Population life tables for the invasive fall armyworm,Spodoptera frugiperda fed on major oil crops planted in China
2021-02-25HELimeiWUQiulinGAOXiwuWUKongming
HE Li-mei ,WU Qiu-lin ,GAO Xi-wu ,WU Kong-ming
1 State Key Laboratory for Biology of Plant Diseases and Insect Pests,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,P.R.China
2 College of Plant Protection,China Agricultural University,Beijing 100193,P.R.China
Abstract The fall armyworm (FAW),Spodoptera frugiperda,is a newly invasive,widespread agricultural pest in China.Understanding the suitability of the main field crops in Chinese agricultural system as host for this polyphagous herbivore is especially important for making control strategy.Here,after FAWs were fed three important oil-bearing crops (oilseed rape,soybean and sunflower)planted in China and resultant population parameters were compared using the age-stage,two-sex life table method,survival of larvae on soybean was significantly lower than that on oilseed rape and sunflower.Developmental duration of larvae on soybean was also the longest (23.3 days).The highest pupation rate was recorded on sunflower.The highest pupal mass (0.19 g)was attained on oilseed rape,significantly higher than on the other host plants; the lowest mass was on soybean (0.15 g).On soybean,oilseed rape and sunflower,respectively,the average generation period was 42.21,39.10 and 40.44 d; the intrinsic rate of increase (r) was 0.0844,0.1041 and 0.1134; the finite rate of increase (λ) was 1.0881,1.1098 and 1.1202.While the most suitable host plant overall was sunflower,S. frugiperda completed development and increased its population on all three host plants.Thus,soybean,oilseed rape and sunflower were all suitable for FAW,and population monitoring and management of FAW in these crops should be increased.
Keywords:developmental duration,fecundity,life history,oil-bearing crops,biology,invasion biology
1.Introduction
The fall armyworm (FAW),Spodopterafrugiperda(J.E.Smith) (Lepidoptera:Noctuidae) (Smith and Abbott 1797),previously known asLaphygmafrugiperda(Vickery 1929; Wilson 1933) was first recorded in 1797 in the United States (Johnson 1987).This highly migratory species is a polyphagous herbivore native to tropical and subtropical America (Luginbill 1928; Sparks 1979) and has a high reproductive rate,relatively short generation time (approximately 30 days) under suitable temperature conditions,high dispersal ability (Luginbill 1928; Dingle 1972),wide host range and distribution during the crop growing season (Knipling 1980).In January 2016,S.frugiperdainvaded the western Africa and rapidly attained outbreak population levels in local maize crops (Goergenet al.2016; Midegaet al.2018),then quickly invaded most African countries,India,Myanmar,Thailand and other Asian countries (CABI 2017; Earlyet al.2018; FAO 2018; Nakweta 2018).On December 11,2018,adult FAWs invaded Yunnan Province in western China (Sunet al.2021).In January 2019,damage fromS.frugiperdalarvae was found in winter corn fields of Jiangcheng County,Yunnan (Yanget al.2019).This insect then spread rapidly to 26 provinces (autonomous regions,municipalities),causing major damage not only to economically important cereal crops such as maize,wheat and sorghum,but also to sugarcane,potato and vegetables(Jianget al.2019).
Spodopterafrugiperdalarvae feed on the leaves,stems and reproductive parts of more than 350 plant species,causing major damage to economically important cultivated grasses such as maize,rice,sorghum,sugarcane and wheat but also other vegetable crops and cotton (Midegaet al.2018; Montezanoet al.2018; Jianget al.2019).At least two strains ofS.frugiperdaexist in nature,i.e.,the “rice-strain”and “corn-strain”,which are defined by their host plant preferences.Larvae of the “corn-strain” likely feed on maize,cotton and sorghum,while those of the “rice-strain” feed on rice and various pastures (Saldamando and Vélez-Arango 2010; Dumaset al.2015).Zhanget al.(2019a) compared the population genetic characteristics of 318 samples of FAWs from 131 counties and cities in Yunnan Province,China,the “corn-strain” accounted for less than 4% of the total samples based on mitochondrialCOIgene,whereas the use ofTpigene indicated the haplotype characteristics of all samples was the “corn-strain”.Genome-wide resequencing of 105 samples from 16 provinces in China further revealed that the FAW population comprises a complex interstrain hybrid,primarily with the “corn-strain” genetic background and less of the “rice-strain” background (Zhanget al.2019b).Tanget al.(2019) found that all FAWs identified using the mitochondrial genes in Chongqing are the “ricestrain”,while the subtypes based on theTpigenes are the“corn-strain” and “rice-strain”.These studies show that the invasive FAWs in China are likely from the offspring of the hybrid population,which may have the biological attributes of both strains and be a potential risk for outbreaks on different host plants.
Of the crops damaged byS.frugiperdalarvae in China,maize is the main host plant (Jianget al.2019; Liu Jet al.2019; Liu Y Qet al.2019; Xuet al.2019; Zhaoet al.2019;Zhou and Yang 2019).In addition,in Yunnan,Hainan,Hunan,Hubei and other areas in China,S.frugiperdalarvae were also found on gramineous weeds such asDigitariasanguinalis(L.) Scop.,Sorghumsudanense(Piper) Stapf.,andEleusineindica(L.) Gaertn.(Jianget al.2019).At present,the risk of FAW damage to oil-bearing crops such as soybean,oilseed rape and sunflower in newly invaded areas in Asia and particularly in China is still unclear.The three agricultural host plants of FAW are cultivated across the word; soybean,oilseed rape and sunflower are also important oil bearing crops widely planted in China.In this study,we assessed developmental variables ofS.frugiperdalarvae after they were fed on soybean,oilseed rape and sunflower in the laboratory and thus established experimental population life tables for the three nongramineous hosts.These life tables will permit the further development of area-wide integrated pest management strategies againstS.frugiperdain soybean,sunflower and oilseed rape fields.
2.Materials and methods
2.1.Larval feeding trials
Laboratory trials were carried out at the Xinxiang Experiment Station of the Chinese Academy of Agricultural Sciences(CAAS; 35°18´13.71´´N,113°55´15.05´´E) in Henan Province,China.Fourth or 5th instar FAW larvae were fieldcollected in early 2019 in Dehong Autonomous Prefecture(Yunnan) and reared for 10 consecutive generations in the laboratory.Insects were kept at (25±1)°C,(75±5)%relative humidity (RH),and 16 h L:8 h D photoperiod.For experimental purposes,larvae were exposed to three different diets (240 larvae for each diet):oilseed rape plants(provided by a farmer in Qiliying Town,Xinxiang County,Henan Province,China),soybean plants (Hongruijidou 17),and sunflower plants (Zhongkeyouba DW776).Eggs that were spawned at the same time were collected and observed daily until hatching,so that larvae could be gently collected individually in 45-mL plastic cups and provided each day with fresh seedlings of the different host plants until pupation.More specifically,greenhousegrown seedlings of sunflower and soybean (20–30 days after sowing) and field-grown plantlets of 4-or 5-leaf stage oilseed rape were exposed to FAW larvae.Larval development and survival were recorded daily until death,pupation or pupal emergence.On the 3rd day after pupation,pupae were weighed on an electronic balance (Mettler Toledo ME204; Beijing Haitian Youcheng Technology Co.,Ltd.,China),then sexed and separated by male and female; the sex of pupae was used to deduce the sex of larvae and eggs.
2.2.Adult development and fecundity
Following pupal emergence,adults (♀:♂=1:1) were kept in 500-mL plastic cups with a sterile gauze stopper and fed daily with 5% (v/v) locust honey water.Moths were kept at (25±1)°C,(80±5)% RH and 16 h L:8 h D photoperiod.On a daily basis,the eggs on the plastic cup or gauze stopper were counted and removed.Eggs were placed in a separate 12 cm×17 cm zip-lock bag to determine eclosion rate.For each FAW individual,we documented pre-oviposition time,daily egg deposition,egg hatching rate and adult survival.Dead female moths were dissected using a stereoscope to count the number of spermatophores present within the spermathecae and thus ascertain mating history.
2.3.Life table parameters
Next,net reproductive rate (R0),generation time (T),intrinsic rate of natural increase (r),and finite rate of increase (λ)of the FAW experimental populations were calculated according to the following equations (Chi and Liu 1985;Chi 1988):
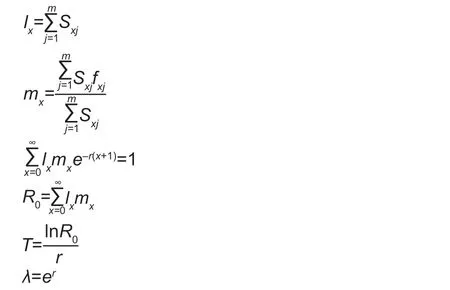
wherexis the time interval in days (d),mis the number of stages,Sxjis the survival rate of FAW from egg development toxdays old and developmental stagej,lx(population agespecific survival rate) is the survival rate of FAW from egg toxdays old,fx10is the age-specific fecundity at agex,mx(population age specific fecundity) is the average population fecundity from egg toxdays old,andlxmx(population agespecific maternity) is the product oflxandmx.
2.4.Statistical analysis
For the three diet treatments,differences in life history parameters were analyzed using a one-way analysis of variance (ANOVA),with proportional data first arcsine square-root-transformed to meet assumptions of normality and heteroscedasticity.Tukey’s honestly significant difference (HSD) was used as a post hoc test.Student’st-test was used to test for differences in developmental duration between different genders.Log rank was used to test the difference and linear trend of survival curves of FAW fed on three host plants,and a logistic model was used to fit the survival rate of FAW population at a specific age.The model equation was:
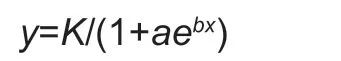
wherea,b,andKare model parameters,yis the survival rate of FAW from egg development toxdays old,respectively.
The program SPSS version 20 (IBM,Armonk,NY,USA)was used for all tests,except for the application for the log rank test in GraphPad Prism 7 (GraphPad Software Inc.,San Diego,CA,USA) and calculation of life table parameters in TWOSEX-MSChart (Chi 2019).
3.Results
3.1.Larval and adult development
FAW successfully completed its development on all host plants,with larval diet greatly affecting various developmental parameters (Tables 1 and 2).More specifically,significant differences were recorded in the developmental duration of 1st instar larvae (F2,716=7.329,P=0.001),2nd instar larvae(F2,615=3.399,P=0.034),3rd instar larvae (F2,570=39.773,P<0.001),4th instar larvae (F2,531=69.602,P<0.001),5thinstar larvae (F2,471=63.120,P<0.001),6th instar larvae(F2,343=5.662,P=0.004),larval stage (1st–7th instar)(F2,321=120.304,P<0.001),pupae (F2,230=43.988,P<0.001)and a combined larva–adult stage (F2,221=8.196,P<0.001)on different diets.Larval development on soybean was considerably slower than on oilseed rape and sunflower,and the shortest larval developmental duration was recorded on oilseed rape,while pupal developmental duration was the longest on oilseed rape and the shortest on soybean.Furthermore,the ratio of 7th instar larvae fed on soybean,oilseed rape and sunflower were 15.42,0.42 and 7.08%,respectively.
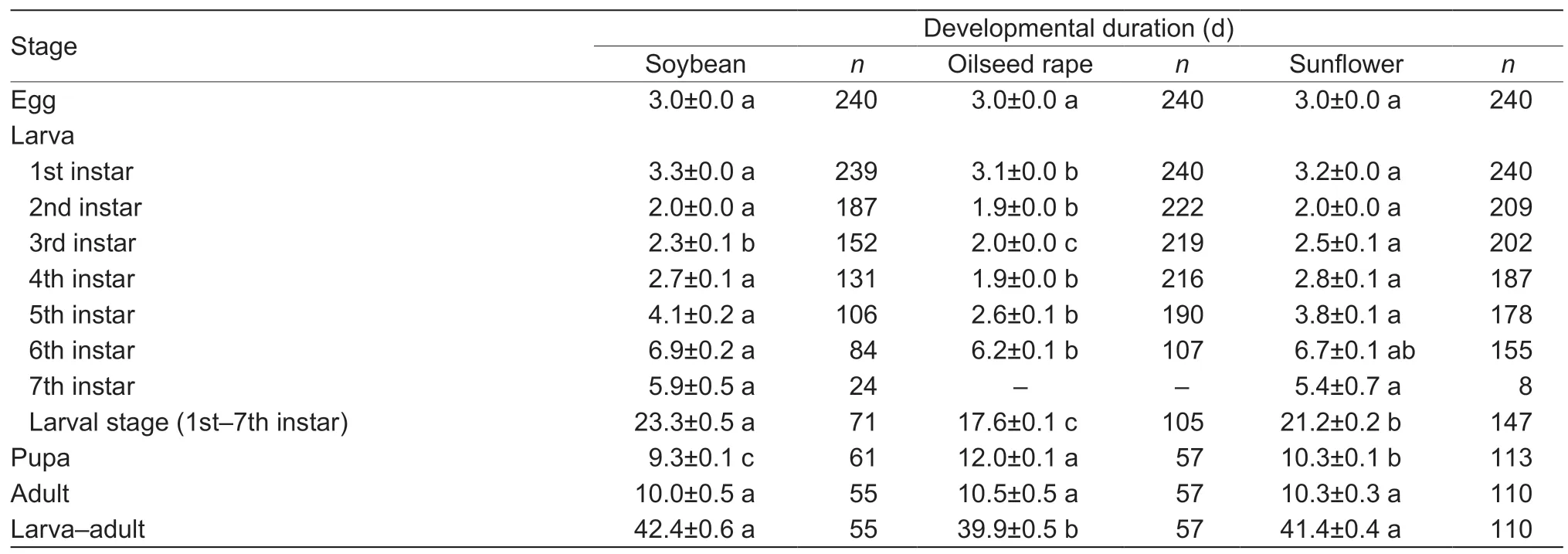
Table 1 Mean duration of developmental stages of Spodoptera frugiperda larvae and adults after larvae fed on different host diets in the laboratory
The developmental duration of larvae,pupae and adults fed on these three host plants differed between sexes (Table 2).With the soybean diet,the developmental duration of 7th instar larvae (t=–2.71,df=23,P<0.05) and pupae (t=–2.70,df=59,P<0.05) of females was significantly shorter than that of the males,while the longevity of female moths (t=2.77,df=53,P<0.05) was significantly longer than that of the male moths.With the oilseed rape diet,the developmental duration of 6th instar larvae (t=–3.03,df=98,P<0.05),larval stage (t=–2.42,df=98,P<0.05) and pupae (t=–7.23,df=55,P<0.05) of males was significantly longer than those of females.For the sunflower diet,pupal developmental duration of females was significantly shorter than that of males (t=–8.56,df=111,P<0.05),while the longevity of female moths was significantly longer than that of male moths (t=2.06,df=108,P<0.05).When larvae fed on soybean,oilseed rape and sunflower,female moths appeared 3,6 and 5 days,respectively,earlier than the male moths.
3.2.Pupal and adult reproductive characteristics
Larval diet greatly affected pupation rate (F2,6=14.766,P=0.005),survival rate of pupa (F2,6=21.138,P=0.002),pupal emergence (F2,6=12.620,P=0.007) and pupal mass(F2,314=47.275,P<0.001) of FAW (Table 3).The pupation rate of FAW was the highest on sunflower,which was significantly higher than on soybean and oilseed rape.The pupal mass of FAW fed on oilseed rape was 0.1925 g,significantly higher than on the other two hosts,and the pupal mass of females fed on soybean (t=–3.29,df=68,P<0.05)and sunflower (t=–2.93,df=145,P<0.05) was significantlylower than that of males on the respective diets (Table 3).Sex ratio of pupa,pre-oviposition,total pre-oviposition and oviposition period,oviposition number per female,hatching rate of eggs and mating frequency of female did not differ significantly among the three diets (Tables 3 and 4).
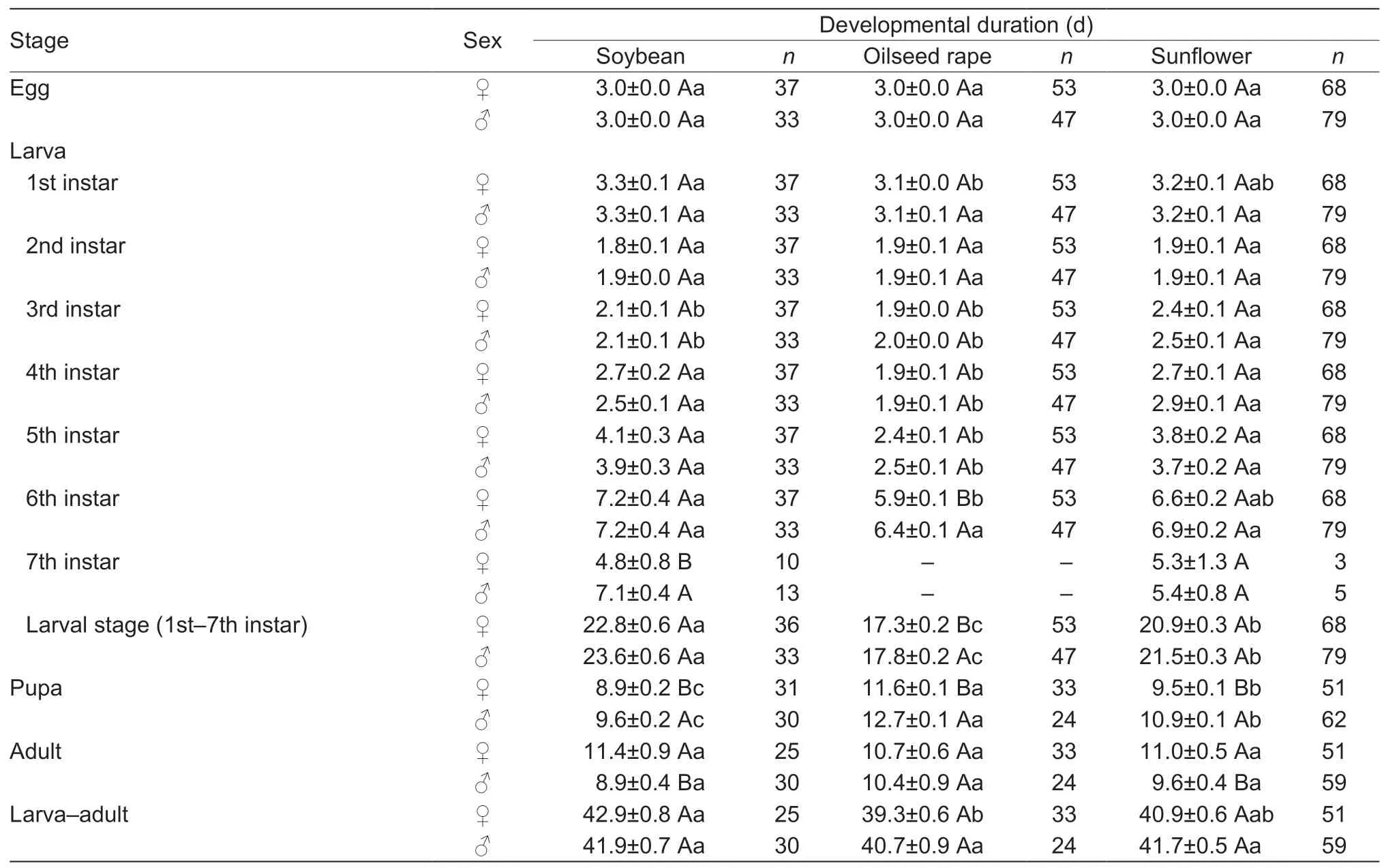
Table 2 Mean duration of developmental stages of male and female Spodoptera frugiperda after larvae fed on different host diets in the laboratory
3.3.Survival and fecundity
Larval diet greatly affected the age-stage specific survival rate ofS.frugiperda(Fig.1).The survival rate of FAW larvae was the lowest for all ages on soybean,and the highest wasrecorded on oilseed rape.From egg development to mature larvae,the survival rate of FAW on oilseed rape,sunflower and soybean was 0.79,0.74,and 0.44,respectively,whereas from egg development to pupae,the highest survival rate (0.58) was recorded on sunflower,which was 0.33 and 0.19 higher than on soybean and oilseed rape.To the adult stage,the survival rate was 0.21,0.23 and 0.46 on oilseed rape,sunflower and soybean,respectively.
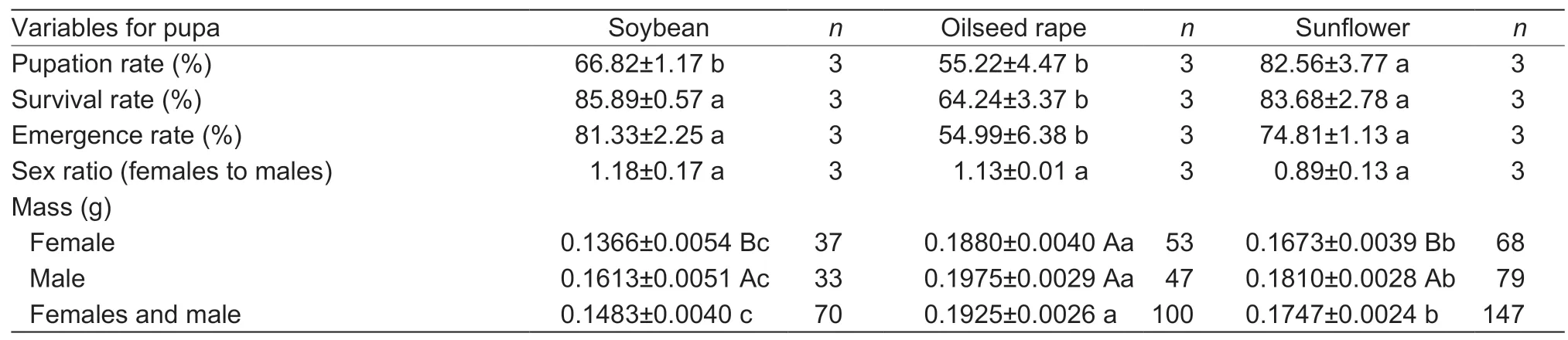
Table 3 Developmental variables for pupa of Spodoptera frugiperda after larvae fed on different host diets in the laboratory
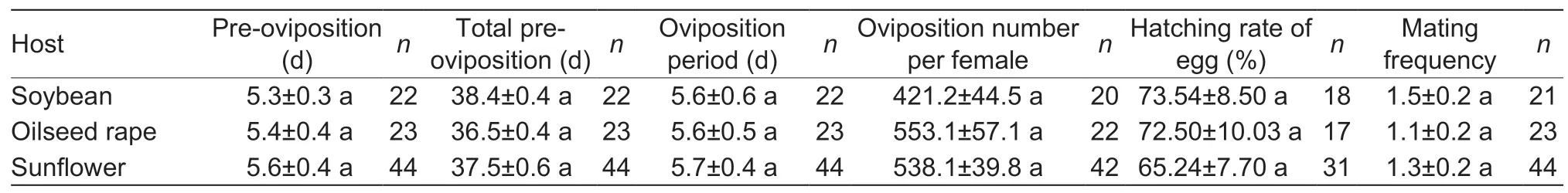
Table 4 Fecundity variables for adult Spodoptera frugiperda after larvae fed on different host diets in the laboratory
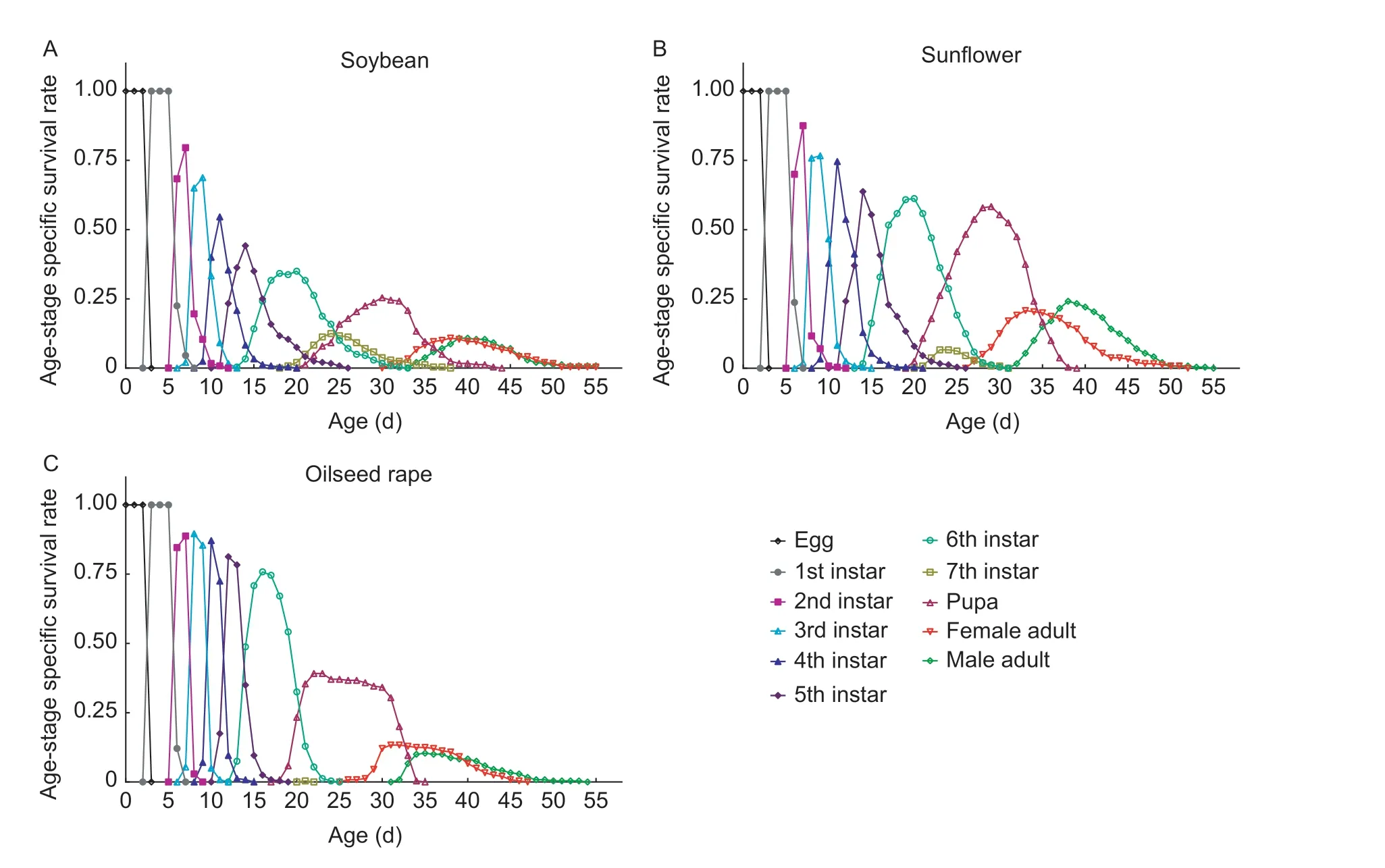
Fig.1 Age-stage specific survival rate of Spodoptera frugiperda after larvae were reared in the laboratory on soybean (A),sunflower(B) and oilseed rape (C).
There was a significant difference in the curveslxof FAW fed among the three hosts (χ2=30.33,df=2,P<0.001),and they had a significant linear trend (χ2=23.64,df=1,P<0.001).Thelxcurves of FAW exhibited a downward trend over time on soybean (y=3.149/(1+1.842e0.058x),R2=0.975,Fig.2-A),oilseed rape (y=1.196/(1+0.121e0.106x),R2=0.982,Fig.2-B) and sunflower (y=0.912/(1+0.013e0.124x),R2=0.957,Fig.2-C).Thelxcurve of FAW on soybean was declined rapidly,and the survival rate had decreased by more than 0.5 on days 4 to 14.Thelxcurves for FAW on oilseed rape and sunflower decreased slowly at the early stage (from zero to 15th day).From day 16 to 21(late larval development),thelxcurve for FAW on oilseed rape was declined rapidly,which indicated an extremely high mortality rate at this stage.In the adult stage,thelxcurves of FAW on these three hosts showed a similar downward trend.
The curves of population age-specific fecundity (mx)and population age-specific maternity (lxmx) showed that the peak spawning day on soybean,oilseed rape and sunflower was on days 39,34 and 34,respectively (Fig.2).The maximum value for the age-specific fecundity of female adult (fx10) fed on soybean,oilseed rape and sunflower was 61.74,95.27 and 69.53,respectively (Fig.2).
3.4.Population dynamics
Life table parameters reflected howS.frugiperdaattained the longest generation time,and the smallest net reproductive rate,intrinsic rate of natural increase,finite rate of increase on soybean (Table 5).Conversely,on sunflower,FAW attained the fastest population growth,with second shortest mean generation time,and the largest net reproductive rate,intrinsic rate of natural increase and finite rate of increase.
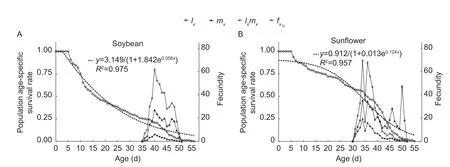
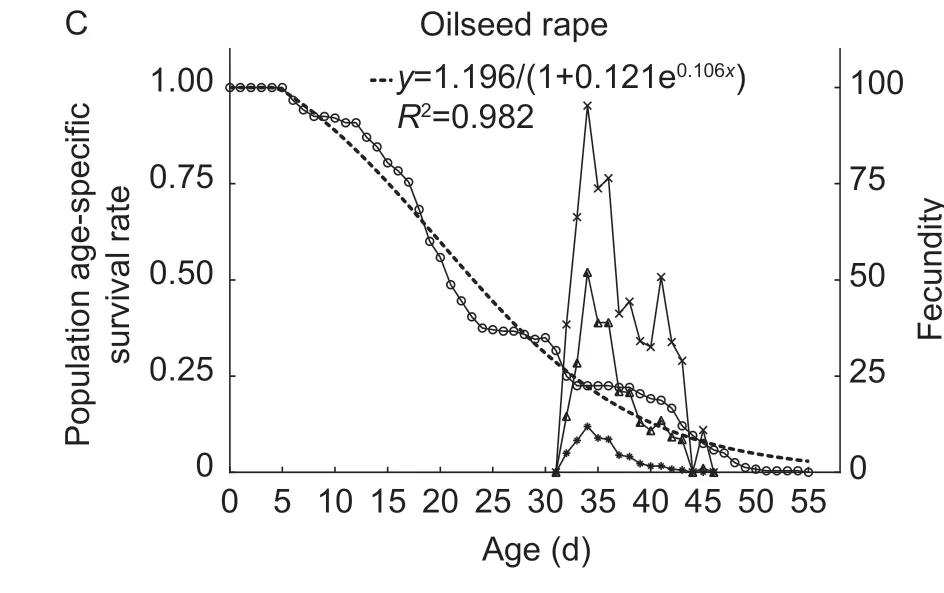
Fig.2 Population age-specific survival rate (lx),population agespecific fecundity (mx),population age-specific maternity (lxmx)and age-specific fecundity of female adult (fx10) of Spodoptera frugiperda after larvae were reared in the laboratory on soybean(A),sunflower (B) and oilseed rape (C).
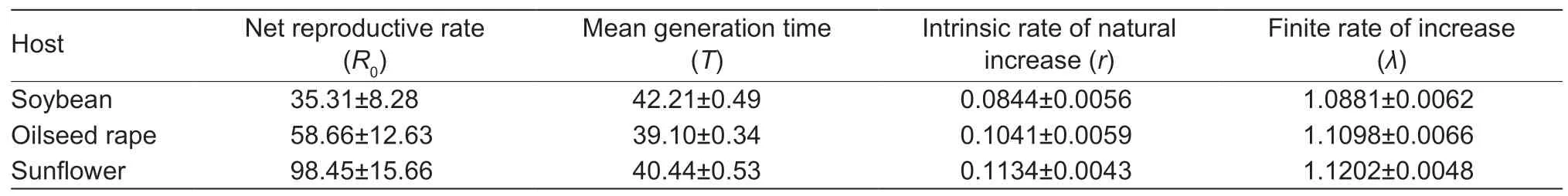
Table 5 Life table parameters of Spodoptera frugiperda after larvae fed on different host diets in the laboratory
4.Discussion
FAW is a polyphagous pest with extensive adaptability to more than 350 host plants.The broad host range of polyphagous insects includes plants of varying palatability,nutritional content and secondary metabolites;thus,the plant species can differentially impact growth,development,reproduction and overall population fitness of the lepidopterans (Hou and Sheng 2000; Montezanoet al.2018).The higher survival rate and shorter developmental duration of phytophagous insects in their larval stage are important measures of host fitness (Moreauet al.2006).Here,we empirically demonstrated the effects of larval diet onS.frugiperdadevelopment.The FAWs completed development on soybean,oilseed rape and sunflower plants,with the longest larval developmental duration and the lowest survival on soybean plants.However,survival rate was higher and developmental duration was shorter on oilseed rape and sunflower plants.These findings were similar to previous studies that the FAW larvae fed on soybean had longer larval development cycles and lower larval survival rate than on maize,while the FAW larval survival rate on sunflower was higher than on maize (Barroset al.2010;Diaset al.2016; Silvaet al.2017).FAW larvae normally complete six larval instars,but they may complete between six and seven depending on temperature and host plant availability (Luginbill 1928).The proportion of 7th instar larvae on soybean was 2.18 and 36.71 times higher than that on sunflower and oilseed rape,respectively.These results indicated that oilseed rape and sunflower are more suitable host plants for increasing the FAW population(Yunnan,China) than soybean.During the trial,we found that soybean leaves with thinner mesophyll lose water faster than oilseed rape and sunflower leaves with thicker mesophyll,which might be responsible for the lower survival rate on soybean plants.Maize,wheat,sorghum and other gramineous plants are widely considered as highly suitable host plants for FAW (Barroset al.2010; Wu Z Wet al.2019; Baet al.2020; Heet al.2021),and our trials indicate that oilseed rape,soybean and sunflower are also suitable host plants for one of the Chinese strains ofS.frugiperda.The survival rates of larvae on sunflower and oilseed rape are almost the same as those on sugarcane and wheat (Wu Z Wet al.2019; Baet al.2020).The curves for the age-stage survival rate of various FAW instar larvae on all host plants were overlapping,similar to the results on maize,wheat,rice and sugarcane (Wu Z Wet al.2019; Baet al.2020) because individual development was inconsistent,which also coincides with the overlapped generations in field.
Overall nutritional status and fitness of larvae are likely to be mirrored by characteristics of the pupal stage (e.g.,pupation rate,pupal duration,emergence,mass,size)(Eduardoet al.2018; Liet al.2019).In our study,FAW attained the highest rate of pupation and survival rate of the pupae on sunflower but developed the most rapidly and attained the highest pupal mass on oilseed rape.Furthermore,there was a significant difference in pupal duration between genders of FAW fed on the same host plants,and female pupae emerged 1–3 days earlier than the male pupa did,as also observed for FAW that fed on peanut (Heet al.2020).Adult fecundity mainly depends on the accumulated nutrition in the larval stage (pupal mass)and supplementary nutrition in the adult stage (Houet al.2000; Awmack and Leather 2002; Jabraeilet al.2014).In our study,higher (though not statistically different) fecundity was recorded for the oilseed rape and sunflower plants.On soybean,FAW attained the longest development duration and the lowest pupal mass and fecundity,which may be due to a possible imbalance in the C/N ratio and a related metabolic burden on larval development (Wu and Liet al.1993),as also found forHelicoverpaarmigera(Hübner)(Houet al.2000).
Under optimal larval feeding conditions,insects will improve their fitness (Barroset al.2010).The suitability of particular (larval) host plants for insect development can be assessed suing different fitness currencies or life table parameters,e.g.,birth rate,mortality,or development rate.In this study,randλof the FAW experimental population fed on all diets were lower than those that fed on maize,sorghum,wheat,rice,sugarcane and peanut (Wu Z Wet al.2019; Baet al.2020; Heet al.2020,2021).Values forrandλwere greater than 0 and 1,respectively,indicating that oilseed rape,sunflower and soybean were suitable for FAW population development.In addition,the largestrandλfor the FAW experimental population were attained on sunflower,which indicated sunflower was the most suitable host in our study.
Since its invasion of western Yunnan (China) on December 11,2018 (Sunet al.2021),FAW has formed a source base in tropical and southern subtropical areas of China (Jianget al.2019).Wu Q Let al.(2019) reported that FAW adults in tropical and subtropical areas migrated mainly toward the northeast.In March to May,south of the Yangtze River was the source site and the main landing area for the northward movement of the moths.After two nights,they can reach the northern part of the Yangtze River to the southern part of the Yellow River.In June to July,with three consecutive nights of flight,the moths can reach a wide area stretching from the northern part of the Yellow River to Inner Mongolia and the southeastern section of northeastern China.In February to April,oilseed rape in the drainage areas of the Yangtze River has entered a critical period of growth and development,and thus provides a suitable host (adult oviposition or larval food) for FAW immigrants.From the oilseed rape field,FAW can then spread to other host plants (e.g.,maize,wheat) or continue to migrate toward the northeast.In April to July,sunflower and spring soybean (planted in the southern and northern drainage areas of the Yangtze River and northeastern China) and the spring oilseed rape (planted in northwestern and northeastern China) can provide abundant food for the population development and reproduction of FAW.From August to September,FAW can move to summer soybean in the Yellow and Huai River Valleys in China.After maize,soybean and sunflower are harvested in October,winter oilseed rape (planted in Central China and the drainage areas of the Yangtze River) provides food for the local FAW population or autumn migrants.The growth period of these three oil-bearing crops in China changes with the seasons and the latitudes from south to north.The resources of the hosts complement each other in time and space and thus provide sufficient food for the regional migration of FAW.Thus,we should strengthen monitoring and early warning work based on the growth period of soybean,oilseed rape and sunflower and the migration trajectory of FAW.
5.Conclusion
Here we demonstrated that invasiveS.frugiperdapopulations from Yunnan (China) can complete their development on oilseed rape,soybean and sunflower plants,and sunflower plant was the best diet for FAW growth,development and reproduction.Therefore,in the absence of maize,sorghum,sugarcane and other highly suitable hosts,the potential for an outbreak of FAW is high for soybean,sunflower and oilseed rape fields.Population monitoring in these fields needs to be strengthened to better determine the incidence of FAW over time and implement preventive and control strategies in advance.As FAW is used to migrate toward other regions from tropical and sub-tropical areas each year in eastern Asia,much in-field population ecology research is needed,and its importance should not be disregarded.Ultimately,field-derived data can be used with our present findings to generate robust predictive models as a foundation for area-wide integrated pest management programs for this newly invasive pest.
Acknowledgements
This work was supported by the National Key R&D Program of China (2019YFD0300102),the earmarked fund for China Agriculture Research System (CARS-15-19) and the Central Public-interest Scientific Institution Basal Research Fund,China (CAAS-ZDRW202007).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China
- Biology,invasion and management of the agricultural invader:Fall armyworm,Spodoptera frugiperda (Lepidoptera:Noctuidae)
- Case study on the first immigration of fall armyworm,Spodoptera frugiperda invading into China
- Windborne migration routes of newly-emerged fall armyworm from Qinling Mountains–Huaihe River region,China
- Laboratory-based flight performance of the fall armyworm,Spodoptera frugiperda
- Adult nutrition affects reproduction and flight performance of the invasive fall armyworm,Spodoptera frugiperda in China
