Mesenchymal stem cell-derived small extracellular vesicles in the treatment of human diseases: Progress and prospect
2021-02-23JieShiYuChenZhaoZhiFangNiuHaoJunFanShiKeHouXiaoQinGuoLuSangQiLv
Jie Shi, Yu-Chen Zhao, Zhi-Fang Niu, Hao-Jun Fan, Shi-Ke Hou, Xiao-Qin Guo, Lu Sang, Qi Lv
Jie Shi, Yu-Chen Zhao, Hao-Jun Fan, Shi-Ke Hou, Xiao-Qin Guo, Lu Sang, Qi Lv, Institute of Disaster Medicine, Tianjin University, Tianjin 300072, China
Jie Shi, Yu-Chen Zhao, Hao-Jun Fan, Shi-Ke Hou, Xiao-Qin Guo, Lu Sang, Qi Lv, Department of Biomaterials and Regenrative Medicine, Tianjin Key Laboratory of Disaster Medicine Technology, Tianjin 300072, China
Zhi-Fang Niu, General Hospital, Tianjin Medical University, Tianjin 300052, China
Abstract Mesenchymal stem cells (MSCs) are self-renewing, multipotent cells that could differentiate into multiple tissues.MSC-based therapy has become an attractive and promising strategy for treating human diseases through immune regulation and tissue repair.However, accumulating data have indicated that MSC-based therapeutic effects are mainly attributed to the properties of the MSC-sourced secretome, especially small extracellular vesicles (sEVs).sEVs are signaling vehicles in intercellular communication in normal or pathological conditions.sEVs contain natural contents, such as proteins, mRNA, and microRNAs, and transfer these functional contents to adjacent cells or distant cells through the circulatory system.MSC-sEVs have drawn much attention as attractive agents for treating multiple diseases.The properties of MSC-sEVs include stability in circulation, good biocompatibility, and low toxicity and immunogenicity.Moreover, emerging evidence has shown that MSC-sEVs have equal or even better treatment efficacies than MSCs in many kinds of disease.This review summarizes the current research efforts on the use of MSC-sEVs in the treatment of human diseases and the existing challenges in their application from lab to clinical practice that need to be considered.
Key Words: Mesenchymal stem cells; Small extracellular vesicles; Exosomes; Human diseases; Therapeutics; Prospects
INTRODUCTION
Mesenchymal stem cells (MSCs) are self-renewing cells that can differentiate into multiple tissues, including muscle and fat cells, and connective tissues[1].MSC-based therapy could regulate immune reaction and promote tissue regeneration, and has become a promising and important strategy for treating diseases.However, MSCbased cell therapy is limited because of its safety.Some studies showed that MSCs may cause tumorigenesis[2]or abnormal differentiation after ectopic engraftment[3].In addition, MSCs might aggregate to form pulmonary emboli or infarctions, and thus result in infusional toxicity in patients[4].Accumulating data have indicated that MSC derived secretome, which consists of soluble components and encapsulated extracellular vesicles (EVs), could contribute to most MSC- base effects[5-7].
EVs are small membrane vesicles composed of a phospholipid bilayer that are released by all kind of cell types.EVs influence recipient cell functions by exchanging components between cells.There are proteins, nucleic acids, and lipids in EVs.EVs are recognized as signaling vehicles in intercellular communication under normal or pathological states.At present, according to their biogenesis, EVs can be classified into three main categories, exosomes, microvesicles, and apoptotic bodies, each with specific characteristics (Table 1).
Researchers have invented dozens of different names for secreted vesicles.The International Society for EVs (ISEV) updated Minimal Information for Studies of EVs (MISEV) guidelines in 2018[8]and suggested that authors should pay attention to use of accurate terms for EV subtypes.Based on the physical characteristics of EVs, small EVs (sEVs) have a diameter < 100 nm or < 200 nm.In most of the studies cited in this review, exosomes were defined by the size of particles.Hence, we will here use the term sEVs instead of exosomes as ISEV suggested.MSC derived sEVs (MSC-sEVs) may transfer into special cells, induce appropriate cellular responses, and contribute to the therapeutic potency by regulating angiogenesis, tissue repair, and inflammation reaction[9-12].Recent studies have indicated that MSC-sEVs can be used to treat multiple diseases, including cardiovascular diseases[13], bone damage[14], cutaneous wounds[15], and acute lung injury (ALI)[16].Furthermore, MSC-sEVs are convenient to preserve in that they are smaller and simpler than MSCs.MSC-sEVs can be stored in mechanical -70 ℃ freezers without affecting therapeutic efficacy, whereas MSCs are best stored in liquid nitrogen at -196 ℃ and can be subject to irreversible damage during the freezing or thawing process, which may result in impaired therapeutic properties[17-19].
This review mainly focuses on summarizing the current progress on the use of MSC-sEVs in the treatment of diseases and discusses the existing challenges in the application of MSC-sEVs from lab to clinical practice.
THERAPEUTIC POTENTIAL IN DIFFERENT DISEASES
MSC-sEVs in treatment of cardiovascular disease
Cardiovascular disease (CVD) seriously threatens the health and living quality of human beings worldwide.Accumulating evidence indicates that MSC-sEVs protect ischemic cardiomyocytes from death, enhance the cardiac repair process, preserve cardiac function, and play an important role in CVD therapy[20,21].
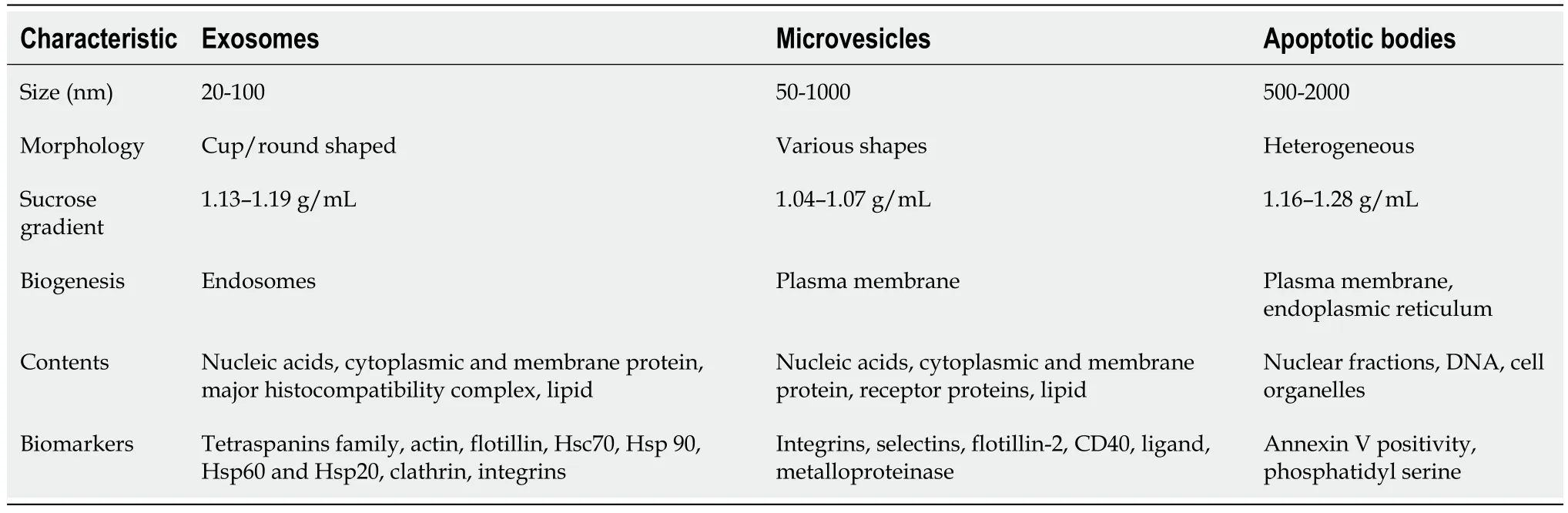
Table 1 Classification of different types of extracellular vesicles
MSC-sEVs in myocardial infarction:Acute myocardial infarction can lead to most of cardiovascular deaths.One study found that sEVs derived from hypoxic murine MSCs facilitated ischemic heart repairviaantiapoptotic miR-125b-5p[22].Furthermore, it has been shown that MSC-sEVs have the ability to regulate immune reactions and improve the myocardial microenvironment by reducing tissue inflammation and promoting tissue regeneration.Zhaoet al[23]indicated that MSC-sEVs alleviated myocardial ischemia-reperfusion injury in mice by intramyocardial injection.It was mainly because that miR-182 packaged in MSC-sEVs could promote the polarization of M1 macrophages to M2 macrophages[23].Wanget al[24]found that endometriumderived MSC-sEVs could significantly promote the recovery of cardiac function after myocardial infarction.They suggested that miR-21-containing MSC-sEVs improved the cardiac function by increasing the levels of vascular endothelial growth factor (VEGF) and enhancing neovascularization in rat ischemic hearts, and improved the cardiac function after acute myocardial infarction[24].Luoet al[25]designed a synthetic MSC (synMSC) in which human MSC secreted factors (containing both soluble factors and sEVs) were packaged into poly(lactic-co-glycolic acid) microparticles with MSC membrane coatings.This exciting synMSC exhibited superior cryostability, and transplantation of synMSCs inhibited cardiac dysfunction after myocardial infarction in mice[25].
MSC-sEV therapy plays a multifaceted role in promoting heart regeneration and repair after pathological damage.However, there are some limitations in the application of sEVs for the treatment of myocardial infarction.Direct intramyocardial injection is the commonly used route for sEV delivery, increasing cardiac localization.In contrast, systemic administrationviaintravenous infusion may result in off-target effects in organs other than the heart[26].
MSC-sEVs in vascular regeneration:Several types of signaling molecules in MSCsEVs could mediate angiogenesis, such as VEGF and miR-126.Weiet al[27]fabricated MSC-sEV-functionalized vascular grafts and evaluated the vascular regeneration in a rat model of hyperlipidemia.The results indicated that MSC-sEVs could effectively promote the vascular smooth muscle and endothelium regeneration.It was demonstrated that the bioactive molecules within the sEVs, including VEGF, miR-126, and miR-145, may participate in the process of regeneration.Furthermore, MSC-sEVs could induce macrophage polarization from a proinflammatory (M1) phenotype to an anti-inflammatory (M2) phenotype[27].At the same time, the microenvironment of original cells also could influence the contents of MSC-sEVs.Duet al[28]indicated that human placenta-derived MSC-sEVs (hp-MSC-sEVs) stimulated by nitrogen oxide could promote human umbilical vein endothelial cell (HUVEC) tube formation.hp-MSC-sEVs could rescue limb function in a mouse model of hind limb ischemia[28].
MSC-sEVs in treatment of neurological diseases
MSC-sEVs in spinal cord injury:Spinal cord injury (SCI) is the most serious complication of spinal injury, and it often causes serious dysfunction of the limb below the injured segment.SCI will not only cause serious physical and psychological harm to the patient, but also cause a huge economic burden on the entire society.To date, the treatment of SCI remains a huge challenge for clinicians[29].MSCs have been widely used in the treatment of nerve injury, but their effects are not obvious, mainly because MSCs are often trapped in the pulmonary vascular bed, and the mechanism of how MSCs work is still unclear[30].
MSC-sEVs reduce pathological changes after SCI and improve motor function, blood flow, and hypoxia.In addition, MSC-sEVs improve the ability of the endothelium to modulate blood flow, maintain the blood spinal cord barrier, eliminate edema, downregulate the expression of matrix metallopeptidase 2, Bax, hypoxia inducible factor-1 (HIF-1α), and Aquaporin-4, and upregulate the level of bcl-2, and decrease cell apoptosis[29].Riazifaret al[31]showed that MSC-sEVs protect nerves, reduce inflammation, and promote angiogenesis in a mouse model of autoimmune encephalomyelitis (EAE)-induced SCI.MSC-sEVs stimulated by interferon γ (IFN-γ) reduce the clinical score of EAE mice, alleviate demyelination, inhibit neuroinflammation, and increase the number of Treg cells in the spinal cord[31].Zhouet al[32]have found that miR-21-5p is one of the most abundant microRNAs (miRNAs) in MSC-sEVs, and miR-21-5p/FasL axis is recognized as a potential mechanism to improve motor function and inhibit apoptosis in MSC-sEVs for SCI[32].Totally, these results demonstrate that MSC-sEVs inhibit neuroinflammation and promote nerve regeneration in SCI.
MSC-sEVs in brain injury:Hypoxic ischemia (HI) is closely related to mortality in preterm infants.MSC-sEVs have neuroprotective potential in treating hypoxicischemic injury.Systemic administration of MSC-sEVs rather than intact MSCs promotes the recovery of brain function in preterm infants after hypoxic ischemia and prevents structural damage[33].MiR-133b was reported to be the foremost mechanism involved in the enhancement of brain recovery in brain injury.MSCs rebalance miR-133b expression in ischemic brain tissue.It was observed that the ischemic environment leads to abundant expression of miR-133b in MSC-sEVs, and therefore, neuronal growth was enhanced by inhibiting the expression of Ras homolog family member A[34].
Traumatic brain injury (TBI) is a common injury in neurosurgery worldwide.There are no effective medications to reduce TBI mortality and improve functional recovery.Cell therapy, including MSCs, has shown promise for TBI.However, relatively few MSCs can be injected intracranially.Intra-arterial injection of MSCs may cause cerebral ischemia.Intravenous injection can cause the distribution of MSCs throughout the body.Recent studies have indicated that MSC-sEVs reduce cognitive impairment in TBI mouse models.sEVs may be safer, and do not induce microvascular embolism.This may open up new clinical applications for TBI intervention[30].
MSC-sEVs in neurodegenerative diseases:Alzheimer’s disease (AD) is a kind of neurodegenerative disease resulting from progressive neuronal death in the hippocampus and cerebral cortex.The accumulation of β-amyloid peptide (Aβ) can cause neuroinflammation, which in turn leads to memory impairment and AD[35].In recent years, cell therapy has become a potential treatment for AD.Injection of MSCs can reduce the accumulation of Aβ and alleviate the inflammation in a mouse model[36].MSC-sEVs reduce neuroinflammation and promote nerve regeneration by clearing Aβ.Neprilysin (NEP) is the most important metalloendopeptidase related to Aβ proteolysis.MSC-sEVs contained enzymatically active NEP that prompts the possibility of reducing Aβ accumulation[37].
Parkinson's disease (PD) is a motor dysfunction caused by the decrease of dopaminergic neurons in the midbrain.Researchers found that injection of the human bone marrow-derived MSC (hBMSC) secretome have neuroprotective effects in a rat model of PD.Granulocyte colony-stimulating factor and hBMSC combination therapy has beneficial effects on a PD model.Proteomic analysis showed that hBMSC-sEVs but not hBMSC transplantation have positive effects[38,39].Sadanet al[40]utilized engineered MSCs producing and secreting high levels of factors such as brain-derived neurotrophic factor and glial-derived neurotrophic factor.The transplantation inhibited dopamine depletion to 72% in the contralateral striatum, which provided a treatment strategy for PD using-modified MSC-sEVs[40].
MSC-sEVs in treatment of orthopedic diseases
MSC-sEVs in fractures:Accidents, sports injuries, and other reasons lead to many fractures each day in the world.Bone tissue engineering has emerged as an attractive strategy for fracture repair.This method involves the combination of cells and bioactive factors with bone substitutes to enhance their capacity of osteogenesis and angiogenesis[41].Liuet al[42]found that hypoxia-treated MSC-sEVs have a therapeutic role in fracture healing.MSC-sEVs under hypoxia exert their proangiogenic effect by transporting exosomal miR-126 to endothelial cells in an extracellular regulated protein kinases (ERK) pathway-dependent manner[42].Lianget al[43]found that MSCsEV transplantation improved bone regeneration in rats.Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is known as a tumor suppressing gene.Lack of PTEN will lead to the formation of new blood vessels.MSC-sEVs could downregulate PTEN and promote angiogenesis.In addition, dimethyloxaloylglycinestimulated human bone marrow MSC-sEVs promote bone regeneration through angiogenesis[43].
MSC-sEVs in cartilage regeneration:MSCs have the potential to regenerate cartilage in animal and preclinical studies[44].Direct action of MSC-sEVs on chondrocytes triggers a series of positive responses from chondrocytes.CD73 carried by MSC-sEVs regulates the adenosine-activated AKT and ERK signaling pathways, promotes the proliferation and migration of chondrocytes, and inhibits apoptosis.MSC-sEVs promote the expression of chondrocyte genes (collagen and proteoglycan) and inhibit the expression of cartilage matrix metabolism and inflammation markers such as inducible nitric oxide synthase[45].
Cartilage usually needs a long time to regenerate.Local MSC-sEV injection that was conducted weekly increased the pain of patients.Liuet al[46]developed a light-induced imine-crosslinked hydrogel that was easy to use, biocompatible, integrated with cartilage, and functionalized with human induced pluripotent stem derived MSC-sEVs to make cell-free tissue patches for cartilage regeneration.It was found that cell-free tissue patches retain MSC-sEVsin vitroand actively regulate chondrocytes and bone marrow stem cells.In addition, cell-free tissue patches could be combined with natural cartilage matrix and promote the deposition of cells at the site of cartilage defects, ultimately promoting the repair of cartilage defects[46].
MSC-sEVs in osteoarthritis:Osteoarthritis (OA) is a common degenerative disease caused by obesity, labor injury, and old age.The mechanism of osteoarthritis is complex and involves multiple processes and tissues.Autologous chondrocytes represent an important cell type in cartilage, which can provide a safe and effective solution.However, the inherent disadvantages are limited availability duringin vitroexpansion, dedifferentiation, and loss of function.MSC-based therapy represents a new and promising treatment strategy for OA in recent years.It is reported that cartilage in joints could be protected from degeneration by intra-articular injection of bone marrow MSCs, which could delay the development of OA.In addition, several clinical trials have shown that MSC-based treatment is a well-tolerated cell-based therapy by reducing inflammation of OA[47].
Human embryonic stem cell-induced MSC (ESC-MSC) derived exosomes increase the expression of collagen type II (the main component of the cartilage matrix) in the cartilage matrix and decrease the expression of ADAMTS5 (a disintegrin and metalloprotease with thrombospondin-like repeat family of enzymes)[48].MSC-sEVs have some particular effects, such as promoting angiogenesis and inhibiting cell apoptosis and oxidative stress, which can help rescue of OA.MSC-sEVs promote the proliferation and migration of chondrocytes[49].Some miRNAs in MSC-sEVs can exert a therapeutic effect against OA through their regulatory role in proliferation and cartilage formation.For example, miR-92a in MSC-sEVs can alleviate OA by upregulating chondrocyte proliferation and matrix synthesis through the PI3K/AKT/mTOR pathway.In vivo,enhanced cartilage regeneration and prevention of OA were demonstrated in rats that were treated with miR-140-5p-transported MSCsEVs[47].
MSC-sEVs in treatment of lung diseases
MSC-sEVs in acute respiratory distress syndrome: The clinical manifestations of acute respiratory distress syndrome (ARDS) are progressive hypoxemia and respiratory distress.The clinical features include injury of alveolar epithelial and capillary endothelial cells, leading to diffuse pulmonary interstitial and alveolar edema.However, there is still no specific therapy for ARDS[50].MSC therapy has the ability to regulate immunity and inflammation, and prevent lung injury caused by infection and regeneration through differentiation or paracrine mechanisms[51].There is increasing evidence that stem cell-derived conditioned medium and/or extracellular vesicles may be convincing alternatives[52].Zhuet al[53]found that MSC-sEVs have a therapeutic effect on endotoxin-induced ALI, partly attributed to the expression of keratinocyte growth factor mRNA in the injured alveoli[53].Khatriet al[54]showed that transfer of RNA from EVs to epithelial cells is the primary cause of the anti-influenza property of MSC-sEVs.In a swine influenza virus model, intratracheal injection of MSC-sEVSsignificantly reduced the release of virus in nasal swabs from infected pigs at 12 h after infection, inhibited replication of influenza virus, and downregulated virus associated pro-inflammatory cytokines.Histopathological results showed that MSC-sEVs could reduce the lung injury of pigs caused by influenza virus[54].Stoneet al[55]found that sEVs derived from MSCs reduce lung inflammation and injury after ischemia-reperfusion and promoteex vivolung perfusion-mediated donor lung repair.The therapeutic effect of MSC-sEVs is partly mediated by reducing the antiinflammatory mechanism of immune cell activation and protecting the integrity of the endothelial barrier to prevent pulmonary edema[55].
MSC-sEVs in pulmonary fibrosis:Pulmonary fibrosis is a serious consequence of changes in normal lung tissue structure and loss of function.Mansouriet al[56]examined the therapeutic effect of human bone marrow MSC-sEVs in a bleomycin induced pulmonary fibrosis model and explored the mechanism.They found that MSC-sEVs effectively prevent or reverse bleomycin-induced pulmonary fibrosis through systematic regulation of the monocyte phenotype[56].MSC-sEVs are profitable in ALI and lung fibrosis.Shentuet al[57]demonstrated that MSC-sEVs but not fibroblast sEVs (fsEVs) inhibit transforming growth factor (TGF-β1) induced myofibroblast differentiation or idiopathic pulmonary fibrosis lung fibroblasts.Compared with fsEVs, MSC-sEVs showed a time and dose-dependent increase in cell uptake.Downregulating Thy-1 (CD90) or blocking Thy-1-integrin interactions reduced MSCsEVs uptake and prevented the inhibition of myofibroblast differentiation[57].
MSC-sEVs in pulmonary hypertension:Pulmonary hypertension (PAH) is defined as an average resting pulmonary arterial pressure of ≥ 25 mmHg[58].PAH is often a progressive and ultimately fatal disease.Adipose-derived mesenchymal stem cells (ADMSCs) and ADMSC-derived sEVs (ADMSC-sEVs) have protective effects in PAH.ADMSCs increased the proliferation of monocrotaline pyrrole (MCTP)-treated human pulmonary artery endothelial cells (HPAECs) through coculture of ADMSCs and MCTP-treated HPAECs.The expression of bone morphogenetic protein receptor 2 (BMPR2) in HPAECs and PAH mice was inhibited by miR-191 in ADMSCs and ADMSC-sEVs[58].Hoganet al[59]found that MSC-sEVs recovered the mitochondrial dysfunction that is associated with PAH.MSC-sEVs improve energy balance and ameliorate O2consumption, which plays a role in enhancing mitochondrial function in pulmonary arterial hypertension[59].Lung morphology, pulmonary fibrosis, right ventricular (RV) hypertrophy, right ventricular systolic pressure, RV/body weight ratio (RV:BW), and pulmonary vascular remodeling can be significantly improved by MSC-sEVs derived from either human bone marrow or the umbilical cord Wharton’s jelly[60].
MSC-sEVs in bronchial dysplasia:Bronchopulmonary dysplasia (BPD) is a chronic lung disease that appears during infancy with high morbidity in premature infants.Premature babies with conditions such as respiratory distress syndrome are at an increased risk of developing BPD.Despite improvements in clinical treatment, the incidence of BPD has not decreased.MSC-sEVs significantly increase the tubular network of HUVECs, partly through a VEGF-mediated mechanism.Daily intraperitoneal injection of MSC-sEVs increases the number and size of pulmonary vessels by promoting angiogenesis[61].The therapeutic effect of MSC-sEVs was blocked by tumor necrosis factor (TNF)-stimulated gene-6 (TSG-6) gene knockout in MSCs or injection of TSG-neutralizing antibody in BPD mice.The levels of the proinflammatory cytokines such as interleukin-6 (IL-6), TNF-α, and IL-1 in peripheral blood and TSG-6-treated BPD mice were decreased, suggesting their regulatory role in lung injury[62].The effect of MSC-sEVs on the pulmonary macrophage phenotype is the basis of their therapeutic effect by regulating hyperoxia (HYRX, 75% O2)-induced bronchopulmonary dysplasia.Early intervention and slowing the early inflammatory phase induced by HYRX are critical in maintaining normal lung development[63].
MSC-sEVs in treatment of liver diseases
MSC-sEVs has produced profitable effects in various animal models of hepatic disease, such as acute liver injury and liver fibrosis[64].Tanet al[65]demonstrated that MSC-sEVs can protect the liver from toxic injury by activating proliferation and regeneration[65].Yanet al[66]demonstrated that glutathione peroxidase 1 (GPX1) derived from human umbilical MSC-sEVs can detoxicate carbon tetrachloride (CCl4) and H2O2, and alleviate oxidative stress and apoptosis.Silencing GPX1 in hucMSCs reduced the antioxidant and anti-apoptotic abilities of hucMSCs-sEVs, and decreased the hepatoprotective effect of hucMSCs-sEVsin vitroandin vivo[66].Liet al[67]showed that human umbilical cord MSC-sEVs suppressed liver fibrosis through inhibition of epithelial mesenchymal transition and collagen deposition in a CCl4-induced liver injury mouse model.The potential mechanism is related to decreasing TGF-β1, the phosphorylation of Smad2, and the expression of collagen types I and III[67].MiR-122 target genes were found to participate in hepatic stellate cell (HSC) proliferation and collagen maturation.MiR-122 modification increased the therapeutic efficacy of AMSCs on CCl4induced liver fibrosis by inhibiting HSC activation and alleviating collagen sedimentation.Therefore, delivery of miR-122 through exosome mediated communication is a promising strategy for the treatment of liver fibrosis[68].
MSC-sEVs in treatment of skin diseases
Extensive burns and trauma could lead to skin damage and result in acute or chronic wounds[69].In recent years, MSCs have been used in wound healing and regeneration, increasing angiogenesis, resolving wound inflammation, favorably improving extracellular matrix (ECM) remodeling, and promoting skin tissue regeneration[70].Recently, MSC-sEVs have gained much attention in the field of skin repairing.
There are three overlapping stages during wound healing, including the inflammation, proliferation, and remodeling[71].MSC-sEVs possess effective antiinflammatory properties and promote the macrophages toward M2 phenotype which is beneficial for wound healing[72].MSC-sEVs are enriched in angiogenesis associated miRNAs and proteins[73,74].Lianget al[75]indicated that miR-125a, which was enriched in ADMSC-sEVs, could improve endothelial cell angiogenesis by upregulating the levels of the angiogenic inhibitor[75].One study revealed that sEVs from hypoxiatreated human ADMSCs could significantly promote angiogenesis by upregulating VEGF-R/VEGF[76].The migration of fibroblasts could be regulated by MSC-sEVs[15,77].ECM reconstruction plays an important role in the process of wound healing.Zhanget al[78]found that human induced pluripotent stem cell-derived MSC-derived sEVs (hiPSC-MSC-sEVs) were beneficial for cutaneous wound healing in a rat model by improving collagen synthesis and angiogenesis[78].In a word, MSC-sEVs play a vital role in wound healing.
THERAPEUTIC CONSIDERATIONS AND PROSPECTS
As described above, the future of MSC-derived sEV therapy has great potential.However, there are some challenges from lab to clinical practice that need to be considered.
Standard methods for separation and purification of MSC-sEVs
The guidelines of MISEV 2018 provide recommendations in six major areas: (1) Nomenclature; (2) Collection and preprocessing of fluids for EV extraction; (3) EV preparation and concentration; (4) EV characterization; (5) Functional studies; and (6) Reporting[8].
In the clinical setting, it is very important that MSC-sEV preparations are manufactured reproducibly[79].To date, there is no perfect technology to separate EVs for either clinical or basic research.The conventional and most widely used method to isolate EVs is differential centrifugation[8,80].Although ultrafiltration concentrates conditioned medium into a sucrose cushion after ultracentrifugation[81-83], this method cannot produce highly pure EVs.Moreover, ultracentrifugation may result in sEV aggregation and poor resuspension[84].Precipitation with polyethylene glycol or other polymers is a reproducible and scalable way to enrich EVs, which has been used in EV-based clinical trials[85,86].However, because of abundant coprecipitates, this kind of method cannot produce pure EVs.Recently, tangential flow filtration and size exclusion chromatography have gained increasing attention.These size-based fractionation methods are adopted as highly scalable and GMP-compatible technologies.Compared with legacy methods, these methods could produce more comparable, superior purity, and functional EVs at the same time[85,87].Finally, these methods should also be standardized to ensure the purity, reproducibility, and maintenance of EV functional properties.
Characterization and quality control of MSC-sEVs
There are no gold standards for EV identification and analysis currently.MISEV 2018 defined some minimal requirements for identifying EVs[8].MSC-sEV preparations must first correspond to the International Society for Cellular Therapy (ISCT) minimal criteria: (1) At least one protein of each category 1 to 3 must be evaluated in any EV preparation; (2) At least one negative protein marker; (3) Electron or atomic force microscopy; and (4) Single particle analyzers.Moreover, MSC-sEV-specific antigens need to be identified.According to the ISCT minimal criteria, MSC surface antigens, such as CD73, CD90, and CD105, have been found in many published MSC-EV proteomics datasets.In contrast, three non-MSC surface antigens (CD14, CD34, and CD11b) from the ISCT minimal criteria were not found in these datasets[88].
As a prerequisite for the clinical use of EV agents, quality control criteria must be established that include not only the physicochemical and molecular parameters described above but also functional parameters.Because of the diversity of active constituents, EVs are thought to act in a complex manner, so therapeutic activity cannot be proposed solely through molecular analysis for pharmaceutical characterization.Biological assays should be developed that allow for the prediction of EV functional properties.Moreover, MSC-sEVs have therapeutic potential in many kinds of diseases.We think that the biotherapy activity of MSC-sEVs for specific diseases should be separately tested in qualified biological assays.
Enhancement of therapeutic potential of MSC-sEVs
MSC-derived sEVs are enriched with growth factors, cytokines, lipids, mRNAs, and therapeutic miRNAs.Multiple kinds of stimulation of MSCs, such as biophysical or biochemical methods, as well as cellular reprogramming, have been shown to influence the contents and enhance the therapeutic efficacy of subsequent MSCsEVs[89,90].
Multiple different biophysical stimuli have been tested in MSCs, including electric pulsing, low-power laser irradiation, 2D and 3D culture, and magnetic forces[90].MSCs are normally cultured on 2D plastic surfaces, which lack the conditions of physiological niche of MSCs.3D bioreactor culture increases the production of sEVs from MSCs; furthermore, EVs from hMSCs cultured in 3D scaffolds showed better outcomes in a model of traumatic brain injury than those from hMSCs cultured in 2D conditions[91,92].Hypoxic conditions (5% O2) increased the proliferation and viability of MSCs.MSC-sEVs under hypoxic conditions showed increased vascular tube formation compared to that of normoxic MSC-EVs[93].
Pretreatment of MSCs with cytokines and other biochemical agents has been widely studied.Recent studies revealed that proinflammatory cytokines such as IL-1β, IL-6, TNF-α, TGF-β, and IFN-γ could enhance the therapeutic efficacy of MSCs effectively[94,95].Pre-conditioning of ADMSCs with platelet-derived growth factor stimulated the secretion of EVs with enhanced angiogenic potential[96].
Immortalized MSCs by genetic modification could continuously produce sEVs, which will improve the batch-to-batch reproducibility of sEVs[84].Chenet al[97]showed that the functionality of MSC-sEVs is preserved after immortalization[97].sEVs secreted by GATA-4-overexpressing MSCs improved cardiac function in a myocardial infarction mouse model[98].Likewise, sEVs from chemokine (C-X-C motif) receptor 4 (CXCR4)-overexpressing MSCs promoted tube formation of HUVECs and exhibited a cardioprotective effect in a myocardial infarction rat model[99].sEVs from HIF-1αoverexpressing MSCs increased angiogenic activity, which promoted cardiac tissue repair in a mouse model[100].
EVs can avoid immune responses, penetrate the blood-brain barrier, and avoid the degradation by RNase during migration[101,102].These characteristics make them an attractive and promising drug delivery tool[103].Chemicals, RNAs, and peptides can be delivered as therapeutic agents to patients.MSCs pretreated with paclitaxel showed strong antitumor activity by uptaking and then releasing the drug[104].MiRNAs show therapeutic potential for many diseases by targeting transcriptional and posttranscriptional regulation.EVs prove to be an effective vehicle for miRNA delivery.MiR-93-5p-overexpressing MSC-sEVs showed a myocardial protective effect by inhibiting inflammatory response and autophagy[105].MiR-122 inhibits liver fibrosis by inhibiting the proliferation of hepatic cells.EVs from ADMSCs overexpressing miR-122 alleviate collagen deposition and enhance the therapeutic efficacy of ADMSCs for the treatment of liver fibrosis[68].EVs from miR-133b-overexpressing MSCs have increased neuroprotective and regenerative activity[106].
CONCLUSION
As a cell-free therapy, EVs minimize safety concerns with the administration of live cells.MSC-derived sEVs have therapeutic potential in brain, heart, liver, lung, skin, and bone diseases.Next, guidelines and standards for purity and quality control of isolated MSC-derived sEVs will be the main challenge in establishing platforms for clinical grade sEV production.Standardized and improved protocols for EV isolation and storage, as well as quantifiable, robust, and reproducible assays that predict the therapeutic capacity of MSC-sEVs, will promote the application of MSC-sEVs from the laboratory to the clinic.
杂志排行
World Journal of Stem Cells的其它文章
- Perspectives of pluripotent stem cells in livestock
- Adipose-derived stem cells: Pathophysiologic implications vs therapeutic potential in systemic sclerosis
- Pancreatic β cell regeneration induced by clinical and preclinical agents
- Stem cell transplantation and/or adenoviral glial cell line-derived neurotrophic factor promote functional recovery in hemiparkinsonian rats
- Vascularization and osteogenesis in ectopically implanted bone tissue-engineered constructs with endothelial and osteogenic differentiated adipose-derived stem cells
- Proliferation and tenogenic differentiation of bone marrow mesenchymal stem cells in a porous collagen sponge scaffold
