked from colony edge of M.fructicola cultured for 5 d with a puncher(diameter 5 mm).The fungal cake was placed in the center of drug-containing medium.Each treatment replicated four times,and the PDA plate without fungicides was used as blank control.The plate was cultured at 25℃under alternating light and dark(12 h/12 h)for 5 d,and colony diameter was measured by crossing method.The colony diameters of various treatment and inhibition rate of mycelial growth were calculated.The inhibition rate of mycelial growth was calculated according to formula(1).

1.2.4 Spore germination method.M.fructicola was cultured on PDA plate at 25℃under alternating light and dark(12 h/12 h)for 7 d.Conidia were rinsed with sterile deionized water,and filtered by sterilized gauze.After fully shaken,the spore suspension was obtained.The concentration of spore suspension was adjusted to 105-106spore/mL by counting with blood cell counting plate.
The 100 μL of spore suspension was evenly spread on WA medium plate,and cultured at 25℃under alternating light and dark(12 h/12 h)for 20 h.Spore germination was observed under microscope,and the field of view was changed to ensure the total number of spores examined not less than 200 per replicate.The total number of spores and the number of spore germination were recorded,and the length of germ tube reaching 1/2 of short diameter of spores was regarded as germination.Each treatment was replicated four times,and those without drugs were set as blank control.Spore germination rate and inhibition rate of spore germination were calculated according to formulae(2)and(3).

1.2.5 Data processing.All data were statistically analyzed using DPS software.Using the logarithm of set mass concentration as the abscissa(x)and the probability of inhibition rate as the ordinate(y),the toxicity regression equation of each fungicide to tested strain y=a+bx,and median effective concentration(EC50value)were obtained.
In order to compare the EC50value of each fungicide more intuitively,the relative toxicity multiple of the fungicide with the largest EC50value among 16 fungicides measured by each method was set as 1.00,and the relative toxicity multiple of each fungicide was calculated.Relative toxicity multiple was calculated by formula(4).
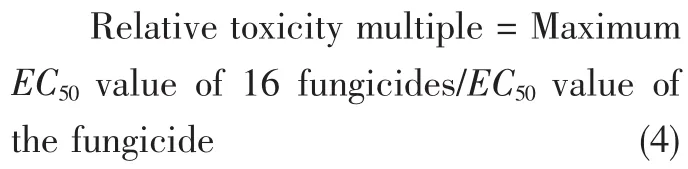
2 Results and Analysis
2.1 Inhibitory effect on mycelial growth The inhibitory effects of 16 fungicides on mycelial growth of M.fructicola were determined(Table 2).

Table 2 Inhibitory effects of 16 fungicides on mycelial growth of Monilinia fructicola
As shown in Table 2,the EC50values of 16 fungicides against mycelial growth ranged from 0.018 4 and 61.530 5 mg/L.Prochloraz,tetramycin,fenbuconazole and fludioxoni had strong inhibitory activity against mycelical growth,and the EC50values were 0.018 4,0.045 6,0.053 1 and 0.081 4 mg/L,respectively,significantly lower than 0.1 mg/L.Mancozeb,chlorothalonil,azoxystrobin and pyraclostrobin had relatively low inhibitory activity against mycelial growth,and the EC50values were 61.530 5,29.732 1,25.532 6 and 17.501 0 mg/L,respectively,significantly greater than 10 mg/L.
2.2 Inhibitory effect on spore germination The inhibitory effects of 16 fungicides on spore germination of M.fructicola were determined (Table 3).As shown in Table 3,the EC50values of 16 fungicides against spore germination ranged from 0.008 4 to 189.393 8 mg/L.Tetramycin and chlorothalonil had strong inhibitory activity against spore germination,and the EC50values were 0.008 4 and 0.037 8 mg/L,respectively,significantly lower than 0.1 mg/L.Tebuconazole,difenoconazole,fenbuconazole,prochloraz,carbendazim,iprodione and procymidone had relatively low inhibitory activity against spore germination,and the EC50values were 189.393 8,143.422 1,96.639 3,90.332 0,77.296 7,44.750 8 and 31.774 3 mg/L,respectively,significantly greater than 10 mg/L.
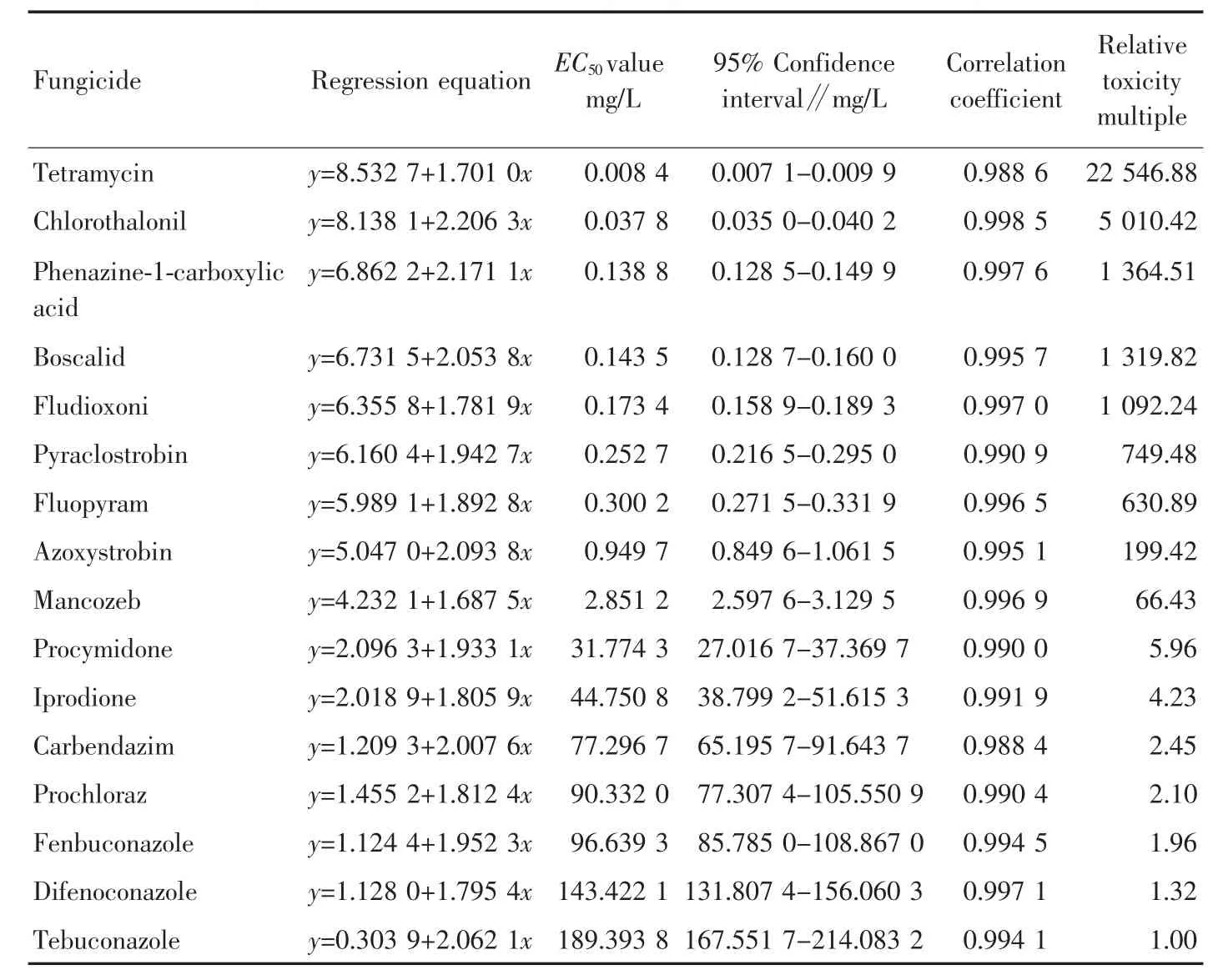
Table 3 Inhibitory effects of 16 fungicides on spore germination of Monilinia fructicola
2.3 Inhibition characteristics of eight types of fungicides The inhibitory effects of eight types of fungicides on mycelial growth and spore germination of M.fructicola were compared(Table 4).The results showed that benzimidazoles,ergosterol inhibitors,and diformimides had strong inhibitory activity against mycelial growth,but weak inhibitory activity against spore germination.Protective fungicides,strobilurins and succinate dehydrogenase inhibitors had strong inhibitory activity against spore germination,but weak inhibitory activity against mycelial growth.Agricultural antibiotics (tetramycin and phenazine-1-carboxylic acid)and pyrrole fungicide(fludioxoni)had strong inhibitory activity against mycelial growth and spore germination.

Table 4 Inhibitory effects of eight types of fungicides on mycelial growth and spore germination of Monilinia fructicola
3 Conclusion and Discussion
From the mechanism of action,MBCs mainly inhibit cell mitosis;SBIs mainly inhibit the synthesis of biofilms;and DCFs are signal transduction inhibitors that increase intracellular osmotic pressure and eventually lead to cell death.The three types of fungicides had strong inhibitory effect on mycelial growth of M.fructicola,but relatively weak inhibitory effect on spore germination,indicating that mitosis,biofilm synthesis and signal transduction activities of M.fructicola in mycelial growth stage were more active than those in spore germination stage,and the inhibitory effect was more obvious.QoIs and SDHIs are respiratory inhibitors,while protective fungicides act on multiple sites.The three types of fungicides had strong inhibitory effect on spore germination,but relatively weak inhibitory effect on mycelial growth,indicating that the respiration of M.fructicola in spore germination stage was more active than those in mycelial growth stage,and it was more sensitive and less tolerant to protective fungicides,and the inhibitory effect was more obvious.Tetracycin and phenazine-1-carboxylic acid are multi-site fungicides,and their inhibitory activities on mycelial growth and spore germination of M.fructicola were significantly higher than those of protective fungicides mancozeb and chlorothalonil,indicating that M.fructicola was more sensitive to the two antibiotics.Pyrrole fungicide fludioxoni has similar action mechanism with DCFs and it is also a signal transduction inhibitor.The inhibitory effect of fludioxoni on mycelial growth and spore germination of M.fructicola was significantly higher than those of iprodione and procymidone,indicating that M.fructicola was more sensitive to fludioxoni.
Our research results showed that MBCs,SBIs and DCFs had strong inhibitory activities on mycelial growth,but weak inhibitory activities on spore germination.Therefore,such fungicides should be applied in early incidence stage of mycelial growth.Protective fungicides,QoIs and SDHIs had strong inhibitory activity on spore germination,but weak inhibitory activity on mycelial growth.Therefore,these fungicides should be applied in early incidence stage of spore germination.Tetracycin and phenazine-1-carboxylic and fludioxoni had good inhibitory activity against mycelial growth and spore germination.Therefore,these fungicides could be applied in each growth stage of M.fructicola.However,the bactericidal spectrum and bactericidal characteristics of the same type of fungicides may be greatly different among different varieties,and the bactericidal varieties determined in this test could not represent all the varieties of this type of fungicides.The inhibitory characteristics of other varieties of fungicides against M.fructicola still need to be further determined.
QoIs fungicides belong to mitochondrial respiratory inhibitors,which mainly block the production of ATP and lead to cell death by inhibiting electron transfer between cytochrome b and c1.Fang et al.[6]found that when normal electron transport pathway was inhibited,plant pathogenic fungi could obtain energy from bypass oxidation pathway.In the field,host plants would secrete flavonoids to inhibit bypass oxidation pathway of pathogens,but the pathway could not be inhibited in medium culture.Therefore,100 mg/L salicylhydroxamic acid(SHAM)was usually added to culture medium to prevent bypass oxidation pathway in indoor determination,but the premise was that SHAM at the dosage had no obvious inhibitory activity against the pathogen and could effectively inhibit bypass oxidation pathway.Fang et al.[6]also found that 40-100 mg/L SHAM had significant inhibitory effect on mycelial growth of most M.fructicola,so it was not suitable for determination of mycelial growth of M.fructicola[6].In addition,although 60 mg/L SHAM did not affect the maximum concentration of spore germination,the inhibition degree of the concentration on bypass oxidation pathway has not been determined.Therefore,neither PDA nor WA medium was added with SHAM in this study,and the impact of SHAM on bypass oxidation pathway in indoor toxicity determination of M.fructicola in Feicheng area need to be further studied.
Due to different application patterns and environment,indoor toxicity test results were often different from field test results.The indoor toxicities of 16 fungicides against M.fructicola were determined in the test,and the field control efficacy should be further tested and verified.Flowering stage and fruit maturity stage are two peak incidence periods of brown rot.Generally,incidence is not observed in young fruit stage.After flower drop,only small spots are observed by naked eyes on surface of infected young fruits,and symptoms will not be seen quickly until maturity stage.This phenomenon is called"dormant infection or latent infection"[2],and the reasons for dormant infection have not been reported yet.Dormant infection also brings about difficulties in field efficacy test.Fungicide administration in flowering period will affect pollination and easily cause chemical injury,while administration after flowering period can not effectively control the infection of pathogen in flowering period,so fungicides are forced to be used in advance during bloom-red period.Practice has shown that it is not easy to investigate the results of peach brown rot test in flowering stage in northern regions.Tests carried out before fruit maturity can only control secondary infection of pathogenic bacteria,but show no effect to pathogens that have revived after dormancy in fruits,and easily cause excess pesticide residues in harvested peach.How to formulate feasible field efficacy test criteria for peach brown rot still needs to be further studied.
M.fructicola was measured in the test,but there are at least three pathogenic bacteria causing peach brown rot[1].Whether there are other two pathogenic bacteria in Feicheng area and whether the toxicity of fungicides to the two pathogenic bacteria are consistent with the results of our study remains to be further studied.
The inhibitory effects of fungicides on mycelial growth and spore germination were determined in the test,and the effect on sporulation still needs to be further studied.In addition,the toxicity was determined by finished preparations,which was more helpful to guide field application than active compound determination.However,fillers and auxiliars in preparation processing may affect determination results,and the influence of different preparation processing technologies on M.fructicola need to be further determined.
杂志排行
植物病虫害研究(英文版)的其它文章
- Integrated Prevention and Control Techniques of Infectious Diseases of Vegetables
- Disease-resistant Mechanism of Pepper against Root-Knot Nematode(Me1oidogyne spp.)
- Research on Chemical Prevention and Control against Stem Base Rot of Sweet Potato
- Effects of Different Amino Acid Fertilizers on Growth,Development and Quality of Mini Watermelon under Substrate Cultivation
- Crystal Structure of Poly[(2,6-di(2’,4’-dicarboxylphenyl)pyridine-κ4 O:O′:O″:O′′′)-(μ2-1,4-bis(1-imidazolyl)benzene-κ2N:N’)cobalt(II)],C39H24Co2N7O8
- Morphological and Biological Characteristics of Rhynchaenus empopulifolis in Tai’an,China
