Larval diet affects development and reproduction of East Asian strain of the fall armyworm,Spodoptera frugiperda
2021-02-25HELimeiWANGTengliCHENYuchaoGEShishuaiKrisWYCKHUYSWUKongming
HE Li-mei ,WANG Teng-li ,CHEN Yu-chao ,GE Shi-shuai ,Kris A.G.WYCKHUYS ,WU Kong-ming
1 State Key Laboratory for Biology of Plant Diseases and Insect Pests,Institute of Plant Protection,Chinese Academy of Agricultural Sciences,Beijing 100193,P.R.China
2 State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops,Institute of Applied Ecology,Fujian Agriculture and Forestry University,Fuzhou 350002,P.R.China
Abstract In December 11,2018,the fall armyworm (FAW),Spodoptera frugiperda invaded China and has since impacted local maize,sorghum and other crops.Here,we draw on laboratory experiments to show how different host crops (i.e.,maize,sorghum,wheat and rice) and artificial diet affect larval growth and adult reproduction of one local FAW strain.Larval diet affected development duration,pupation rate,survival and emergence rate of pupae,and S. frugiperda adult fecundity.FAW attained the slowest larval development (19.4 days) on sorghum and the fastest (14.1 days) on artificial diet,with larvae attaining 99.6% survival on the latter food item.On rice,FAW larvae attained survival rate of 0.4% and were unable to pupate successfully.Pupation rate and pupal survival varied substantially between artificial diet and live plantlets at different phenological stages.Pupal weight was the highest (0.26 g) on artificial diet and the lowest (0.14 g) on sorghum,while FAW females reached the highest fecundity (699.7 eggs/female) on 2-leaf stage maize.Egg hatching rate equaled 93.6% on 4-or 5-leaf stage maize and 36.6% on artificial diet.FAW intrinsic rate of natural increase and the finite rate of increase varied between larval diets,reflecting how young maize leaves are the most suitable diet.Our findings can help to refine laboratory rearing protocols,devise population forecasting models or guide the deployment of ‘area-wide’ integrated pest management (IPM) modules in FAW-invaded areas of China and other Asian countries.
Keywords:developmental duration,development,fecundity,life history,insect mass-rearing,biology,invasion biology
1.lntroduction
The fall armyworm (FAW),Spodoptera frugiperda(J.E.Smith) (Lepidoptera:Noctuidae),is a highly-mobile and polyphagous herbivore native to tropical and subtropical America (Smith and Abbottet al.1797; Luginbill 1928;Sparks 1979; Johnson 1987; Montezanoet al.2018).In 2016,S.frugiperdainvaded the African continent and rapidly attained outbreak population levels in local maize crops (Goergenet al.2016; Midegaet al.2018).Over a 2-year time span,FAW spread across most of sub-Saharan Africa (Earlyet al.2018) and got intercepted in Europe,with specimens collected in Germany and the Netherlands(CABI 2017).By mid-2018,S.frugiperdahad equally made its unfortunate arrival in the state of Karnataka (India) and subsequently spread to Myanmar,Thailand and other Southeast Asian countries (FAO 2018; Nakweta 2018).In December 11,2018,the FAW moth was captured in western Yunnan Province (Sunet al.2021).In January 2019,the FAW larvae was recorded in maize fields of Yunnan Province (Yanget al.2019) and has since fast expanded its in-country distribution,impacting local maize,sugarcane,sorghum and other crops (Huet al.2019; Liuet al.2019; Yanget al.2019).
As a highly polyphagous pest,S.frugiperdalarvae feed on a total of 353 different host plants belonging to 76 plant families,including Poaceae,Asteraceae and Fabaceae(Montezanoet al.2018).ThoughS.frugiperdaconsists of two genetically-distinct host-adapted strains,i.e.,so-called“rice-strain” and “corn-strain” (Dumaset al.2015),the pest primarily inflicts economically-relevant damage in maize,sorghum,cotton and soybean crops (Pitre and Hogg 1983;Casmuzet al.2010; de Freitas Buenoet al.2011; Hardkeet al.2015).Polyphagous insects such as FAW exhibit variable growth,development and reproduction depending on host plant consumption during the larval stage (Hou and Sheng 2000; Yinet al.2004; Tuet al.2008; Tenget al.2012;Liet al.2013; Huanget al.2018a,b),with FAW larval diet affecting multiple fitness parameters,e.g.,larval survival,body weight and adult oviposition (Barroset al.2010).
The four main host plants ofS.frugiperdaare agricultural crops of global allure; rice,maize and wheat are cultivated across the world,while sorghum is widely grown in different parts of Asia and Africa (Casmuzet al.2010; Liuet al.2014;Diaset al.2016; Montezanoet al.2018).For invasive FAW populations,maize is thought to be a highly-suitable host crop as compared with millet,rice or tobacco (Barroset al.2010; Wuet al.2019; Xuet al.2019).Earlier (laboratory)work has shown how F1offspring of invasive FAW populations in Guangdong Province,China,successfully completed its development on both sugarcane and rice(Wuet al.2019).Though the differential impacts of those crops on life history parameters have been thoroughly investigated in the FAW region of origin (Andrews 1998; de Sáet al.2009; Barroset al.2010),no work is known from its newly-invaded range in Asia.Follow-up research on the developmental impacts of different host plants is especially warranted,given the complex (inter-strain hybrid) genetic make-up of invasiveS.frugiperdapopulations (Zhang Let al.2019).
In this study,we systematically assess biological characteristics ofS.frugiperdawhen fed with either maize,rice,sorghum and wheat plantlets or with artificial diet under laboratory conditions.Field observations and preliminary laboratory trials revealed howS.frugiperdalarvae mainly consume the apex or young leaves of the plant,and often do not complete their development when fed with older leaves of sorghum,wheat and maize.Hence,2-leaf stage and 4-or 5-leaf stage maize were used as separate diet treatments in our study.Our findings permit the establishment of experimental population life tables on its three main host plants,and the further development of (area-wide) integrated pest management strategies,amongst others.
2.Materials and methods
2.1.Larval feeding trials
Laboratory trials were carried out during June–August 2019 at the Xinxiang Experiment Station of the Chinese Academy of Agricultural Sciences (CAAS; 35°18´13.71´´N,113°55´15.05´´E) in Henan Province,China.FAW individuals were field-collected in early 2019 in Dehong Autonomous Prefecture,Yunnan,and reared for four consecutive generations in the laboratory.Insects were kept at(25±1)°C,(75±5)% relatice humidity (RH),and 16 h L:8 h D.For experimental purposes,larvae were exposed to six different diets:artificial diet (a soybean flour and wheat bran diet; Greeneet al.1976),2-leaf stage and 4-or 5-leaf stage maize plants (Zhengdan 958),2-leaf stage wheat plants(Jimai 22),2-leaf stage sorghum (dwarf red sorghum),and 2-leaf stage rice (Zhenghan 10).More specifically,1st to 4th instar FAW larvae were reared individually on artificial diet in 24-well plates and 5th instar larvae were then individualized in glass tubes (7.5 cm×1.5 cm) until pupation.Greenhouse-grown seedlings of the different host plants (at their respective phenological stage) and field-grown 4-or 5-leaf stage maize plantlets were exposed to FAW larvae 1–4 days after hatching.More specifically,leaves of each host plant at a specific phenological stage were immersed into a thin layer of 1% agar in tubes (12.0 cm×3.3 cm) and exposed to early-instar FAW larvae (20 larvae per tube).Once larvae were 5 days old,they were gently collected,individualized in 25-mL plastic cups and provided on a daily basis with fresh seedlings of the different host plants until larval pupation.Every day,larval development and survival were recorded until death,pupation or pupal emergence.On the 3rd day after pupation,pupal weight was determined with an electronic balance (Mettler Toledo ME204,Beijing Haitian Youcheng Technology Co.,Ltd.,China),and pupae were sexed and separated by male and female.Each diet treatment consisted of 80 FAW larvae and was replicated three times.
2.2.Adult development and fecundity
Following pupal emergence,adultswere kept in 460-mL plastic cups with a sterile gauze stopper,and fed daily with 5% (v/v) locust honey water.Moths were kept at (25±1)°C,(80±5)% RH and 16 h L:8 h D.On a daily basis,deposited egg masses were collected and recordings were made of pre-oviposition time,daily egg deposition,egg hatching rate and adult survival.Dead female moths were dissected under a stereoscope to count the number of spermatophores present within the spermathecae and thus ascertain mating history.
2.3.Life table parameters
Next,net reproductive rate (R0),generation time (T),intrinsic rate of natural increase (r),and finite rate of increase (λ)of the FAW experimental populations were calculated according to the following equations (Chi and Liu 1985;Chi 1988):
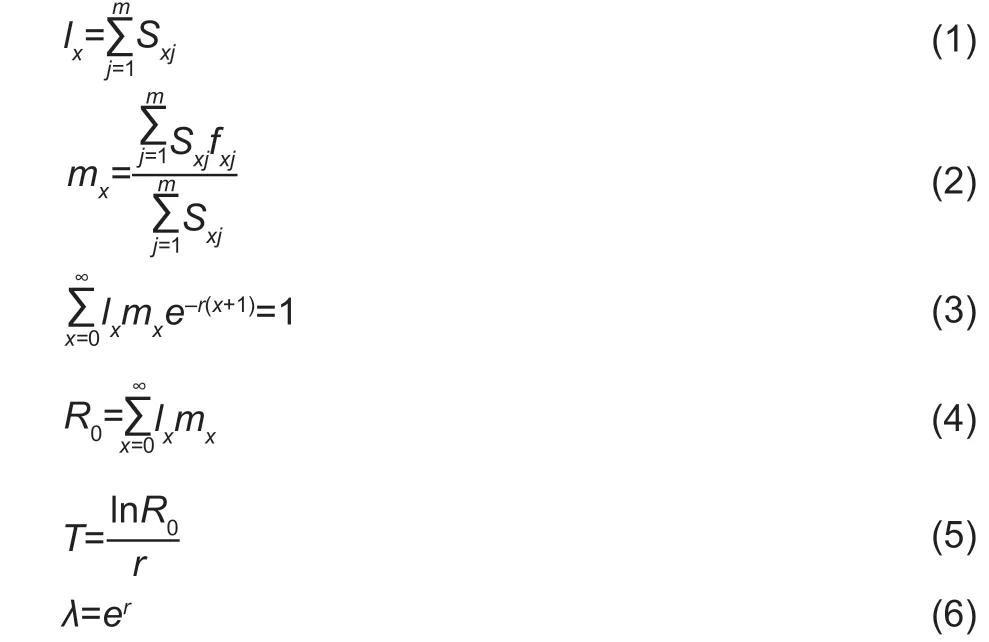
wherexis the time interval in days (d),mis the number of stages,Sxjis the survival rate of FAW from egg development toxdays old and developmental stagej,lx(population agespecific survival rate) is the survival rate of FAW from egg toxdays old,fx9is the age-specific fecundity at agex,mx(population age-specific fecundity) is the average population fecundity from egg toxdays old,andlxmx(population agespecific maternity) is the product oflxandmx.
2.4.Statistical analysis
For the five diet treatments,differences in life history parameters were analyzed using One-way analysis of variance (ANOVA),with proportional data first arcsine square root transformed to meet assumptions of normality and heteroscedasticity.Duncan’s honestly significant difference (HSD) was used as a post-hoc test.To fit the survival rate of FAW population at a specific age,a logistic model was used with equation:y=K/(1+aebx),wherea,b,andKare model parameters,yis the survival rate of FAW from egg development toxdays old.Pearson linear correlation was used to assess the correlation between individual development variables.All data were analyzed using IBM SPSS Statistics ver.20 (IBM,Armonk,NY,USA),except for the calculation of life table parameters in TWOSEX-MSChart (Chi 2019).
3.Results
3.1.Larval and adult development
Fall armyworm successfully completed its development on all diets except rice,with larval diet greatly affecting various developmental parameters (Tables 1 and 2).More specifically,significant differences were recorded in the developmental duration of 1st to 2nd instar larvae(F5,1110=123.214,P<0.001),3rd instar larvae (F5,996=132.942,P<0.001),4th instar larvae (F5,928=36.877,P<0.001),5th instar larvae (F4,919=57.439,P<0.001),6th instar larvae(F4,919=118.172,P<0.001),larval stage (F4,919=348.505,P<0.001),pupae (F4,785=39.170,P<0.001),adults(F4,711=30.820,P<0.001) and a combined larva-adult stage(F4,710=69.081,P<0.001) on different diets.Larval development on sorghum and 4-or 5-leaf maize was considerably slower than on other diet treatments,while pupal development duration was the longest on sorghum.Adult longevity and larva-adult development was the longest on wheat,while the shortest adult longevity was recorded on 4-or 5-leaf stage maize and the shortest larva-adult development on artificial diet.
3.2.Survival rate and pupal weight
Larval diet greatly affected survival and pupal weight ofS.frugiperda,with significant effects on survival rate of 3rd to 6th instar larvae,total larval stage and pupal stage(P<0.05; Table 2).Larval diet equally affected pupation rate (F4,10=5.586,P=0.013),pupal emergence (F4,10=4.557,P=0.024) and pupal weight (F4,914=765.230,P<0.001).FAW survival rate was the lowest on rice (i.e.,0.42%) and no larval pupation was recorded on this host plant,while larvae attained the highest survival rate on artificial diet.FAW equally attained its highest pupation rate and pupal weight on artificial diet,though the respective pupal survival and emergence rate were low.
Survival curves of the the FAW population exhibited a downward trend on artificial diet (y=1.024/(1+0.019e0.116x),R2=0.962; Fig.1-A),2-leaf stage maize (y=0.994/(1+0.015e0.113x),R2=0.984; Fig.1-B),sorghum (y=0.984/(1+0.006e0.117x),R2=0.981; Fig.1-C),wheat (y=1.572/(1+0.455e0.057x),R2=0.981; Fig.1-D) and 4-or 5-leaf stage maize (y=0.977/(1+0.003e0.148x),R2=0.959; Fig.1-E).
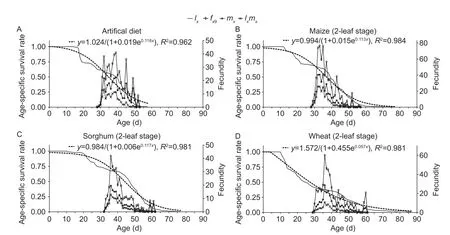
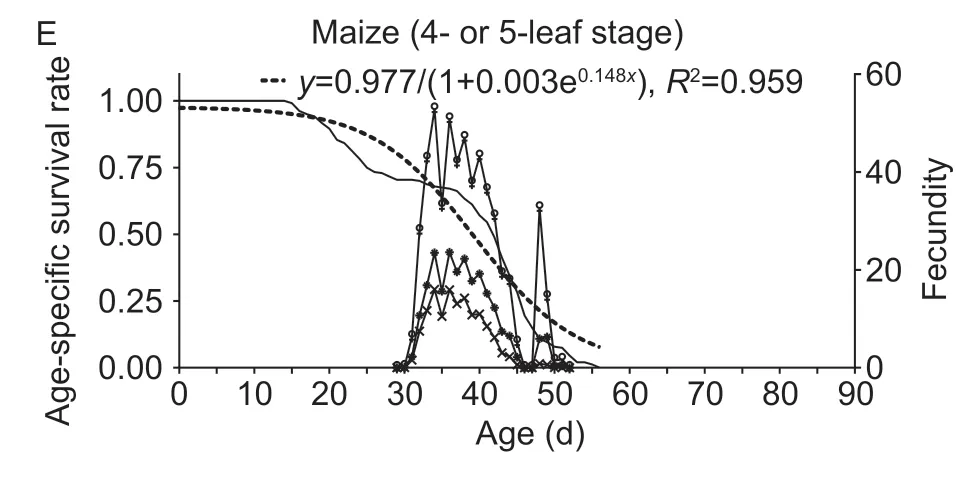
Fig.1 Population age-specific survival rate (lx),population agespecific fecundity (mx),population age-specific maternity (lxmx)and age-specific fecundity of female adult (fx9) of Spodoptera frugiperda after larvae were reared in the laboratory on artifical diet (A),2-leaf stage maize (B),2-leaf stage sorghum (C),2-leaf stage wheat (D),and 4-or 5-leaf stage maize (E).
3.3.Adult reproductive characteristics
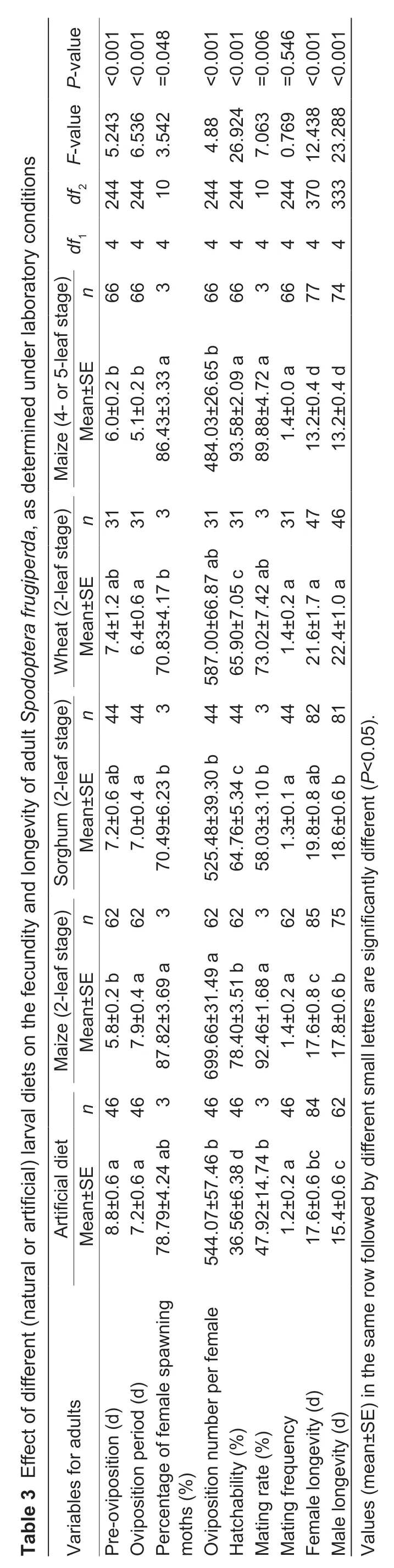
Larval diet greatly affected fecundity and longevity ofS.frugiperdaadults (Table 3),with statistically significant effects on pre-oviposition and oviposition period,percentage of egg-laying females,fecundity and egg hatching rate,and female or male longevity between diet treatments.Diet-related differences were equally recorded for female mating rate and mating frequency (Table 3).When reared on artificial diet,the longest pre-oviposition period was recorded.The shortest oviposition period was attained on 4-or 5-leaf stage maize,and female fecundity (and mating rate) was the highest on 2-leaf stage maize.Themxcurve showed several oviposition peaks on artificial diet though only one peak oviposition period on the various plant hosts(Fig.1).Female larval duration on all diets,except for artificial diet,was negatively correlated with pupal weight(r=–0.983,P<0.05) and female fecundity (r=–0.953,P<0.05),while female pupal weight was positively correlated with female fecundity (r=0.992,P<0.01).
3.4.Population dynamics
Life table parameters reflected howS.frugiperdaattained the smallest net reproductive rate and intrinsic rate of natural increase,finite rate of increase on wheat (Table 4).Conversely,on 2-leaf stage maize,FAW attained the fastest population growth,with the shortest mean generation time,and the largest net reproductive rate and intrinsic rate of natural increase,finite rate of increase.
4.Discussion
Like several other lepidopteran pests,S.frugiperdaexhibits a broad,and expanding geographical distribution,and relies upon myriad host plant species for its larval development.The broad range of FAW host plants comprises plants of varying palatability,nutritional content and presence of secondary metabolites,with different plant species differentially impacting growth,development and overall population fitness of their lepidopteran herbivores(Hou and Sheng 2000; Zhanget al.2013; Wanget al.2015; Montezanoet al.2018; Zhang Set al.2019).Here,we empirically demonstrate how larval diet affectsS.frugiperdadevelopment.Though artificial diet resulted in the fastest larval development,young maize plants equally benefited development and survival of various FAW stages.Maize is widely considered as a highly-suitable host plant for invasive FAW populations (Barroset al.2010; Wuet al.2019; Xuet al.2019),and our trials confirm these results specifically for one Chinese strain ofS.frugiperda.Yet,as F1offspring of invasive FAW populations in China’s Guangdong Province successfully completed its development on rice (Wuet al.2019),it is likely that this (potential) rice–corn hybrid differs from the one examined in our study.In our work,the superior performance of Yunnan populations on maize and their inability to complete development on rice are indicative of the “corn-strain”,and thus herald potentially severe damage on this crop in China (Megersa and Tamiru 2018; Baudronet al.2019; Yanget al.2019).
Overall nutritional status and fitness of larvae are likely to be mirrored by characteristics of the pupal stage (e.g.,pupal emergence,weight,size)(Eduardoet al.2018; Liet al.2019).In our study,FAW exhibited the most rapid development and attained the highest (female) pupal weight on artificial diets and 2-leaf stage maize.Furthermore,a negative correlation was detected betweenS.frugiperdafemale pupal weight and larval duration,as also observed in lepidopteran herbivores such asMythimna separata(Walker)andSpodoptera litura(Fabricius) (Tuet al.2008; Huanget al.2018b).Hence,pupal weight possibly reflects FAW larval nutritional status but it is not indicative of pupal emergence for larvae reared on artificial diets.For plant-based diets,higher -though not statistically different -pupal emergence rates were recorded on 4-to 5-leaf stage and 2-leaf stage maize.
Larval diet can equally shape adult fecundity (Awmack and Leather 2002;Wanget al.2008; Zhanget al.2011; Jabraeilet al.2014; Blairet al.2019),as also mirrored in the long FAW oviposition period,high percentage of ovipositing females and high fecundity on 2-leaf stage maize and artificial diet.For plantbased diets,a higher egg hatching rate and mating rate were also recorded on different maize phenological stages.Furthermore,a negative correlation was observed between larval development duration and adult fecundity,as equally recorded forM.separata(Huanget al.2018b).For artificial diets,the lowered adult fitness may be due to a possible imbalance in its C/N ratio and a related metabolic burden on larval development,as also found forM.separataandHelicoverpa armigera(Hübner) (Zhanget al.2005; Huanget al.2018b).Yet,under field conditions,many of our laboratory-derived patterns possibly don’t hold as adult fecundity is shaped by factors such as carbohydrate consumption,exposure to secondary metabolites in floral nectar and adverse abiotic factors (e.g.,temperature,air humidity) (Adler 2000; Mevi-Schütz and Erhardt 2005; Levinet al.2017)
Under optimum larval feeding conditions,insects will improve their fitness(Barroset al.2010; Xuet al.2019).To assess the suitability of particular (larval)food items for an insect’s development,one can draw upon different fitness currencies or life table parameters,e.g.,birth rate,mortality or development rate.In this study,rand λ of the FAW experimental population were greater than 0 and 1,respectively,thus indicating that all food items (except rice) were suitable for FAW population development.Both parameters are calculated under conditions in which the population reaches a stable age group over time and can be used to compare population growth potential under different conditions.Though such parameters can be used to develop forecasting and‘early warning’ models,a pest’s development will ultimately be affected by adult food,agro-climatic conditions,influx or efflux through (active or wind-mediated)colonization,biological control and other environmental factors (Huang and Chi 2012; Liuet al.2014; Panet al.2014; Shiet al.2017; Zhouet al.2018; Jonaset al.2019; Romoet al.2019).Furthermore,one needs to account for egg hatching rate in addition to female fecundity(Jhaet al.2012).Future work can involve the construction of life tables under field conditions (Cañaset al.2002; Naranjoet al.2005; Asiimweet al.2007),and thus help gain a more grounded understanding of FAW population ecology under the specific agro-climatic and ecological conditions of Asian maize cropping.
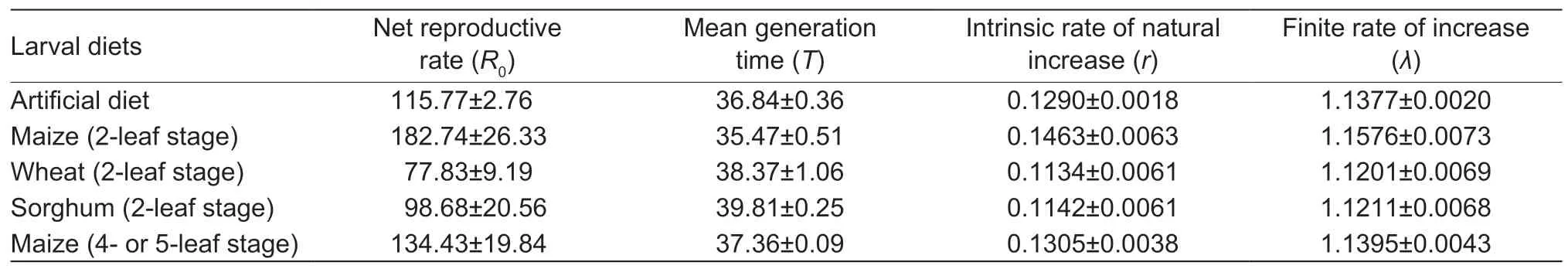
Table 4 Life table parameters of Spodoptera frugiperda when reared on five different (natural or artificial) diets
5.Conclusion
Our work demonstrates how invasiveS.frugiperdapopulations from Yunnan,China,likely belong to the “cornstrain”,and successfully complete their development on a range of natural and artificial diets.Early phenological stages of maize proved the most suitable natural diet for FAW growth,development and reproduction.Yet,as empirical work was conducted under artificial (laboratory) conditions,due caution needs to be taken when extrapolating findings to field conditions or when using them for the development of population forecasting models.With the 2019 arrival of fall armyworm in eastern Asia,much in-field population ecology research waits to be conducted and its importance cannot be disregarded.Ultimately,these field-derived data,together with our findings,could underbuild robust predictive models and underpin ‘area-wide’ integrated pest management programs for this newly-invasive pest.
Acknowledgements
We would like to thank Ms.Gao Qiuyan,Ms.Yue Shunli and Ms.Hang Xuebing from the Henan Institute of Science and Technology,for their assistance with insect rearing.This work was supported by the earmarked fund for China Agriculture Research System (CARS-15-19) and the Central Public-interest Scientific Institution Basal Research Fund,China (Y2019YJ06).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China
- Biology,invasion and management of the agricultural invader:Fall armyworm,Spodoptera frugiperda (Lepidoptera:Noctuidae)
- Case study on the first immigration of fall armyworm,Spodoptera frugiperda invading into China
- Windborne migration routes of newly-emerged fall armyworm from Qinling Mountains–Huaihe River region,China
- Laboratory-based flight performance of the fall armyworm,Spodoptera frugiperda
- Adult nutrition affects reproduction and flight performance of the invasive fall armyworm,Spodoptera frugiperda in China
