Effect of Foliar GA3 and ABA Applications on Reactive Oxygen Metabolism in Black Currants (Ribes nigrum L.) During Bud Paradormancy and Secondary Bud Burst
2021-02-21HuoJunweiFeiXiaohuiGangHuixinZhangYanGongXiaonanLiSonglinBianChunyangYuGaryGaoandQinDong
Huo Jun-wei, Fei Xiao-hui, Gang Hui-xin, Zhang Yan, Gong Xiao-nan, Li Song-lin, Bian Chun-yang,Yu Gary-Gao, and Qin Dong*
1 College of Horticulture & Landscape Architecture, Northeast Agricultural University/Key Laboratory of Biology and Genetic Improvement of Horticultural Crops (Northeast Region), Ministry of Agriculture, Harbin 150030, China
2 OSU South Centers, College of Food, Agricultural and Environmental Sciences, The Ohio State University, Piketon, Ohio 45661, USA
Abstract: The secondary bud burst can cause around 10%-20% yield losses in black currants, an economically important crop in parts of Europe, Asia and North America. The metabolism of reactive oxygen species (ROS) has been linked to bud dormancy and its early release (secondary bud burst) in several fruit crops. But the relationship between ROS metabolism and the secondary bud burst is still not well understood in black currants. In the present study, two black currant cultivars (Adelinia and Heifeng) with opposing tendency of exhibiting the secondary bud burst were sprayed with abscisic acid (ABA) and gibberellic acid (GA3) to either inhibit or induce the secondary bud burst. The results showed that ABA inhibited the secondary bud burst by reducing the contents of ROS (H2O2, ) in buds; decreasing the activities of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT); and increasing the contents of oxidized glutathione (GSSG) and ascorbic acid (AsA). GA3 effectively induced the secondary bud burst by increasing ROS contents; increasing the activities of several antioxidant enzymes, such as SOD, POD, CAT, glutathione reductase (GR), ascorbate peroxidase (APX) and the contents of reduced glutathione (GSH); and decreasing the contents of AsA. The experimental results showed that GA3 treatment increased the content of ROS, accelerated the metabolism of reactive oxygen species, and promoted the second burst of black currants. However, ROS metabolism was at a low level under ABA treatment, and the buds remained dormant. These results suggested that ROS metabolism might play an important role in the two black currants of the secondary bud burst.
Key words: Adelinia and Heifeng, AsA-GSH cycle, concentration, reactive oxygen species (ROS), enzyme activity
Introduction
Black currant (Ribes nigrumL.) is a perennial deciduous shrub that is native to northern Europe and northern Asia (Barneyet al., 2005). Black currants are grown commercially in more than 30 countries. The bulk of production is in Europe and the total world production is around about 180 000 kg (Eurofresh, 2016). In China, this species is mainly grown in Heilongjiang Province, which accounts for about 70% of the total production (Qinet al., 2012). Black currants are known to have high vitamins concentrations, especially vitamin C in some cultivars that contains up to 464 mg · 100 g-1FW in fruits (Qinet al., 2017a). Black currant is very popular among consumers because of its high nutritional and medical values (Huoet al., 2011; Zhanget al., 2015).
However, two leading cultivars of black currants Adelinia and Brodtrop have been shown to have a secondary bud burst, which means some of the buds break paradormancy around late summer and early autumn, and then burst in autumn (Qinet al., 2017b). It is estimated that the secondary bud burst has caused about 10%-20% yield losses in black currants and has led to lower economic returns in northeast China (Qinet al., 2017b). Therefore, it is critical to study the physiological mechanisms of the secondary bud burst in black currants so that practical methods can be developed to prevent the occurrence of the secondary bud burst.
H2O2and O2-· are the two major ROS and can play important roles in the growth and development of higher plants (Nafeeset al., 2019). During the periods of abiotic stresses, several ROS are produced in small amounts and used to regulate the metabolic pathways in their adaption to changes in environmental factors (Choudhury-Lechet al., 2016). Several studies have shown that H2O2acts as a signal during dormancy and germination process in plants (MacRae and Ferguson, 1985; Sudawanet al., 2016; Maksimovet al., 2018).The relationship between reactive oxygen species (ROS) metabolism and bud dormancy and dormancy breaking has been studied in quite a few fruit crop species. H2O2concentration in buds has been shown to reach a high level, during bud dormancy and is maintained at an elevated level throughout the dormancy period, and then decreased gradually, when dormancy is released in crops, such as grapes (Hanet al., 2007), peaches (Wanget al., 2010) and nectarines (Wanget al., 2006). There has not been a published report on changes of H2O2concentrations in black currants buds before, during and after paradormancy. Elevated levels of ROS are closely tied to the increased activities of key enzymes, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), glutathione reductase (GR) and ascorbate peroxidase (APX), thus leading us to conclude that the activation of these enzymes are the causes or requirements for breaking dormancy. The strong correlation between H2O2levels and the antioxidant enzymes in corn seed embryos has indicated that SOD, POD and CAT are important in ROS metabolism (Boseet al., 2005).
Changes in the activities of antioxidant enzymatic systems may play important roles in breaking bud dormancy (Apel and Hirt, 2004). Consequently, the metabolic activities and developmental stages of flower buds can be affected by activities of antioxidant enzymes and antioxidant substances concentrations (Beauvieuxet al., 2018). Ascorbic acid (AsA)-glutathione (GSH) cycle system plays an important role in scavenging ROS and an increase in GSH concentration has been shown to be an important trigger for releasing dormancy (Wanget al., 1991). Respiration metabolism and the associated in cellular ultrastructures in relation to the secondary bud burst have been studied in black currants (Qinet al., 2017b). However, the changes of ROS during the secondary bud burst of black currants have not been examined. Two black currants cultivars Adelinia and Heifeng with known contrasting susceptibilities to the secondary bud burst were used to carry out a systematic study on the relationships between ROS metabolism and the secondary bud burst. The aim of this study was to elucidate the biochemical and physiological mechanisms of the secondary bud burst in black currants and ROS might play important roles in bud dormancy and the secondary bud burst by inducing or inhibiting the secondary bud burst either GA3or abscisic acid (ABA).
Materials and Methods
Plant materials
Ten-year old bushes of the two black currants cultivars Adelinia and Heifeng were used for this study. Adelinia and Heifeng were selected since Adelinia was very prone to have the secondary bud burst and Heifeng was not. The test plants were grown in National and Local Joint Engineering Research Center for Development and Utilization of Small Berry in Cold Regions in Harbin City (45.7325°N, 107.6120°W),Heilongjiang Province, China. The bushes were spaced 1.2 m between plants and 1.5 m between rows. On August 14, 2018, after the fruits were harvested and before the leaves fall (the highest temperature in July could reach 32-35℃), 15 plants of each cultivar with relatively uniform vigor were sprayed with 30 mg · L-1GA3and 50 mg · L-1ABA, respectively, since GA3at this concentration was shown to induce the secondary bud burst and ABA at this concentration was shown to inhibit the secondary bud burst, when compared to water as the control, based on one of the previously published papers (Qinet al., 2017a). GA3and ABA applications were made once per day for three consecutive days, and water was used as the control. Test plants were all sprayed to runoff. The buds were collected randomly every 5 days after treatments, and the buds were immediately placed in liquid nitrogen and transported to the laboratory for storage at -80℃ and subsequent analyses.
Chemical and biochemical essays
POD activity in buds was measured according to a previously published method (Li, 2000) with slight modifications. About 0.2 g sample was weighed and put in a pre-chilled mortar and ground with a chilled pestle. The 4 mL of 50 mmol · L-1pre-chilled phosphate buffer (pH 7.8) was added to the homogenate on ice bath, and then the homogenate was transferred to a centrifuge tube and centrifuged at 4℃ for 20 min at 12 000 g. The supernatant was the crude enzyme solution. The supernatant was immediately used for spectrophotometric determination of POD activity at 470 nm. The measurement of SOD activity was carried out following another published method (Dhindsa and Matowe, 1981). APX activity was measured following the method developed by Ahmadet al(2011). The contents of AsA, GSH and GSSG and the activities of CAT and GR were determined using kits purchased from Beijing Solebo Technology Company (Beijing, China). The measurements were performed according to the kit instructions. In addition, the production rate of O2-· and the contents of H2O2were determined according to the kit instructions. The kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). All of the above mentioned measurements had three biological replicates.
Statistical analyses
Data processing and mapping were performed using Excel 2007 (Microsoft Corporation, Redmond, Washington, USA) and Origin 8.5 (Origin Lab Corporation, Northampton, Massachusetts, USA), and relevant statistical analyses were performed using SPSS 17.0 software (IBM Corporation, Armonk, New York, USA).
Results
Effect of GA3 and ABA on H2O2 concentrations in buds of Adelinia and Heifeng
GA3significantly increased H2O2concentrations in the buds of both Adelinia and Heifeng in comparison to the water control. H2O2concentrations in the buds reached their peak levels were close to or on September 9, which was the date of the complete secondary bud burst in Adelinia with GA3and the water control. On the other hand, some of buds in Heifeng started swelling under GA3treatment while the buds in the control group were very tight and did not show any visible signs of bud swelling or the secondary bud burst on September 9. On September 9, H2O2concentrations in the buds of Adelinia and Heifeng were 17.51 mmol · g-1and 13.38 mml · g-1, respectively, which were 3.67 and 2.98 times of their initial values on August 17 increased by 2.23 mmol · g-1and 2.98 mmol · g-1in comparison to those of the water control. The results showed that GA3treatment significantly increased the content of H2O2in comparison to the water control and was effective in causing the buds of Heifeng to start swelling, the initial stage of the secondary bud burst. It seemed that H2O2concentration in Heifeng needed to reach a threshold of 13.38 mmol · g-1triggered by GA3treatment before the buds started swelling or visible signs of the secondary bud burst started showing. All of the buds in Adelinia reached the secondary bud burst stage on September 9 under GA3treatment or the water control. GA3caused buds to swell 3 days after application in Adelinia and at a much lower threshold of approximately 7 mmol · g-1.
ABA significantly decreased the concentrations of H2O2in the buds of Adelinia and Heifeng (Fig. 1A and B). On September 9, H2O2contents in the buds of Adelinia and Heifeng were at 9.18 mmol · g-1and 4.46 mmol · g-1, respectively. With ABA treatment, the concentrations of H2O2in the buds of Adelinia and Heifeng were approximately half and one third of that of GA3treatment on September 9. The results showed that ABA treatment significantly inhibited the contents of H2O2in the buds of the black currants. ABA application was quite effective in reducing the secondary bud burst in Adelinia where only a small number of bud broke dormancy on September 9. The buds in Heifeng treated with ABA and the water were all quite tight and did not exhibit any visible signs of the secondary bud burst.
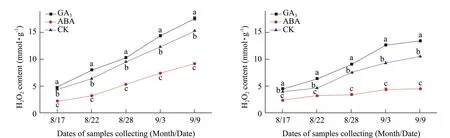
Fig. 1 Changes of H2O2 contents in buds of Adelinia (A) and Heifeng (B) of GA3 and ABA treatment
Effect of GA3 and ABA on superoxide (O2-·) production rate in buds of Adelinia and Heifeng
GA3significantly increased the O2-· production rate in the buds of Heifeng from August 17 to September 3, but not on September 9 in comparison to the water control (Fig. 2A and B). The effect of GA3on O2-· production rate in the buds of Adelinia was not as consistent and only increased the rate on September 3 and 9, when the secondary bud burst had commenced and completed. GA3caused O2-· production rate to reach the peak levels of 159.64 and 135.69 µmol · min-1· g-1in Adelinia and Heifeng, which were 1.88 times and 1.49 times of their initial values, respectively. Under GA3treatment, the results showed that GA3treatment significantly increased O2-· production rate in Heifeng in comparison to the water control, from August 17 to September 3, only from September 3 to 9 in Adelinia. It was possible that the endogenous GA levels might be naturally high in Adelinia, and not as much in Heifeng.
ABA treatment was effective in reducing O2-· production rate in the buds of Adelinia from August 22 to September 3, but not on August 17 (Fig. 2A and B). However, ABA increased O2-· production rate on August 17, had no effect from August 22 to 28 and decreased the rate from September 3 to 9. The results showed that ABA treatment significantly inhibitedproduction rate in comparison to GA3and the water control more consistently in Adelinia than that in Heifeng.
Effect of GA3 and ABA treatments on changes of SOD activities in buds of Adelinia and Heifeng
The activity of SOD in the buds of Adelinia gradually increased from August 17 to 28, peaked on September 3 and dropped on September 9 (Fig. 3A and B). The activity of SOD in the buds of Heifeng gradually increased from August 17 to 22, peaked on August 28 and dropped from September 3 to 9. GA3significantly increased the activity of SOD in the buds of Adelinia from August 17 to 22, and from September 3 to 9, but not on August 28, in comparison to the water control. GA3significantly increased the activity of SOD in the buds of Heifeng from August 17 to 22, and from September 3 to 9, but decreased activity of SOD on August 28 (Fig. 3A and B).
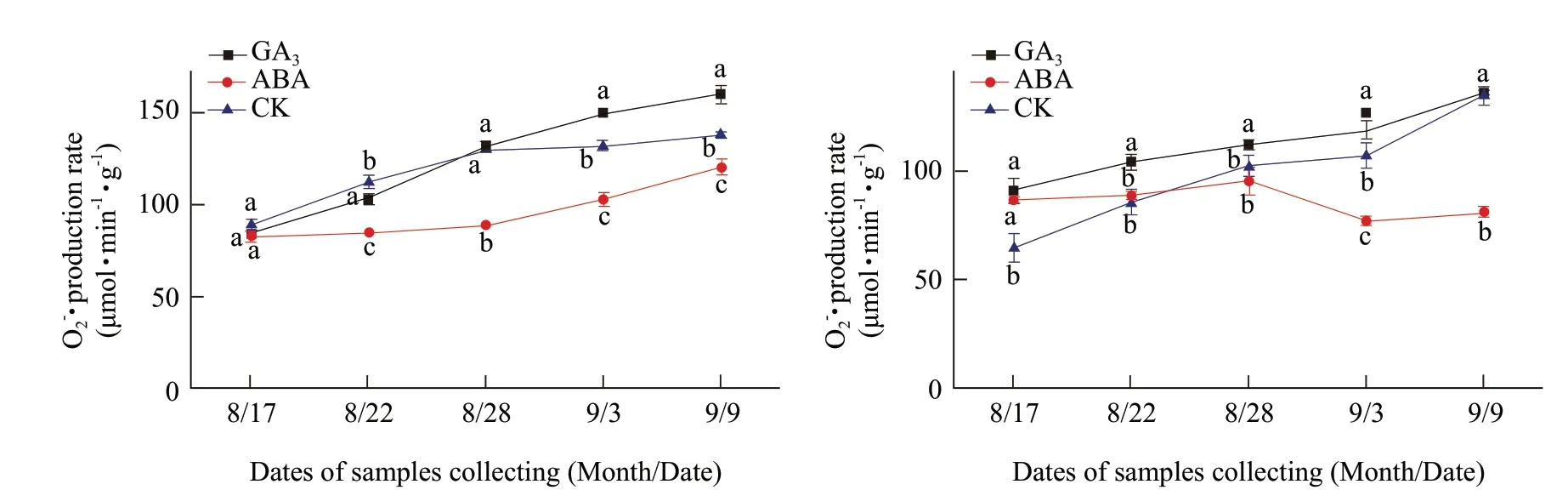
Fig. 2 Changes of O2-· production rate in buds of Adelinia (A) and Heifeng (B) of GA3 and ABA treatment
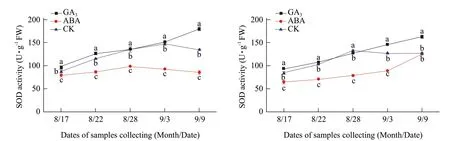
Fig. 3 Changes of SOD activities in buds of Adelinia (A) and Heifeng (B) of GA3 and ABA treatment
ABA decreased the activity of SOD in the buds of Adelinia in comparison to the water control from August 17 to September 9 and in the buds of Heifeng from August 17 to September 3, but not on September 9. The results showed that ABA treatment significantly inhibited the activity of SOD in comparison to GA3and the water control during all the experiments in Adelinia and only from August 17 to September 3 in Heifeng.
Effect of GA3 and ABA on CAT activities in buds of Adelinia and Heifeng
GA3did not significantly increase CAT activity in the buds of Adelinia from August 17 to 28, but did increase it from September 3 to 9 in comparison to the water control. However, GA3significantly increased the CAT activity in the buds of Heifeng from August 17 to September 9 in comparison to the water control. Under GA3treatment, the activities of CAT in the buds of Adelinia and Heifeng were 124.86 and 97.85 U · g-1FW on September 9, which were increased 67.70 and 29.56 U · g-1FW of their initial levels. The activities of CAT in the buds of Adelinia and Heifeng increased by 19.21 and 15.32 U · g-1FW of GA3treatment in comparison to the water control (Fig. 4A and B).
ABA decreased CAT activity in the buds of Heifeng from August 17 to September 9. On September 9, CAT activities in the buds of Adelinia and Heifeng were 72.12 and 53.01 U · g-1FW, respectively. The results showed that ABA could significantly inhibit CAT activities in the buds of Adelinia and Heifeng, when compared to the water control, but the effect of ABA was more consistent on Heifeng than that on Adelinia.
Effect of GA3 and ABA on POD activities in buds of Adelinia and Heifeng
POD activity in the buds of Adelinia showed an increasing trend from August 17 to September 9. In Heifeng, POD activity increased from August 17 to 22, peaked on August 28, then decreased on September 3, and increased on September 9. GA3significantly increased POD activity in the buds of Adelinia from August 22 to 28, and September 3. GA3significantly increased POD activity in the buds of Heifeng from August 17 to September 9.
ABA significantly decreased POD activity in the buds of Adelinia from August 28 to September 9, not from August 17 to 22 (Fig. 5A and B). ABA significantly decreased POD activity in the buds of Heifeng from August 17 to 28, from September 3 to 9, but not on August 22. The effect of ABA on POD activity was more consistent and more pronounced in Heifeng than that in Adelinia.
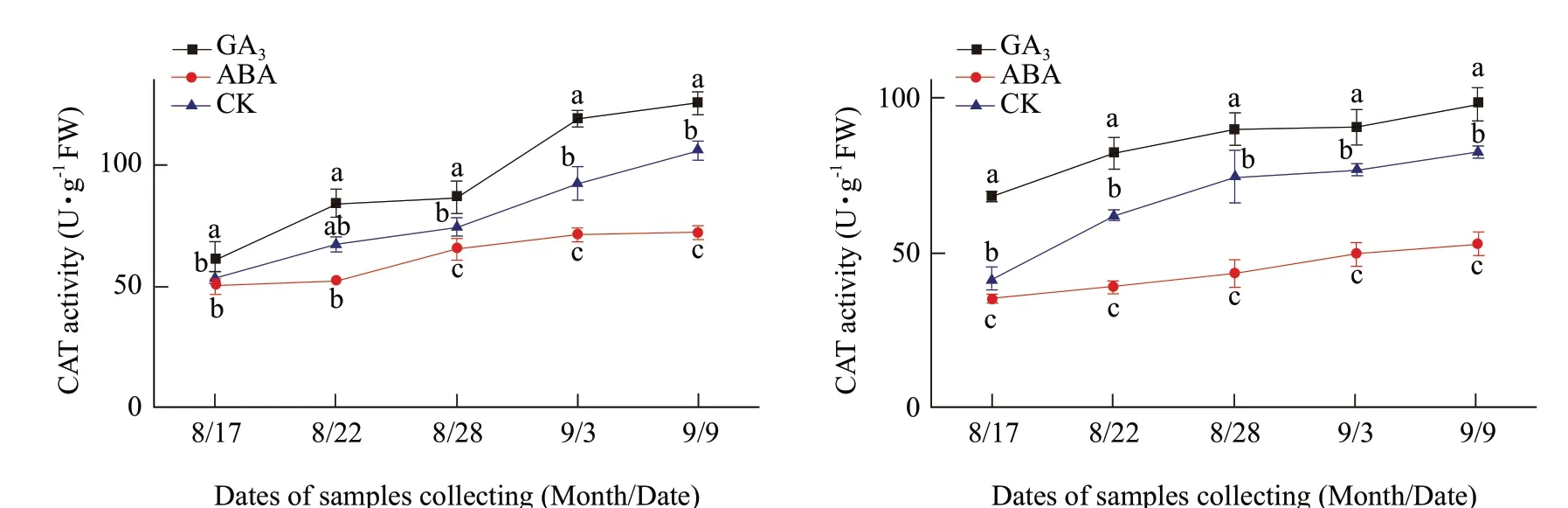
Fig. 4 Changes of CAT activities in buds of Adelinia (A) and Heifeng (B) of GA3 and ABA treatment
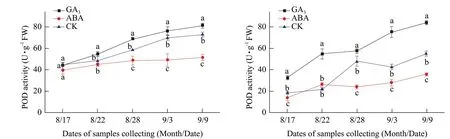
Fig. 5 Changes of POD activities in buds of Adelinia (A) and Heifeng (B) of GA3 and ABA treatment
Effect of GA3 and ABA on GR activities in buds of Adelinia and Heifeng
GR activity in the buds of Heifeng held relatively steady from August 17 to 28 and showed an increasing trend from September 3 to 9 (Fig. 6A and B). GA3increased GR activity in the buds of Adelinia in comparison to that of the water control only on August 17 and September 9 (Fig. 6A and B). GA3increased GR activity in the buds of Heifeng in comparison to that of the water control only on August 28 and September 3 (Fig. 6A and B). ABA significantly increased GR activity in the buds of Adelinia from August 22 to September 3, but not on August 17 and September 9 (Fig. 7A and B). ABA significantly increased GR activity in the buds of Heifeng from August 17 to 22, and from September 3 to 9, but not on August 28.
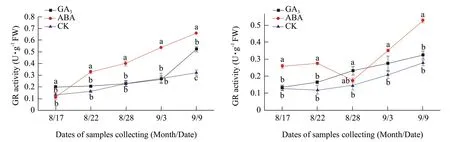
Fig. 6 Changes of GR activitiy in buds of Adelinia (A) and Heifeng (B) of GA3 and ABA treatment
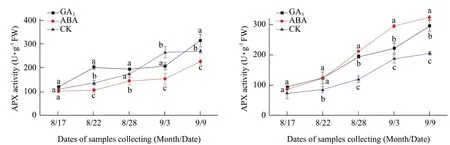
Fig. 7 Changes of APX activities in buds of Adelinia (A) and Heifeng (B) of GA3 and ABA treatment
Effect of GA3 and ABA on APX activities in buds of Adelinia and Heifeng
APX activity in the buds of Adelinia and Heifeng showed an increasing trend from August 17 to September 9 (Fig. 7A and B). The effect of GA3on APX activity in Adelinia was inconsistent. GA3significantly increased APX activity in the buds of Adelinia on August 22 and September 9, decreased APX activity on September 3, and did not significantly affect the APX activity from August 17 to 28. GA3significantly increased APX activity in the buds of Heifeng from August 17 to September 9. The only exception on August 17 when the effect of ABA on APX activities was not statistically significant.
ABA significantly decreased APX activity in the buds of Adelinia from August 22 to September 9, but not on August 17 (Fig. 7A and B). ABA had an opposite effect on Heifeng where it significantly increased APX activity in the buds of Heifeng from August 22 to September 9, but not on August 17.
Effect of GA3 and ABA on reduced GSH contents and GSSG in buds of Adelinia and Heifeng
GA3significantly increased GSH content in the buds of Adelinia on August 28 and September 9, but not from August 17 to 22 and September 3. GA3had significantly increased GSH content in the buds of Heifeng from August 17 to September 3, but not on September 9. ABA significantly decreased GSH content of Adelinia from August 17 to 28, but not from September 3 to 9 (Fig. 8A and B). ABA had significantly decreased the GSH content of Heifeng from August 22 to September 9, but not on August 17. GSSG content in the buds of Adelinia increased from August 17 to 22, decreased slightly on August 28, and then increased on September 3 and 9 (Fig. 8C and D). GSSG content in the buds of Heifeng increased from August 17 to 22, peaked on August 28, and then decreased on September 3 and 9. GA3did not significantly increased GSSG content in the buds of Adelinia from August 17 to 22, but significantly increased GSSG content from August 28 to September 9. GA3did not significantly increase GSSG content in the buds of Heifeng on August 17, but significantly increased GSSG content from August 22 to September 9. ABA significantly increased GSSG content in the buds of Adelinia from August 17 to September 9 (Fig. 8C and D). ABA increased GSSG content in the buds of Heifeng from August 22 to September 9, but not on August 17.
GSH/GSSG ratio in the buds of Adelinia showed a generally decreasing trend from August 17 to September 9, but GSH/GSSG ratio in the buds of Heifeng decreased from August 17 to 28, and then increased from September 3 to 9 (Fig. 8E and F). GSH/GSSG ratio in the buds of Adelinia and Heifeng was significantly decreased with GA3and ABA treatment, and GSH/GSSG ratio decreased faster in the buds of Heifeng than that of Adelinia. GSH/GSSG ratio in the buds of Adelinia and Heifeng treated with GA3was always higher than that treated with ABA.
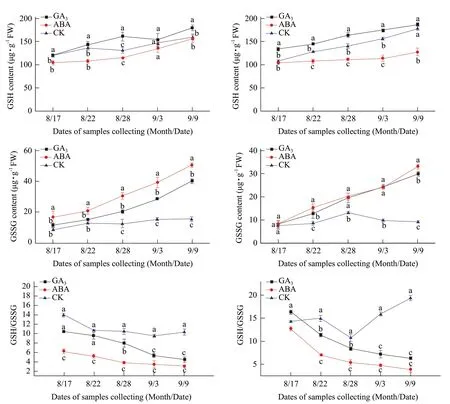
Fig. 8 Changes of GSH, GSSG contents and GSH/GSSG ratio in buds of Adelinia (A C E) and Heifeng (B D F) of GA3 and ABA treatment
Effect of GA3 and ABA on AsA contents in buds of Adelinia and Heifeng
AsA contents in the buds of both Adelinia and Heifeng showed an overall decreasing trend from August 17 to September 9 (Fig. 9A and B). GA3did not significantly affect AsA contents in the buds of Adelinia and Heifeng from August 17 to September 9 (Fig. 9A and B).
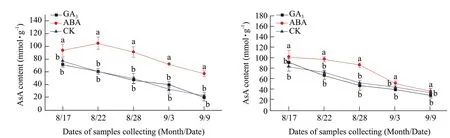
Fig. 9 Changes of AsA contents in buds of Adelinia (A) and Heifeng (B) of GA3 and ABA treatment
ABA significantly increased AsA contents in the buds of Adelinia and Heifeng from August 17 to September 9.
Discussion
Black currants are known to have high nutraceutical values. The secondary bud burst has become a major limiting factor of black currant production in leading commercial cultivars, such as Adelinia and Brodtorp, in China and possible other areas with similar weather conditions. Hence, it is very critical to determine the mechanisms of the secondary bud burst so that practical methods can be developed to reduce the rate of its occurrence to avoid unnecessary yield loss. In the current study. ROS metabolism was systematically examined during the secondary bud burst of the two contrasting black currant cultivars in order to elucidate the underlying physiological and biochemical mechanisms of the secondary bud burst.
In peach and grape, H2O2had been shown to be a 'signaling' chemical in regulating the metabolic pathways in flower buds during their dormancy period and could be involved in promoting the buds breaking dormancy (Gaoet al., 2002). The results from this study showed significant changes in the levels of ROS in Adelinia and Heifeng treated with GA3and ABA. GA3significantly increased the production of H2O2andwhile ABA significantly decreased the production rates of H2O2and O2-· during certain parts of the bud dormancy period. There were definite differences between Adelinia and Heifeng in their response to GA3, ABA and the subsequent secondary bud burst. In Adelinia, the endogenous levels of H2O2and O2-· were higher than those in Heifeng. GA3treatments led to the release of paradormancy in both Adelinia and Heifeng through the increased levels of H2O2and O2-·at different rates, while ABA treatment inhibited the release of dormancy in both Adelinia and Heifeng through the decreased levels of H2O2and O2-· at different rates. These differences might have been caused by the inherent differences in the endogenous levels of ABA and GA3in Adelinia and Heifeng.
In this study, GA3application led to increases in SOD activities and associated increase in O2-· activity though a disproportionation reaction that convertedto H2O2and H2O, reducing the potential harmful effect of O2-·. In Adelinia, ABA reduced SOD activity, increased SOD activity in Heifeng, but to a much extend less than GA3. Similar results have been observed in pears where SOD activity was shown to be quite low, during dormancy and quickly increased, when the bud dormancy was broken (Tesfayet al., 2016).
POD and CAT had been known to be involved in the neutralization of ROS and the levels of ROS were closely related to the activities of antioxidant enzymes (Sheikh-Mohamadiet al., 2018). In this study, GA3treatment led to increases in both the activities of POD and CAT and H2O2concentrations, and the subsequent secondary bud burst. On the other hand, ABA treatment reduced the activities of POD and CAT, and thus inhibited the secondary bud burst. It was safe to assume that POD and CAT were involved in the release of bud dormancy and their low activities were closely tied to the lower tendency of the secondary bud burst.
APX and GR were two key enzymes involved in the neutralization of ROS (Grataoet al., 2008). In this study, both AsA concentrations and APX activities showed differences between Adelinia and Heifeng. GA3increased APX activity in Adelinia while ABA decreased the activity of APX. In Heifeng, both ABA and GA3generally increased APX activity, high APX activity was responsible for the reduction of H2O2into H2O, reducing ROS levels and protecting the plants from damaging the cells by excessive ROS; causing the rapid reduction of AsA. The higher APX activity, the faster the removal of H2O2level in the body (Zhanget al., 2009), keeping the active oxygen level low and staying dormant.
In a study of apple bud dormancy, GSH level had been one of the key indicators for the end of dormancy (Faustet al., 1995). In this study, ABA reduced GSH concentration and inhibited the secondary bud burst, while GA3increased GSH concentration and thus promoted the secondary bud burst. GSH/GSSG ratio was beneficial for maintaining the reducing environment in the cell and improving the efficiency of the glutathione cycle (Zhanet al., 2017). GA3treatment significantly increased GSH contents in the buds of Adelinia and Heifeng, and ABA treatment also increased GSSH contents in the buds of Adelinia and Heifeng, but GSH/GSSG ratios were both decreased under GA3and ABA treatments. GSH/GSSG ratio was higher than that of ABA under GA3treatment in the buds of Adelinia and Heifeng, which was favorable for the secondary bud burst.
Conclusions
In summary, the changes in ROS levels showed strong ties to the release of paradormancy or the secondary bud burst in black currants. It was safe to conclude that ROS served as the signaling chemicals in the regulation of the secondary bud burst in black currants. GA3promoted the secondary bud burst by increasing ROS levels through increasing the enzymatic activities in ROS metabolism. On the other hand, ABA inhibited the secondary bud burst by lowering ROS levels through decreasing the enzymatic activities. The effect of an anti-GA plant growth regulator would be studied to reveal the possible interactions between GA and ABA. ABA application could be used by black currants growers to reduce the secondary bud burst. However, it was possible that an application of ABA plus an anti-GA type plant growth regulator might be even more effective in reducing the secondary bud burst in black currants.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Mining Heat Stress Associated Genes in Tomato Fruit (Solanum lycopersicum L.) Through RNA-seq
- Advances on Current Techniques and Methodologies in Milk Lipidomics
- Cloning of MYB2 Gene from Dryopteris fragrans and Its Response to ABA and Drought Stress
- NIRS Prediction of SOM, TN and TP in a Meadow in the Sanjiang Plain, China
- Screening of Feather Degrading Bacteria and Optimization of Fermentation Conditions from Poultry
- Isolation, Molecular and Phylogenetic Analysis of Porcine Encephalomyocarditis Virus Strain HLJ in China
