Preparation,Structures and Thermal Stabilities of Four Transition Metal Complexes Constructed by 3,7-Di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane Bipyridine Ligand
2021-02-24LILi
LI Li
(School of Chemistry and Life Sciences,Suzhou University of Science and Technology,Suzhou,Jiangsu 215009,China)
Abstract:Four transition metal complexes,[Co(NO3)(H2O)2(L)2]NO3(1),[Co2Cl4(L)2]·CH2Cl2(2),[Cd2(AcO)4(L)2]·4CH3OH(3)and[Cd2(NO3)2(CH3OH)2(H2O)2(L)2](NO3)2·2H2O(4),were synthesized by employing a clamplike bipyridine ligand 3,7-di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane(L).Single-crystal X-ray analysis reveals that complex 1 is mononuclear structure;2 is macrocyclic dimer;wheras 3 and 4 are rectangular dinuclear structures.The ligand molecules in these complexes has shown three types of coordination mode including mono-dentate,trans-bridge and cis-bridge.All of the complexes were also characterized by elemental analysis,IR,thermal stabilities and singlecrystal structure analysis.CCDC:940778,1;915840,2;1446573,3;1446574,4.
Keywords:cobalt;cadmium;3,7-di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane;bipyridine;crystal structure
0 Introduction
Pyridine ligands have been employed extensively in constructing functional materials with transition metal[1-13].They have shown many advantages containing appropriate coordination capability,uncharged property,easy structural design as well as versatile coordination mode.N,N'-bidentate bipyridine has drawn much attention during these decades.Those ligands with larger spacer have been used to construct porous metallocyclic architectures,metal-organic frameworks(MOFs),optical complexes and so on,such as bis(4-(pyridine-4-yl)phenyl)amine[3],N,N'-(1,2-phenylene)diisonicotinamide[4],3-bis(4-pyridyl)propane[7],1,2-dimethoxy-4,5-bis(2-pyridylethynyl)benzene[8],1,8-bis(4-pyridylethynyl)anthracene[9],((pyridinyl)-1H-pyrazolyl)pyridine[10],4,6-bis(4'-pyridylsulfide)dibenzofuran[11],4,6-bis(methylsulfanylmethyl)dibenzofuran[11],1,1'-bis(4-pyridyl)ferrocene[13]and the others[14-16].To date,most of those ligands are flexible.
In our previous research,a clamplike bipyridine ligand 3,7-di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane(L)has been synthesized.We have measured the1H NMR spectra of L in CDCl3in a range of-10 to 50℃.Along with the change of temperature,the rotation of the N-C bonds accompany with alternate between“up,up”and“down,down”conformations in ligand would take place.The bipyridine ligand has shown quasirigid characteristics under room temperature.During the coordinating with copper salts,it acted as acis-bridge ligand[17].Herein,as part of continuing studies on its coordination chemistry,we have built four transition metal complexes[Co(NO3)(H2O)2(L)2]NO3(1),[Co2Cl4(L)2]·CH2Cl2(2),[Cd2(AcO)4(L)2]·4CH3OH(3)and [Cd2(NO3)2(CH3OH)2(H2O)2(L)2](NO3)2·2H2O (4).They have shown diverse coordination chemistry.X-ray single-crystal structure analysis displays that 1 is a mononuclear complex,while 2~4 are dinuclear complexes.The ligand molecule in these complexes has exhibited three types of coordination mode including mono-dentate,trans-bridge andcis-bridge.All of these complexes have been characterized by elemental analysis,IR spectra,thermal gravity analysis and X-ray single-crystal structure analysis.The intra-/inter-molecular hydrogen interactions of these complexes have also been discussed.
1 Experimental
1.1 Reagents and instruments
The ligand L has been prepared and characterized previously[17].Other reagents were purchased from commercial sources and used as received without further purification.Carbon,hydrogen and nitrogen analysis were carried out by direct combustion on an EA1110-CHNSO elementalanalyzer.FT-IR spectra were recorded on a Perkin Elmer Spectrum BXⅡspectrometer.Thermal stability analysis was measured on a PRT-1A Thermogravimetric Analyzer.Powder X-ray diffraction(PXRD)determination was performed on an X-ray diffractometer(X'Pert PRO MPOCPW 3040/60,Panalytical)with CuKαradiation(λ=0.154 06 nm).The operating voltage and current were 40 kV and 40 mA,and the measurement was carried out over a 2θrange of 5°to 50°in continuous scanning mode.
1.2 Preparation of complexes 1~4
Complex 1:To a Pyrex tube(6 mm inner diameter)was added 5.0 mL CH2Cl2solution of L(1.0 mmol,290 mg),then was slowly layed onto 5.0 mL CH3OH solution of Co(NO3)2·6H2O(1.0 mmol,291 mg).The tube was sealed at room temperature for 4 d,and red block crystals of complex 1 were afforded.Yield:481 mg(63% based on Co).Anal.Calcd.for C28H36CoN10O12(%):C 44.04,H 4.75,N 18.34;Found(%):C 44.12,H 4.68,N 18.15.FT-IR(KBr,cm-1):3 412(m),3 042(w),2 963(w),2 935(w),2 902(w),1 602(w),1 578(m),1 522(s),1 496(vs),1 464(w),1 443(w),1 426(w),1 369(vs),1 309(s),1 261(s),1 229(vs),1 193(m),1 167(w),1 136(s),1 058(s),1 029(vs),1 015(s),991(s),942(m),888(m),828(w),799(m),781(w),745(w),696(m),665(m),621(w),569(w),487(w),416(w).
Complex 2:To a Pyrex tube was added 5.0 mL CH2Cl2solution of L(1.0 mmol,290 mg),then was slowly layed onto 5.0 mL CH3CN solution of CoCl2(1.0 mmol,130 mg).The tube was sealed at room temperature for 6 d,and blue block crystals of complex 2 were afforded.Yield:231 mg(52% based on Co).Anal.Calcd.for C29H34Cl6Co2N8O4(%):C 39.17,H 3.85,N 12.60;Found(%):C 40.12,H 3.97,N 12.95.FT-IR(KBr,cm-1):3 413(w),3 112(w),3 077(w),3 048(w),2 980(w),2 940(w),2 903(w),1 600(s),1 577(s),1 495(vs),1 438(s),1 368(vs),1 305(s),1 258(m),1 223(s),1 197(m),1 171(w),1 136(s),1 063(s),1 038(vs),1 015(s),991(s),944(s),889(m),807(s),730(m),692(s),663(s),620(w),568(w),496(w),419(w).
Complex 3:It was prepared as the method for complexes 1 and 2 except that 5.0 mL CH3OH solution of Cd(AcO)2·2H2O(1.0 mmol,267 mg)was used.The tube was sealed at room temperature for 5 d,and colorless block crystals of complex 3 were afforded.Yield:329 mg (58% based on Cd).Anal.Calcd.for C40H60Cd2N8O16(%):C 42.37,H 5.33,N 9.88;Found(%):C 42.12,H 5.08,N 9.70.FT-IR(KBr,cm-1):3 440(vs),2 967(w),2 963(w),2 933(w),1 560(vs),1 498(s),1 420(s),1 364(s),1 306(s),1 261(m),1 231(s),1 199(w),1 166(w),1 134(s),1 063(s),1 033(vs),1 016(s),995(s),942(s),876(m),803(m),790(w),697(m),670(m),),642(w),620(w).
Complex 4:It was prepared the method for 1~3 except that 5.0 mL CH3OH solution of Cd(NO3)2·4H2O(1.0 mmol,308 mg)was used.After one week,colorless block crystals of complex 4 were afforded.Yield:830 mg(72% based on Cd).Anal.Calcd.for C30H48Cd2N12O22(%):C 31.23,H 4.19,N 14.57;Found(%):C 31.12,H 4.02,N 14.30.FT-IR(KBr,cm-1):3 450(vs),2 901(w),1 602(m),1 498(m),1 384(vs),1 305(s),1 261(m),1 233(s),1 198(m),1 167(w),1 135(m),1 062(m),1 033(s),997(m),942(m),900(w),881(w),799(m),692(m),641(w).
1.3 Crystallographic analyses
The single crystals with dimensions of 0.50 mm×0.40 mm×0.35 mm(1),0.25 mm×0.22 mm×0.20 mm(2),0.35 mm×0.25 mm×0.22 mm(3)and 0.40 mm×0.20 mm×0.20 mm(4)were mounted on a glass fiber,relatively.Single-crystal X-ray diffraction measurements were performed on a Bruker APEXⅡ4K CCD area detector equipped with a graphite monochromated MoKαradiation(λ=0.071 073 nm)by using theω-scan mode on all observed reflections in aθrange of 3.06°~25.35°(1),3.07°~26.00°(2),3.05°~25.02°(3)and 3.16°~24.24°(4).All absorption corrections were applied using the SADABS program[18].The structures were solved by direct methods and refined onF2by full-matrix least-squares using the SHELXTL-97 program package[19].Metal atoms were located from the e-maps,and other non-hydrogen atoms were derived from the successive difference Fourier peaks.The nitrate anion was disordered about a twofold rotation axis in complex 4[20].Hydrogen atoms were placed in geometrically idealized positions(C—H 0.095~0.098 nm)and were constrained to ride on their parent atoms withUiso(H)=1.2~1.5Ueq(C).Summary of the crystallographic data for 1~4 are given in Table 1.Selected bond lengths and angles are given in Table 2.Hydrogen bonds geometries are listed in Table 3.
CCDC:940778,1;915840,2;1446573,3;1446574,4.
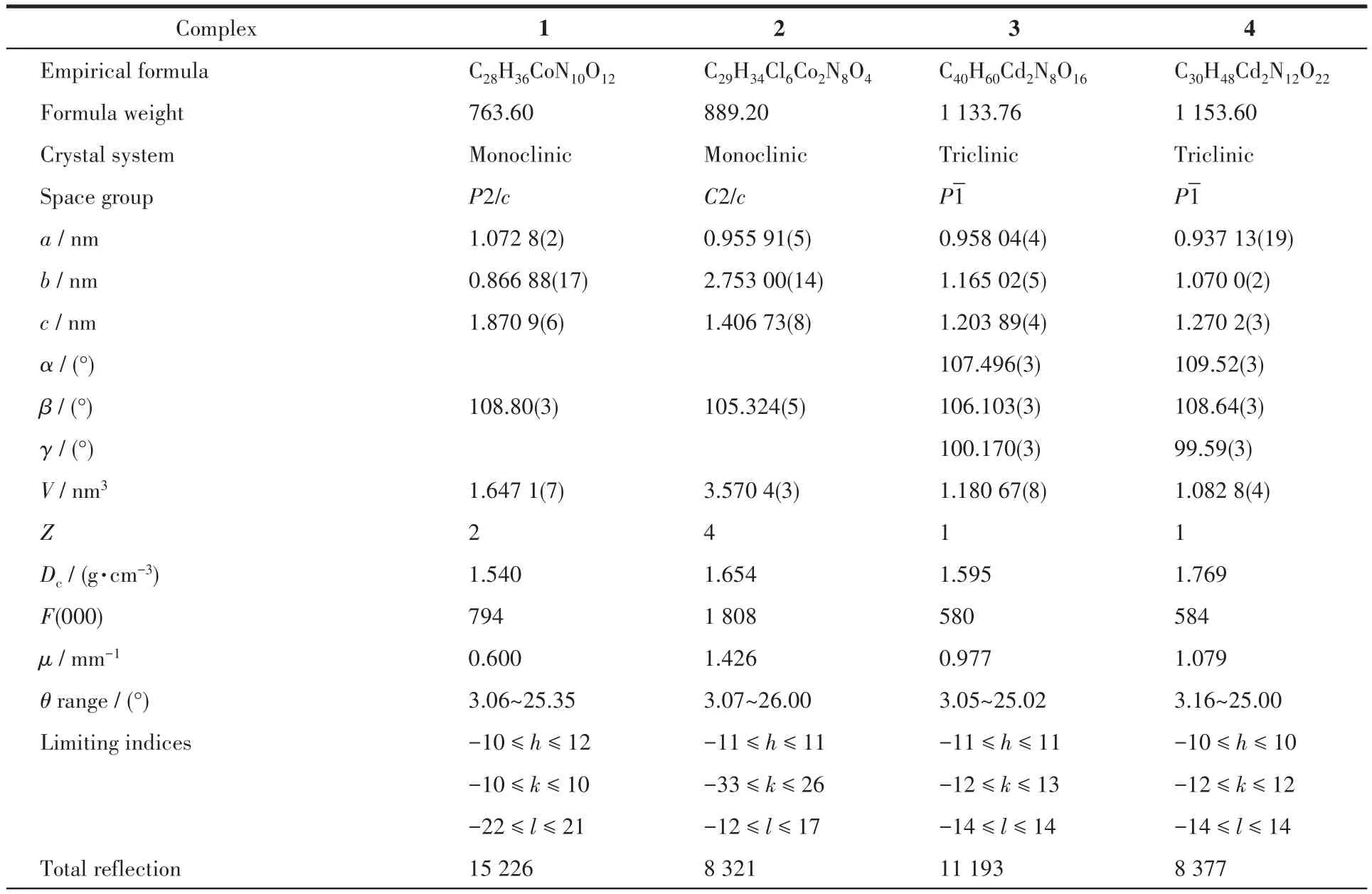
Table 1 Crystallographic and structural refinement details for 1~4
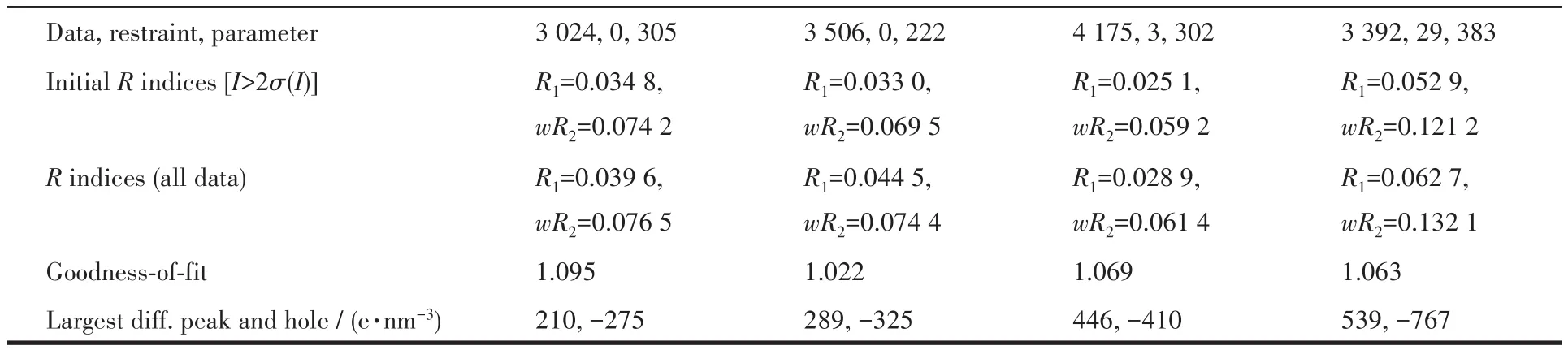
Continued Table 1
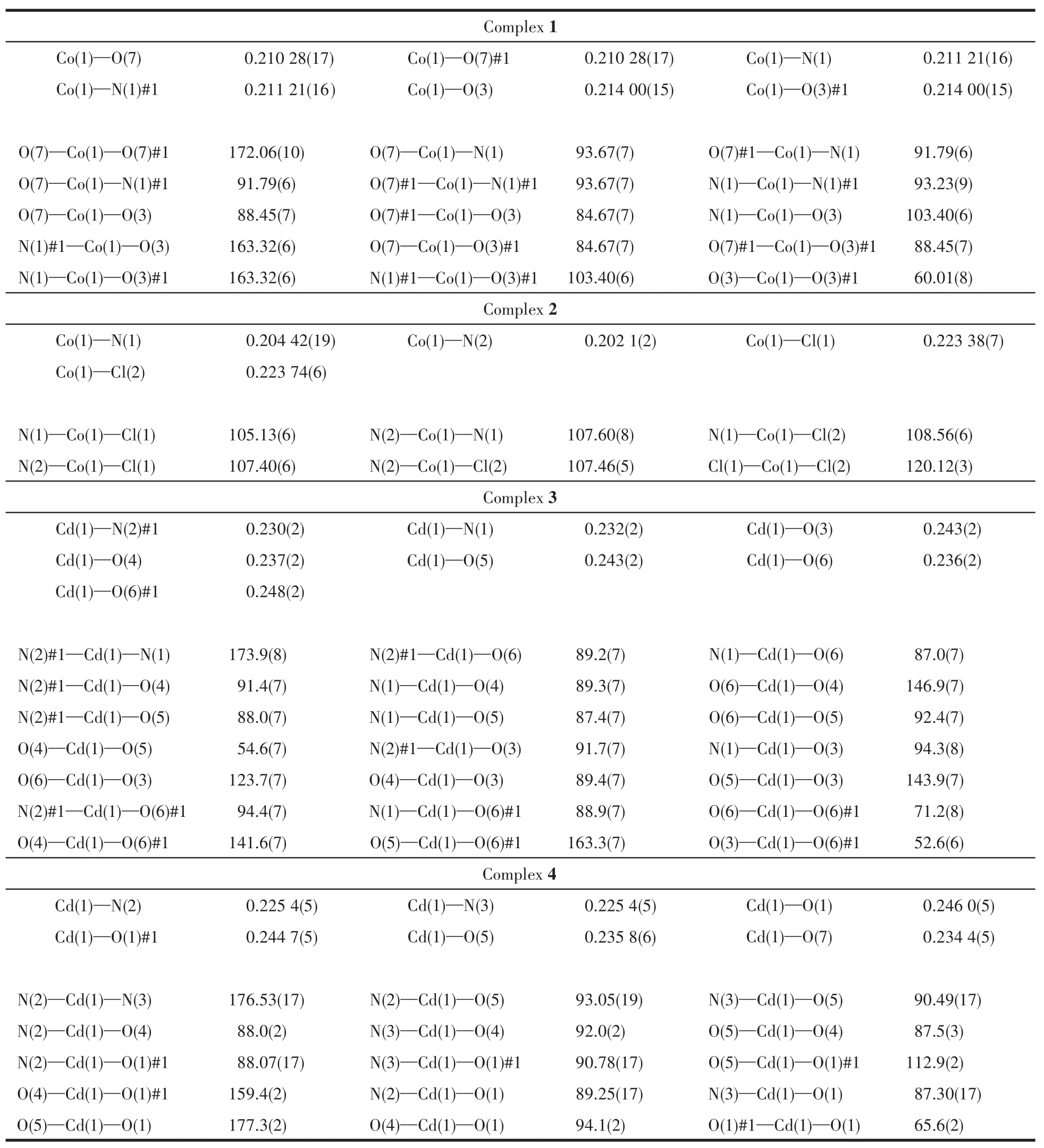
Table 2 Selected bond lengths(nm)and angles(°)for complexes 1~4
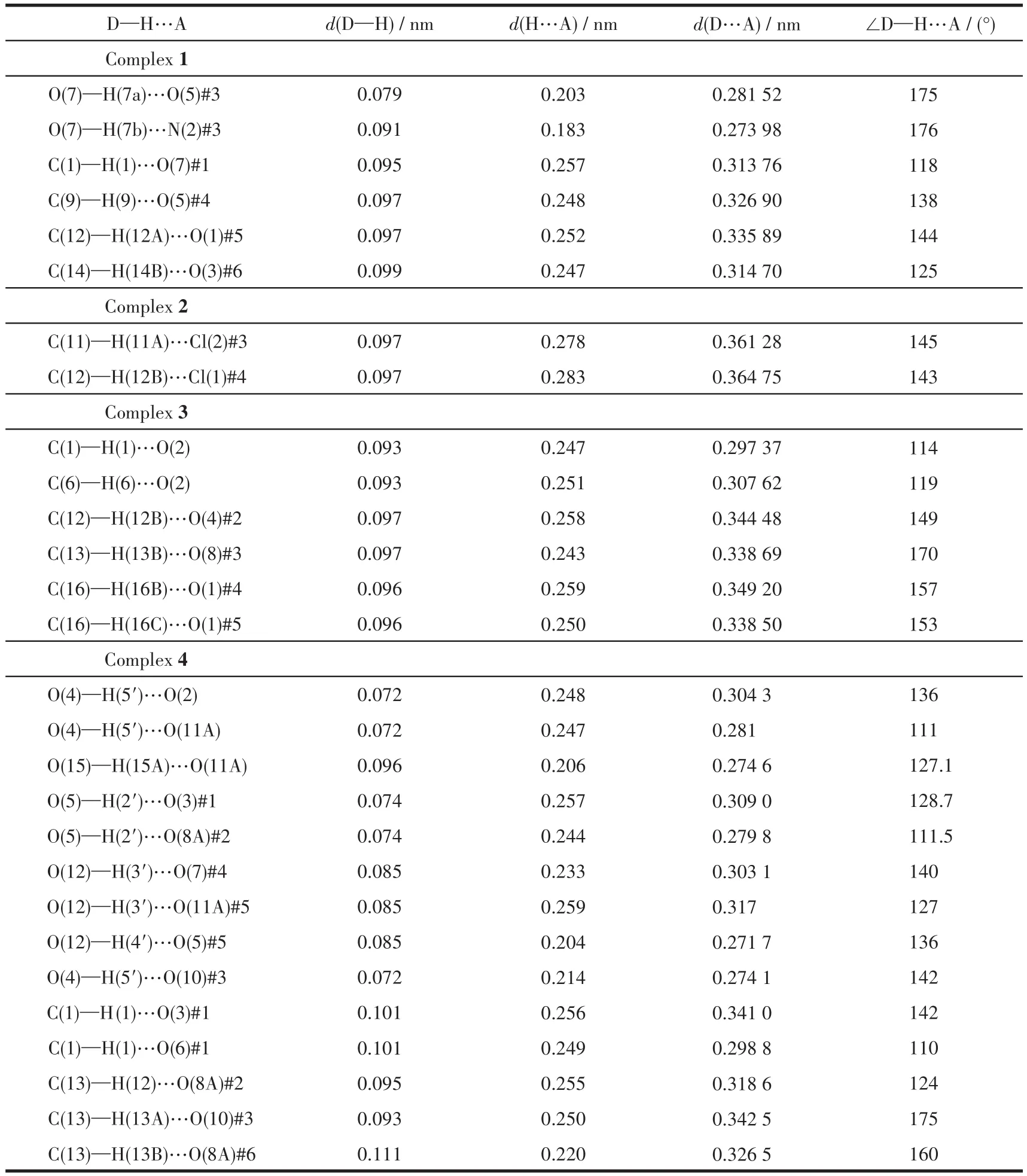
Table 3 Hydrogen bond geometries for complexes 1~4
2 Results and discussions
2.1 Description of crystal structures
2.1.1 Crystal structure of complex 1
Single crystal structure reveals that 1 crystallizes in the monoclinic space groupP2/c.The asymmetric unit consists of a discrete[Co(NO3)(H2O)2(L)2]+cation and a free nitrate anion(Fig.1a).In the coordination unit,the Co center adopts slightly distorted tetragonal biyramid geometry.Two molecules of water occupy the apical sites,and two pyridine nitrogen atoms(from two molecules of ligands)and a chelating nitrate anion are at the ecuatorial plane.Two molecules of L choose a mono-dentated coordination mode in the cation.They lie on the reverse side to avoid steric hindrance.On the equatorial plane,the maximum deviation of Co atom from the mean plane constructed by N1,N1A,O3 and O3A is 0.021 nm.The bite angle of N1—Co1—N1A is 93.23(9)°.It is wider than that of O3—Co1—O3A(60.01(8)°).The average bond lengths for Co—N and Co—O are 0.203 nm and 0.212 nm,separately.They are close with those in[Co(L1)(L2)(H2O)2]·3H2O(L1=terephthalic acid;L2=2,2'-dipyridylamine)of 0.207 and 0.214 nm,respectively[21].The free nitrate anion is stabilized by multiple hydrogen interactions including O…H—C(0.314~0.336 nm)and O…H—O(0.282 nm),as is shown in Fig.1b.
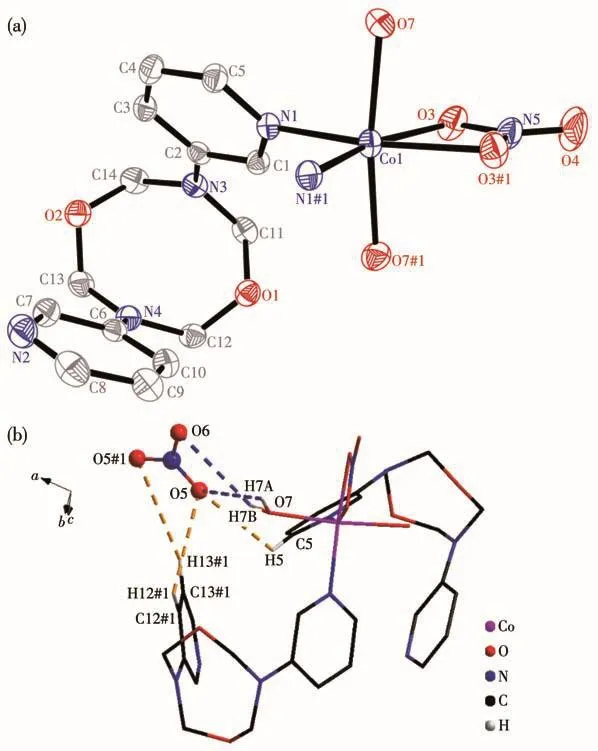
Fig.1 (a)Coordination structural unit with 30% probability ellipsoids in complex 1,where H atoms are omitted for clarify and the symmetry code is#1:-x,y,1/2-z;(b)Interactions of nitrate anion in complex 1 where the symmetry code is#1:-1+x,-1+y,z
2.1.2 Crystal structure of complex 2
Complex 2 crystallizes in the monoclinic space groupC2/c.The asymmetric unit consists of a neutral[Co(L)Cl2]unit and half a CH2Cl2solvent molecule,as shown in Fig.2a.In the coordination unit,each Co atom is coordinated by two nitrogen atoms from pyridine ringsand two Clatoms,adopting a tetrahedral[CoN2Cl2]coordination geometry.The angle of N2—Co1—N1 is 107.60(8)°.It is smaller than that reported in[Co2Cl4(L3)2](L3=1,2-dimethoxy-4,5-bis(2-pyridylethynyl)benzene)of 117.53(9)°[8].This might be due to the tension of octagonal ring in ligand.Two pyridine rings in ligand L arrange in an opposite direction.Ligand L acts as atrans-bridge,and they wrap around two cobalt atoms producing a helical 24-membered macrocycle.The Co…Co distance is 0.700 nm.The distance between the center of heterooctane is 1.019 nm.The average bond lengths for Co—N(0.203 nm)and Co—Cl(0.224 nm)are in agreement with those in[Co2Cl4(L4)2](L4=N,N'-(1,2-phenylene)diisonicotinamide)(Co—N 0.203 nm,Co—Cl 0.223 nm)[4]and[Co2Cl4(L3)2](Co—N 0.203 nm,Co—Cl 0.224 nm)[8].The free solvent molecule CH2Cl2is not eclipsed in the macrocycle,and it is stabilized by three adjacent coordination molecules as is illustrated in Fig.2b.It might result from the gentle reaction condition as well as the intermolecular hydrogen interactions of Cl…H—C(Cl…C 0.305 nm)and O…H—C(O…C 0.277 nm).
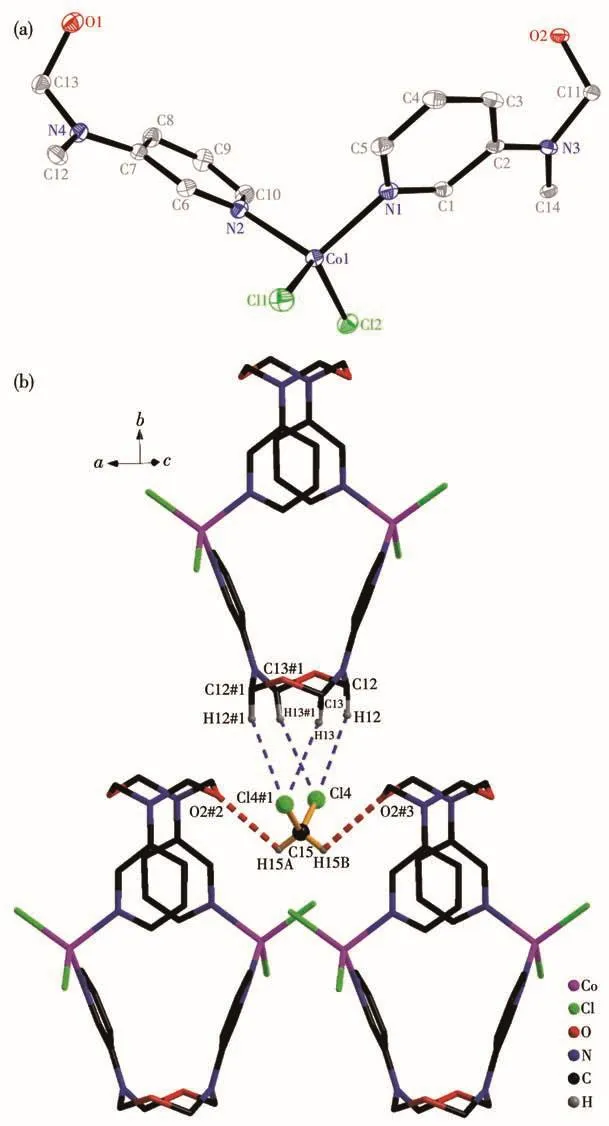
Fig.2 (a)Coordination structural unit with 30% probability ellipsoids in complex 2,where H atoms and solvent molecule are omitted for clarify;(b)Interactions of CH2Cl2solvent molecule in complex 2,where the symmetry codes are#1:-x+1,-y,-z+1;#2:1/2-x,1/2-y,1-z;#3:-1/2+x,1/2-y,1/2+z
2.1.3 Crystal structure of complex 3
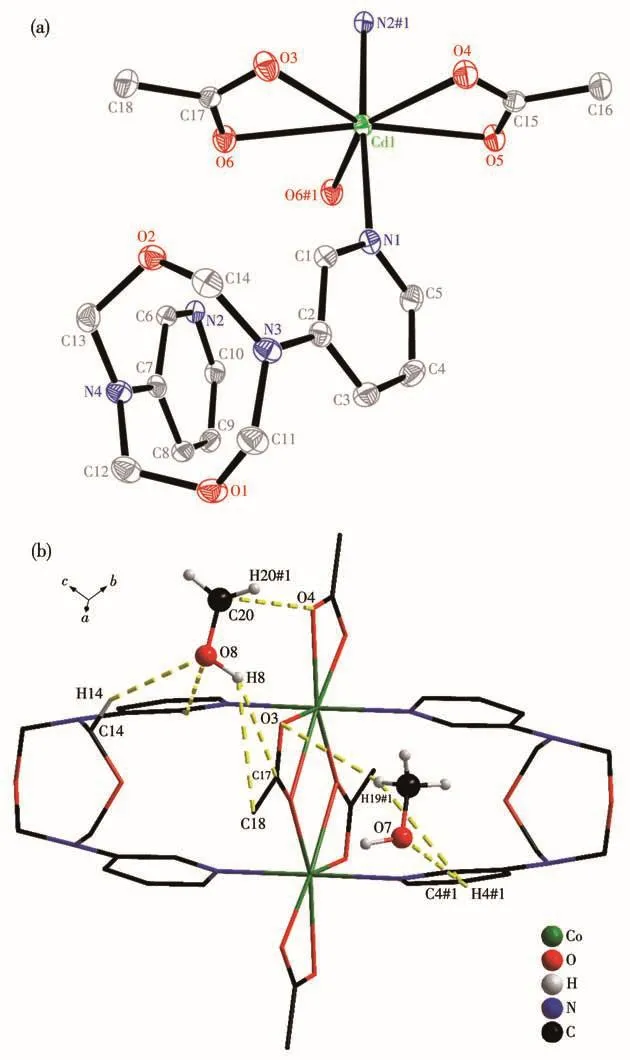
Fig.3 (a)Coordination structural unit with 30% probability ellipsoids in complex 3,where H atoms and solvent molecules are omitted for clarify and the symmetry code is#1:2-x,-y,1-z;(b)Interactions of CH3OH solvent molecules in complex 3,where the symmetry code is#1:1-x,1-y,1-z
Complex 3 crystallizes in the triclinic space groupP.The asymmetric unit consists of a discrete[Cd(L)(μ-OAc)(OAc)](Fig.3a)and two molecules of methanol.The Cd center is seven-coordinated by two separate pyridine ligands,two bridged acetates and another chelated acetate anion forming a distorted pentagonal bipyramidal geometry.The N atoms of the bipyridine occupy the apical sites and the O atoms of the acetate units lie in the equatorial plane.In complex 3,pyridine rings in ligand L arrange parallel to each other.Two molecules of ligand L act ascis-bridge,and link with two Cd(NO3)2units forming two rectangle cavities.The Cd…Cd separation is 0.393 nm.The mean Cd—N(0.231 nm)and Cd—O(0.241 nm)bond lengths are in agreement with those in[Cd2(L5)4(L6)2](L5=bis(4-bromobenzoate),L6=1,3-bis(4-pyridyl)propane)(0.231,0.242 nm)[22].Four free methanol molecules are stabilized by multiple hydrogen interactions of O…H—O(O…O 0.271~0.273 nm)and O…H—C(O…C 0.297~0.349 nm,Fig.3b).
2.1.4 Crystal structure of complex 4
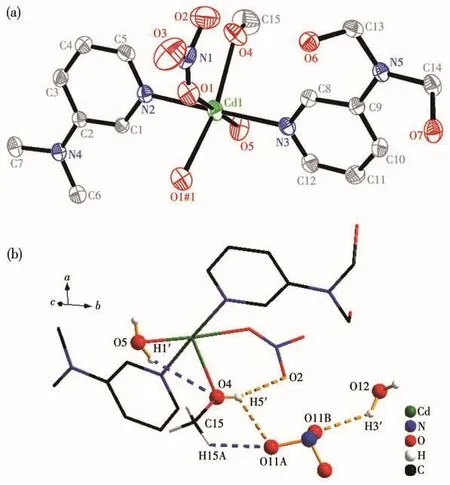
Fig.4 (a)Coordination structural unit with 30% probability ellipsoids in complex 4,where H atoms,counter nitrate anions and solvent molecules are omitted for clarify and the symmetry code is#1:-x+1,y,-z+1/2;(b)Hydrogen bond interactions in complex 4
Complex 4 crystallizes in the triclinic space groupP.The asymmetric unit consists of a[Cd(L)(μ-NO3)(CH3OH)(H2O)]+cation(Fig.4a),one disordered nitrate anion and one molecule of water.The Cd center is sixcoordinated by two separate pyridine ligands,two bridged nitrate anions,one water molecule and one methanol molecule forming a distorted tetragonal bipyramidal geometry.The bipyridine ligand L in complex 4 adopts a similar coordination mode with that in complex 3.The Cd…Cd separation is 0.412 nm.The average bond lengths for Cd—N and Cd—Onitrateare 0.226 and 0.246 nm,separately.They are comparable to those reported in complexes[Fe(η5-C5H4-1-C5H4N)2]2Cd2(NO3)4·CH3OH·0.5C6H6(0.224 and 0.249 nm)[23]and[Cd4(L7)4(NO3)6(MeOH)6]2+(L7=4,4'-bis(4-pyridyl)biphenyl)(0.226 and 0.249 nm)[13].One water molecule and a disordered nitrate anion are stabilized by multiple hydrogen interactions of O…H—O (O…O 0.272~0.317 nm)and O…H—C(O…C 0.299~0.341 nm)(Fig.4b).
In complexes 1~4,the average torsion angles of two pyridyl rings varied from 42.88°(2)to 19.34°(4).The corresponding centre distances of two pyridyl rings range from 0.497 9(2)to 0.382 0 nm(4)(Table 4).Through rotation of the N—C bonds and broaden or compress of two pyridine rings,the ligand L molecule has displayed considerable flexibility to adjust itself to adapt different coordination environment.
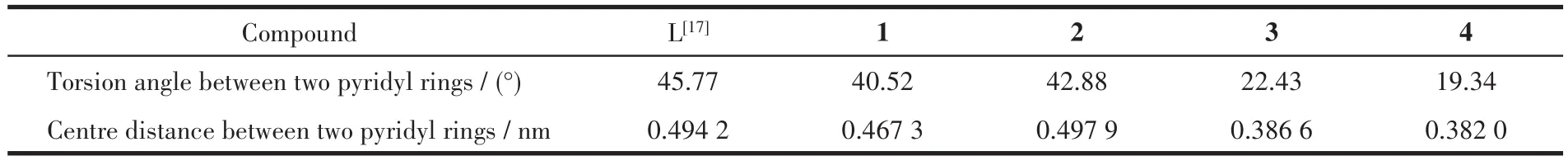
Table 4 Torsion angles and centre distances between two pyridyl rings of ligand L in complexes 1~4
2.2 IR spectra of complexes 1 to 4
In the IR spectra of complexes 1~4(Fig.5),bands around 1 602,1 578,1 495 cm-1(1),1 600,1 577,1 495 cm-1(2),1 560,1 498,1 420 cm-1(3)and 1 602,1 578,1 499 cm-1(4)are assigned to the stretching vibrations of pyridine groups,separately.The strong peaks around 1 136 cm-1in all complexes are attributed to the stretching vibrations of the C—O—C bond.The symmetric and antisymmetric stretchings of C—H bonds(—CH2— units in ligand)in complexes 1~4 are also in agreement with each other.They were in the region of 2 902~3 042 cm-1(1),2 903~3 077 cm-1(2),2 901~2 967 cm-1(3)and 2 901~2 974 cm-1(4),respectively.Strong vibrations of 1 370,1 309 cm-1(1)and 1 364,1 303 cm-1(4)are attributed to the NO-3anions[24].The isolated peak of 731 cm-1in complex 2 is assigned as antisymmetric stretching of C—Cl bonds in CH2Cl2[25].The broad bands at 3 440 cm-1(3)and 3 408 cm-1(4)are attributed the stretching of O—H.The infrared spectra of these complexes are in good accordance with their structural features.
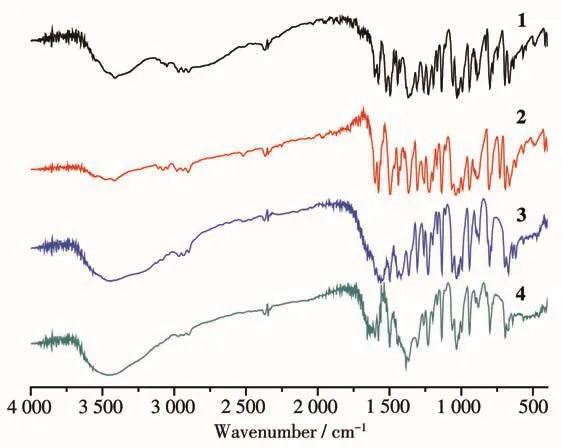
Fig.5 IR spectra of complexes 1~4
2.3 Thermal stabilities of L and complexes 1~4
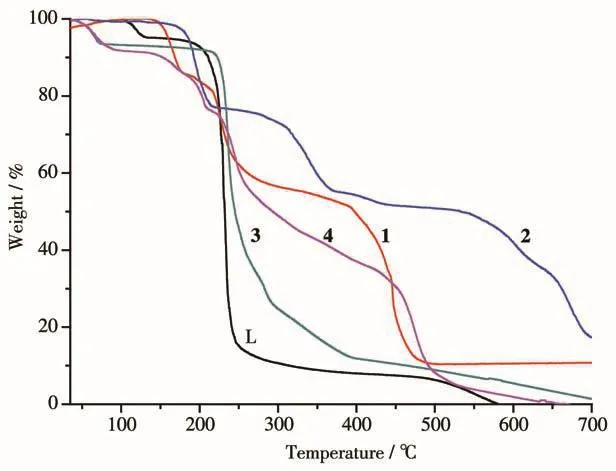
Fig.6 TGA curves of L and complexes 1~4 in air
Thermogravimetric analyses(TGA)of ligand L and complexes 1~4 were carried out under air atmosphere from 30 to 700℃with a heating rate of 10℃·min-1using crystalline samples,and the resulting curves are shown in Fig.6.The skeleton of ligand L decomposed at around 194℃.Correspondingly,curves in 1(178℃),2(174℃),3(214℃)and 4(207℃)have shown dramatic stages of weight loss,which might be attributed to the decomposition of ligand.In complex 1,the fisrt stage of weight loss(150~178 ℃)is attributed to the loss of HNO3and H2O(Obsd.15.66%,Calcd.16.51%),then accompanied by the degradation of ligand gradually.There are two steps of weight loss(in the region of 178~300℃and 400~500℃,respectively),the decomposition residue is in accordance with CoO(Obsd.10.36%,Calcd.9.81%).For complex 2,dichloromethane was unstable and escaped when exposing to the air,it was removed before thermogravimetric test.Ligand L molecules started to degrade at 174℃,and it showed two complicated degradation process(in the region of 174~200℃ and 200~375℃,separately).The final decompsition residue is suggested to be CoO(Obsd.17.54%,Calcd.18.13%).For complex 3,molecules of free methanol were unstable and partially escaped when exposing to the air.The weight loss of the first stage(from 30 to 105℃)corresponds to the loss of methanol molecules(Obsd.7.6%,Calcd.11.3%).The weight loss of the second stage(214~700 ℃)indicates the decomposition of two molecules of ligand L and CH3COO-.For complex 4,the weight loss of the first stage(from 30 to 100℃)corresponds to the loss of free water molecules and methanol(Obsd.8.54%,Calcd.8.68%).The skeleton of ligand L began to decompose at 207℃,and did not end up to 550℃,exhibiting two weight loss platforms in TGA curve.Accompanied by gradually sublimation of CdO,there was almost no residue for complexes 3 and 4.Thermogravimetric analysis indicates that,the bipyridine ligands have shown obvious two steps decomposition in complexes 1,2 and 4,and the ligands incis-bridge complexes are more stable than those in mono-dentated andtrans-bridgecomplexes.
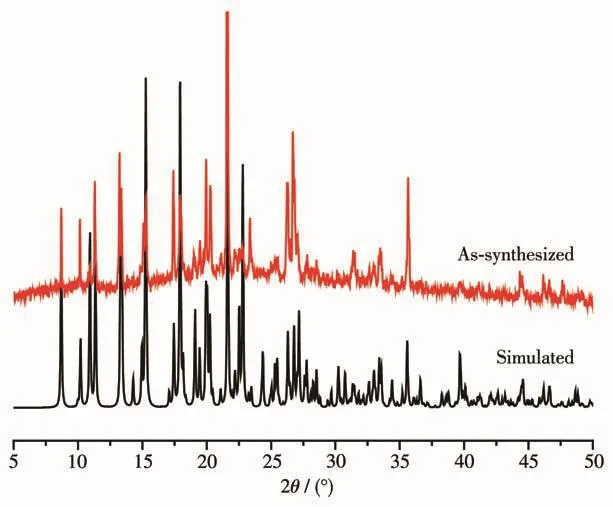
Fig.7 PXRD patterns of complex 1
2.4 Powder X-ray diffraction of complex 1
The phase purity of bulk products of complex 1 was further confirmed by elemental analysis and PXRD.As is illustrated in Fig.7,the PXRD pattern of the synthesized sample was consistent with the simulated one from single crystal structure.
3 Conclusions
Four transition metal complexes were prepared by utility of a clamplike bipyridine ligand 3,7-di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane.Single-crystal structures,IR spectra and thermal stabilities of these complexes have been discussed.The bipyridine ligand molecule has displayed diversity coordination mode in these complexes.All of these architectures have shown considerable stabilties.The study on its coordination chemistry with other metal ions is continuing.
杂志排行
无机化学学报的其它文章
- 《无机化学学报》投稿须知(NOTICE TO AUTHORS)
- 三维α-MnO2@Co3O4异质材料的制备、表征及其催化性能
- Aluminum Amine Compound Protected by β-Diketiminate Ligand:Preparation and Enhanced Performance as Catalyst for Ring-Opening Polymerization of ε-Caprolactone
- 基于1D/0D有序复合SnO2纳米晶的钙钛矿太阳能电池
- 三元混配铜(Ⅱ)配合物的晶体结构、DNA作用及其生物活性
- 巯基/羧基修饰硅藻土及其对Pb(Ⅱ)、Cd(Ⅱ)的吸附性能
