Laboratory-based flight performance of the fall armyworm,Spodoptera frugiperda
2021-02-25GEShishuaiHELimeiHEWeiYANRanKrisWYCKHUYSWUKongming
GE Shi-shuai ,HE Li-mei ,HE Wei ,YAN Ran ,Kris A.G.WYCKHUYS ,WU Kong-ming
1 State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops,Institute of Applied Ecology,Fujian Agriculture and Forestry University,Fuzhou 350002,P.R.China
2 State Key Laboratory for Biology of Plant Diseases and Insect Pests,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,P.R.China
3 College of Plant Protection,Henan Agricultural University,Zhengzhou 450002,P.R.China
Abstract In late 2018,the fall armyworm (FAW) Spodoptera frugiperda Smith (Lepidoptera:Noctuidae) made its arrival in China and its populations have since proliferated across most of eastern Asia.While S.frugiperda exhibits a considerable dispersal capacity and engages in long-distance migration,there’s only scant information on the species’ flight capability.Here,we empirically assessed S.frugiperda flight activity under varying climatic conditions using a flight mill.More specifically,under laboratory conditions,FAW exhibited superior flight performance at 20–25°C and 60–90% relative humidity (RH).When quantifying flight performance over five consecutive nights (i.e.,10 h/night),all flight parameters initially increased and then gradually dropped and FAW adults attained a total flight distance,duration and velocity equal to 63.73 km (48.42–94.12 km)(median,quartile range),24.12 h (20.87–27.73 h) and 2.73 km h–1 (2.13–3.33 km h–1),respectively.Our work constitutes a first comprehensive assessment of S.frugiperda flight performance and provides baseline information for future efforts to forecast spatiotemporal changes in its geographical distribution,movement patterns and invasion trajectories.Such can ultimately permit a timely and targeted deployment of area-wide pest management measures against this newly-invasive pest in China and across eastern Asia.
Keywords:aerobiology,flight mill,flight capacity,migration,invasion biology,movement ecology
1.lntroduction
Originating from the Neotropics,the fall armyworm (FAW;SpodopterafrugiperdaSmith) has recently invaded both the African and Asian continents (Sparks 1979; Goergenet al.2016; Sharanabasappaet al.2018; IPPC 2019).The species’ invasion process and its recurrent population outbreaks in its native range and newly-invaded areas are facilitated by various biological characteristics and life history traits,e.g.,high fecundity,polyphagous feeding habit and ecological plasticity (Muruaet al.2006; Montezano 2018).Also,host-associated genetic differentiation has led to an emergence of two different FAW strains,i.e.,“corn-strain”and “rice-strain”,with the former primarily feeding on corn,cotton,sugarcane and sorghum,and the latter specifically adapted to rice and various grasses (Pashley 1986; Dumaset al.2015).On corn,FAW larvae voraciously feed on foliage and cause extensive damage to seedlings,with pest attack occasionally resulting in total crop loss (Baudronet al.2019).In addition to acting as a ‘cutworm’ on seedlings,S.frugiperdalarvae consume vegetative and reproductive tissues of the corn plant and thus either directly or indirectly lower crop yield and quality of harvested produce (Andrews 1980; Hruska and Gladstone 1988; Wyckhuys and O’Neil 2006; FAO 2017).In FAW-invaded areas of Africa,pest attack reportedly reduces corn production levels by 25–67%(Megersa and Tamiru 2018),though an average 12% pestinflicted yield loss is likely to be a more realistic estimate(Baudronet al.2019).
In late 2018,S.frugiperdamade its arrival in China(Sunet al.2021) and subsequently invaded 26 different provinces (autonomous regions,municipalities) except for Xinjiang,Qinghai and northeastern China (Jianget al.2019).Invasive populations pertain to the “corn-strain” (Zhanget al.2019) and currently affect 1.1 million hectares of China’s corn crop and 15 000 hectares of other locally-grown crops.In 2019,pest-inflicted damage was the highest during summer months (i.e.,June–August) with either southern and western provinces reporting the highest infestation levels (Jianget al.2019).This exceptionally rapid invasion across two continents (or within China’s national territory)can be ascribed to the long-distance dispersal behavior ofS.frugiperda(Dingle 1996).This allows FAW adults to enter the southern Sahara from western parts of the African continent in one single night or over few consecutive nights(Earlyet al.2018).Furthermore,in its native range,FAW migrates northward during spring and reaches Canada’s provinces of Ontario and Quebec by the end of summer(Roseet al.1975; Mitchellet al.1991).Given that FAW exhibits no diapause,its annual northward spread is anticipated to initiate in China’s southern-most provinces or in neighboring Vietnam,Laos and Myanmar potentially aided by the East Asia monsoon circulation (Wuet al.2019).As such,under suitable conditions,S.frugiperdacan enter northern parts of the Yangtze River or northeastern China in as little as two or three nights,respectively (Wuet al.2019).
This long-distance movement is enabled through a particularly strong flight performance of migrant individuals and further modulated by the prevailing meteorological conditions (Kroderet al.2006; Zhanget al.2008; Fuet al.2017).Overall,insect flight performance is shaped by climatic parameters such as temperature or relative humidity (RH),and further aided by wind patterns.Though laboratory-based assessments have been made of wingbeat frequency and flight performance under varying climatic conditions for various insect species (Camposet al.2004;Deakin 2010),these kinds of experimental assays so far have not been conducted forS.frugiperda.
In this paper,we use a flight mill apparatus to assess flight activity of FAW adults under different temperatures and RH levels.Our work allows identifying suitable climatic conditions for long-distance flight of newly-invasive FAW populations and provides valuable insights intoS.frugiperdaflight biology and its associated migration behavior.
2.Materials and methods
2.1.Study insects
In January 2019,S.frugiperdalarvae were hand-collected in corn fields of Dehong Autonomous Prefecture (Yunnan,China) and transferred to the laboratory.Field-collected larvae were fed with corn leaves until pupation.After pupal emergence,adults were mated and the resulting offspring was maintained on a soybean flour and wheat bran-based artificial diet (Lianget al.1999) under controlled conditions at (25±1)°C,(70±10)% RH and 16 h L:8 h D photoperiod.The experimental population was drawn from different generations of this laboratory population.Male and female pupae were separated and placed in glass culture dishes with moistened cotton.Following pupal emergence,male and female moths were prevented from mating,maintained separately within 30 cm×30 cm×30 cm cages and fed on a daily basis with 10% honey water solution.Three-day old adults were then subjected to experimental assays.
2.2.Experimental assays
Flight performance (i.e.,flight distance,duration and velocity) ofS.frugiperdaadults was assessed using a FXMD-24-USB flight mill (Jiaduo Science,Industry and Trade Co.,Ltd.,Henan,China).More specifically,the flight mill was placed in a 70 cm×50 cm×114 cm MGC-450HP artificial climate chamber (Yiheng Technology Instrument Co.,Ltd.,Shanghai,China) andS.frugiperdaadults were individually attached to the apparatus following Fuet al.(2017).In brief,active and undamaged FAW adults were gently collected from the rearing cage and individualized within a 1.5-mL cryopreservation tube.Next,each FAW individual was weighed and lightly anesthetized with ether.Wings of theS.frugiperdaadult were expanded,scales were brushed from the intersect of abdomen and thorax,and tethering was done using a small droplet of 502 glue (Deli Group Co.,Ltd.,Zhejiang,China).After adult recovery,only undamaged and active individuals were attached to the flight mill -as kept under set temperature and RH.Next,the climate chamber was then completely darkened and moths were allowed to engage freely in tethered flight.Wingbeat frequency of tethered individuals was assessed three times before each flight test using a Phaser Strobe PBX stroboscope (Monado Monarch,Amherst,New Hampshire,USA).For each tested individual,Jiaduo Insect Fly Information System Software was used to convert specific recordings (e.g.,number of mill revolutions per second) to different flight parameters such as total flight distance,duration and average velocity.
Flight experiments were either conducted at five different temperatures (i.e.,10,15,20,25 and 30°C) with a fixed 75% RH,or at five different RH regimes (i.e.,30,45,60,75 and 90%) with a fixed temperature of 25°C.Each FAW individual was flown between 8 p.m.and 8 a.m.on the subsequent day,after which the moth was removed from the apparatus and weighed again.Based upon these assays,optimum temperature and RH forS.frugiperdaflight were ascertained.
Next,tethered flight trials were conducted over the course of five consecutive nights under optimum climatic conditions (i.e.,25°C and 75% RH).One-day old female and male moths were selected and subject to tethered flight from 9 p.m.until 7 a.m.on the following day.FAW adults were subsequently removed from the apparatus and allowed to feed on 10% honey water solution.For a given FAW individual,this procedure was repeated over five successive days.
2.3.Data analysis
Earlier work had shown no statistically-significant differences in flight performance betweenS.frugiperdaadult sexes (Geet al.2019).Prior to analysis,all data were tested for normality by Shapiro-Wilk test and for homogeneity of variances by Levene’s test.To assess differences in flight parameters (i.e.,flight distance,flight duration,flight velocity and wingbeat frequency) among treatment groups,a non-parametric Kruskal-Wallis test was performed followed by a Bonferroni-adjusted significance test for pairwise comparison at the 0.05 significance level.The extent of weight loss was either compared using analysis of covariance (ANCOVA) -the latter with temperature as fixed factor,weight loss as response variable,and initial body mass as covariate.All data were analyzed using SPSS 20.0 (SPSS 2011).
3.Results
3.1.Effect of temperature on flight parameters
Over the range of experimental temperatures,significant differences were recorded in flight distance (Kruskal-Wallis,H=56.777,P<0.001),flight duration (H=32.374,P<0.001),mean flight velocity (H=62.781,P<0.001) and wingbeat frequency (H=169.684,P<0.001).Over a 12-h assessment period,all parameters initially increased and then gradually dropped.The longest flight distance and the highest velocity were obtained at 25°C,while flights with the longest duration were recorded at 15°C.Wingbeat frequency increased at higher temperatures with maxima recorded at 30°C (Table 1).Hence,S.frugiperdaadults engaged in normal flight at 15–30°C,with the optimal flight temperature range 20–25°C.
When accounting for initial body mass,the flight-related degree of weight loss was not affected by temperature (ANCOVA,F=1.422,P=0.227).Adjusted weight loss generally increased at higher temperatures,with the highest extent of weight loss observed at 30°C and the lowest at 10°C (Table 2).
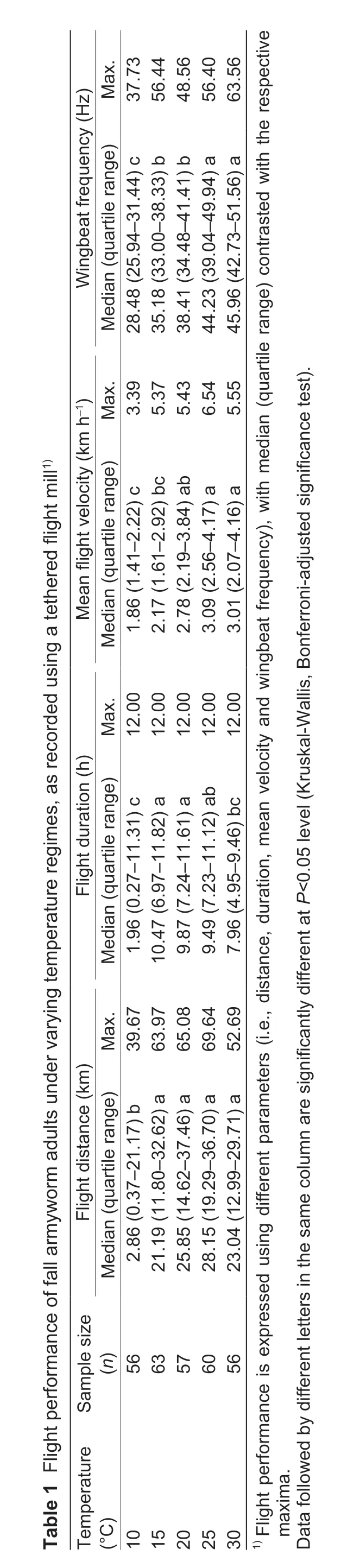
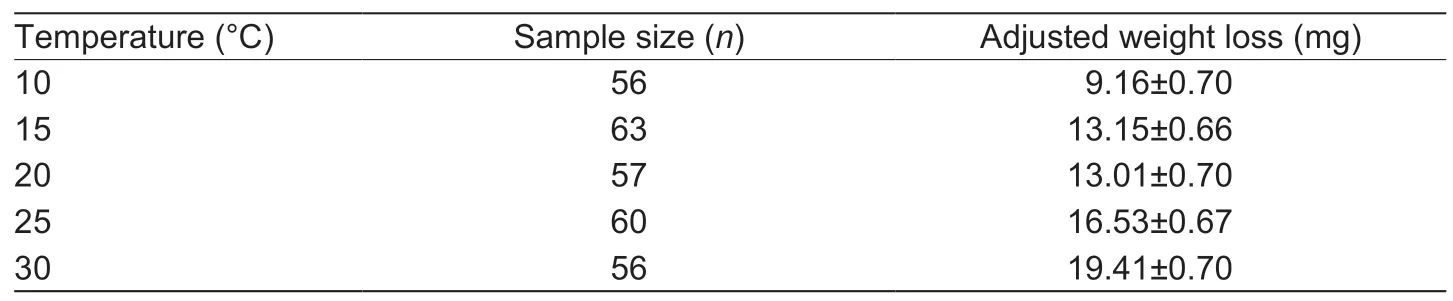
Table 2 Adjusted weight loss of fall armyworm adults,following tethered flight under varying temperature regimes
3.2.Effect of relative humidity on flight parameters
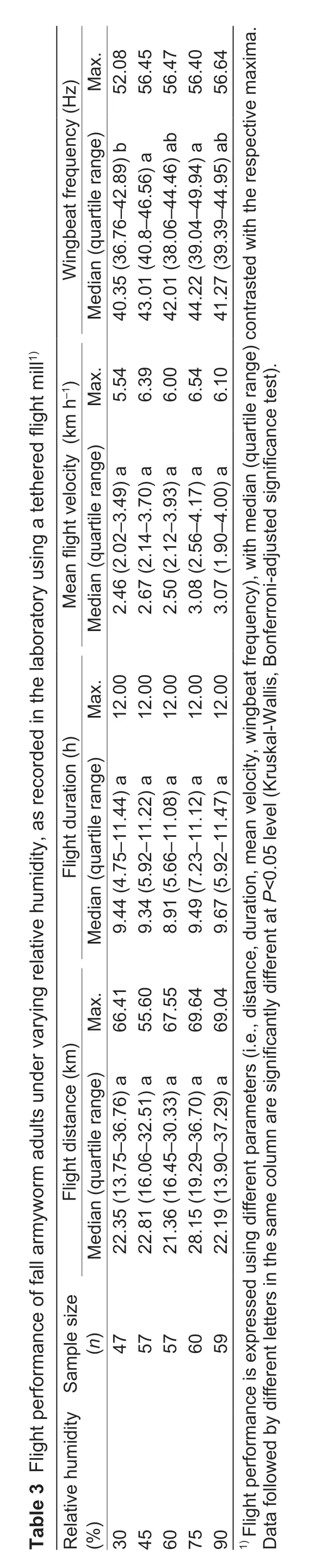
Over the range of experimental humidity regimes,significant differences were recorded in wingbeat frequency (Kruskal-WallisH=13.786,P=0.008),with the frequency at 30%RH lower than at 75% RH (Table 3).Yet,flight distance (H=7.453,P=0.114),duration(H=0.814,P=0.936) or velocity (H=6.778,P=0.148) exhibited no differences between humidity regimes.Hence,S.frugiperdaadults engaged in normal flight over 30–90%RH,with optimum flight performance over 60–90% RH.
When accounting for initial body mass,the flight-related degree of weight loss was not affected by relative humidity (ANCOVA,F=2.325,P=0.057).As such,the highest extent of weight loss was observed at 30% and the lowest at 90% RH (Table 4).
3.3.Flight capacity
When quantifying FAW flight performance over a 5-day period,significant differences were recorded in flight distance (Kruskal-WallisH=31.140,P<0.001),duration (H=23.194,P<0.001) and velocity (H=14.017,P=0.007) between successive nights.Overall,S.frugiperdaadults exhibited the strongest flight performance on the second night and all flight parameters gradually weakened on the subsequent days (Table 5).
Overall,84% adults flew over more than 40 km,with the longest flight distance being 163.58 km.Furthermore,76% adults engaged in flight over more than 20 h,with the longest flight time being 46.73 h (as recorded over a total experimental period of 5×10 h) (Fig.1).
4.Discussion
The FAW,S.frugiperda,has an exceptional capacity for long-distance dispersal,as exemplified by its rapid invasion of both African and Asian continents over the past 3–4 years (Earlyet al.2018; Nagoshiet al.2018) and by its yearly multi-generational migration from the subtropics into North America (Westbrooket al.2019).Though its dispersal ability is well-recognized,so far no empirical assessment has been made ofS.frugiperdaflight behavior.Here,we relied upon laboratory-based flight mill assays to characterize how environmental conditions shape multiple FAW flight parameters.Overall,FAW adults engaged in flight over a range of experimental climatic conditions and exhibited the strongest flight performance under 20–25°C and at 60–90% RH.
Low temperature can reduce flight speed and wingbeat frequency,andS.frugiperdaadults flight performance was particularly weak at 10°C.Wingbeat frequency gradually increased at higher temperatures,reaching maximum level at 30°C.Yet,at these temperatures,average flight speed dropped as adults were unlikely to engage in sustained energy-demanding flight (Farmery 2011).Moreover,water consumption and metabolic rate are also elevated under higher temperatures (Caiet al.2002).In contrast,humidity regimes only had a minor impact on overallS.frugiperdaflight performance in a similar fashion as for other insect species (Druryet al.2016; Venter 2019).Yet,as RH did affect FAW wingbeat frequency and body weight loss,we hypothesize that low humidity conditions accelerate water consumption,ultimately inhibiting metabolism and interfering with flight activity (Suet al.2014).
The above climatic parameters not only shape flight performance and equally impact migration processes in myriad ways.For example,temperature can determine appearance and behavior of certain migratory morphs(Rankin and Burchsted 1992).For the rice leaf rollerCnaphalocrocismedinalis,high summer temperatures induce reproductive stagnation and northward migration(Wuet al.1985) while RH can affect migration direction and flight altitude (Wanget al.2006).For other migratory species,temperature thresholds have been identified for flight initiation (Smith 1998) and prevailing temperatures also determine the exact altitude or atmospheric layer for migratory flight (Woodet al.2006; Reynoldset al.2009).On the other hand,adverse conditions,e.g.,rainfall can alter landing behavior,raise energy consumption and ultimately compromise migration success.
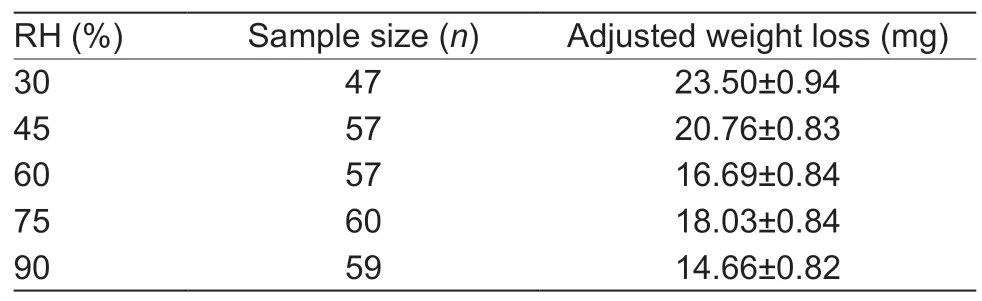
Table 4 Adjusted weight loss of fall armyworm adults,following tethered flight under varying relative humidity (RH) regimes
Our 5-day flight performance trial revealed a strong flight capability ofS.frugiperda,as exemplified by flight distance of 63.73 km (48.42–94.12 km) and maximum of 163.58 km.Also,FAW engaged in flight over 24.12 h(20.87–27.73 h) with the longest flight duration 46.73 h (as recorded over a 50-h experimental period).These findings are similar to those recorded for other migratory insects,such asSpodopteraexigua(Hübner) andMacdunnoughia crassisigna(Jiang and Luo 2010; Fuet al.2017).Given that FAW adults generally exhibited poor flight performanceon the first night,we suspect that either young individuals were physiologically unprepared for flight (Zera and Mole 1994) or the necessary thoracic muscles were not yet fully developed (Dickinson 2006).Following a strong flight performance on the second night,S.frugiperdaflight structure likely degraded and the ensuing flight performance declined (Blackmeret al.2005).However,when allowed sufficient access to carbohydrate-rich food and daytime recovery,S.frugiperdaengages in sustained flight over the course of several nights,as equally recorded for other long-distance migratory insects such asC.medinalisorMythimnaseparata(Yanget al.2016; Liuet al.2017).Yet,other migratory insects such as the planthoppersSogatella furciferaandNilaparvatalugensgenerally only engage in one single long-distance flight (Jianget al.2004; Rosenberget al.2008).
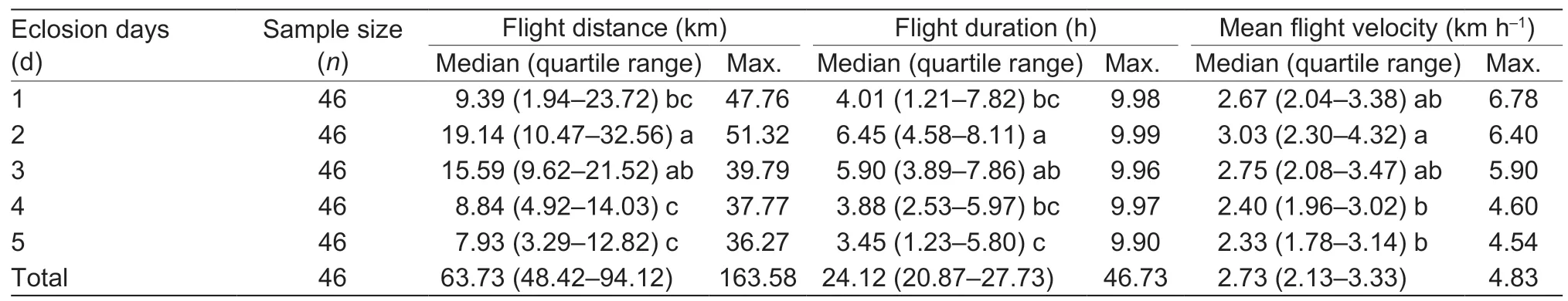
Table 5 Total flight performance of fall armyworm (FAW) adults over a 5-day period,as recorded in the laboratory using a tethered flight mill1)
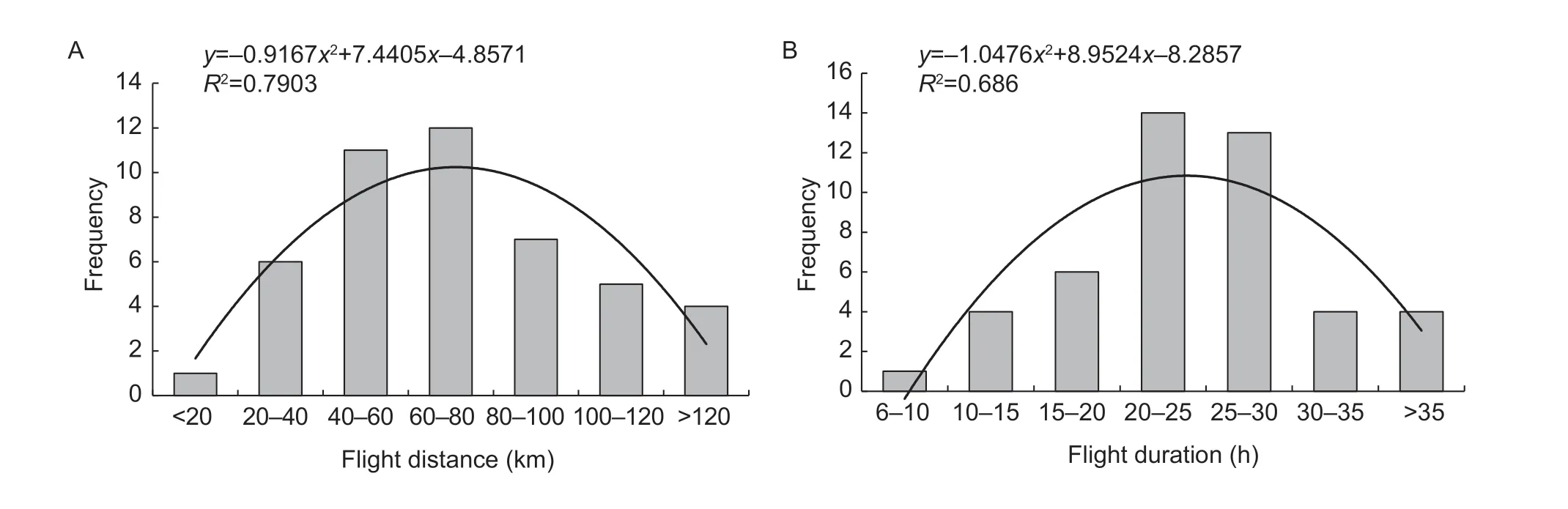
Fig.1 Frequency distribution of total flight distance and duration of fall armyworm adults subjected to tethered flight over five consecutive nights (i.e.,10 h/night).Flight parameters were recorded in the laboratory,under optimum climatic conditions (i.e.,25°C,75% relative humidity).Equation and goodness-of-fit of the fitted curve are indicated on each respective graph.
Previous studies have shown howS.frugiperdamigration is tied to the vertical distribution of temperature and wind speed,while its migration direction relates to the wind direction (Westbrook 2007; Westbrooket al.2016).Our work reveals howS.frugiperdaadults inherently possess a strong flight ability that is further enhanced by wind currents.At present,insect radar can be effectively used to study insect migration (Chapmanet al.2011),while advanced radar can use wingbeat frequency as a signature to identify airborne insects (Drake and Wang 2013; Huet al.2018).Wingbeat frequency signatures,as obtained in our study for FAW adults,thus facilitate radar-based monitoring of migratory FAW populations.
Abundant food resources are readily available along its tentative migration path in mainland China (Changet al.2018),thus allowingS.frugiperdato rapidly migrate over extensive areas,build up its population levels and inflict economically-important crop losses.Based upon existing literature records and laboratory-derived findings (Aliet al.1990; Heet al.2019; Jianget al.2019),we can reasonably assume thatS.frugiperdawill maintain year-long populations in southern China and re-colonize the country’s corn-growing areas on an annually-recurring basis.Hence,extensive FAW monitoring in its overwintering range needs to be complemented with (model-based) immigration forecasting and early-warning assessments in corn production areas nationwide.Our in-depth assessment ofS.frugiperdaflight capacity can hereby be integrated with spatially-explicit data on wind patterns and local (agro-) climatic parameters to devise a reliable and effective forecasting system for FAW in China and across eastern Asia.
5.Conclusion
FAW is a newly-invasive pest in eastern Asia,with its rapid dispersal and continent-wide proliferation threatening local corn production systems,associated farmer livelihoods and rural agro-industries.Here,we employed laboratorybased flight mill assessments to characterize how FAW adults exhibit strong flight performance across a wide range of climatic conditions.Our work identifies 20–25°C and 60–90% RH as the most suitable conditions for its flight,makes inferences regarding the pest’s long-distance migration capability,and generates baseline information for robust population forecasting and area-wide integrated pest management.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31727901).In addition,the authors would like to thank Mr.Sun Yulun and Mrs.Zhang Mengke(Henan Institute of Science and Technology,China),for their contributions in larval rearing and assisting this work.The authors would like to thank Dr.Fu Xiaowei (Henan Institute of Science and Technology,China) and Mrs.Li Aili (Institute of Plant Protection,Chinese Academy of Agricultural Sciences),for their contributions in providing a good experimental environment.
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China
- Biology,invasion and management of the agricultural invader:Fall armyworm,Spodoptera frugiperda (Lepidoptera:Noctuidae)
- Case study on the first immigration of fall armyworm,Spodoptera frugiperda invading into China
- Windborne migration routes of newly-emerged fall armyworm from Qinling Mountains–Huaihe River region,China
- Adult nutrition affects reproduction and flight performance of the invasive fall armyworm,Spodoptera frugiperda in China
- Flight activity promotes reproductive processes in the fall armyworm,Spodoptera frugiperda
