壳聚糖-蒙脱土复合涂膜在常温下对荔枝货架期及品质的影响
2021-02-16闫加桐王雨柔李莉蓉覃宇悦
闫加桐,张 珩,王雨柔,李莉蓉,覃宇悦
(昆明理工大学 农业与食品学院,云南 昆明 650550)
1 Introduction
Litchi,which is known as‘Dan Li’,is one of the subtropical evergreen fruits.China is a major producer of li⁃tchi,with one-third of the world′s total litchi production[1]. In the international market,litchi is a kind of favorite tropical fruit with business value. It is one of the leading export products of China. However,the quality of litchi fruit declines during picking,storage,and distribution,and the pericarp browning occurs within days[2].The short shelf life greatly limits the sales of litchi fruit.
Chitosan is obtained by deacetylation of chitin,which is widely present in nature[3]. Chitosan shows excellent properties such as biofunctionality,antibacterial ability,safety,and degradability. It has been applied in many fields such as medicine,food,chemical,and biomedical engineering[4]. Previous studies indicated that chitosan coating was an effective method to extend the shelf life of fruits like longan fruit,mango,and rambutan[5-6].The chi⁃tosan coating could delay weight loss and maintain the organoleptic quality of fruits[7]. However,the pericarp of li⁃tchi fruit is rough with cristated structure. After litchi was immersed in chitosan solution,the chitosan coating be⁃came brittle after solution drying[8].The shortcoming of pure chitosan coating restricted its functionality[9].
Lately,it has been confirmed that the addition of nanoparticles could improve the performance of chitosan coating[10].Montmorillonite(MMT)is a kind of clay and originates from special organic compounds.The average wa⁃fer thickness of MMT used in this study was less than 25 nm.It has good dispersion performance and is widely used as an additive for polymer. It is the most commercially available inorganic polymer thickener[11]. Moreover,MMT was less used and harmless to the food.MMT had been successfully used in food packaging materials[12-13].
The aim of this work was to study on the physicochemical properties of litchi coated with chitosan and chito⁃san-MMT stored at room temperature for 8 days.
2 Material and methods
2.1 Experimental materials and instruments
Using the‘June Red’litchi as the test material,the place of production is Yunnan. In June 2019,the 2 400 mature litchis were picked from the orchard in Yuxi,Yunnan,China,and carried to the laboratory within 3 h,the condition of conveyance was 1~5 ℃and humidity was 60%.Litchi fruits had consistent maturity,they were singled out by coincident size,shape,colour,and free from physical harm,fungal infection and injury caused by insects.Acetic acid was obtained from Shanghai Chemical Factory (Shanghai,China) and purity of 99.5%,chitosan(Deacetylation degree 95%,molecular mass 1.42 × 106) was obtained from Jinan Shangyou Bio-tech Co.,Ltd.(Shandong,China),MMT was obtained from Hengchang Mineral Products Chemical Co.,Ltd.(Hebei,China).All other required reagents were of analytical grade.
2.2 Preparation of chitosan-MMT coating
The composite coating was prepared according to the experimental protocol[14]. Two grams of chitosan powder was dissolved in 100 mL of 0.126 mol/L acetic acid aqueous solution,and continued stirring for 2 h[11]. 0.1 g of MMT was dissolved in 100 mL of 0.126 mol/L acetic acid aqueous solution.The solution mixed vigorously for 1 h[13].Then,adding MMT suspensions to chitosan solution,the chitosan-MMT solution was degassed under vacuum for 30 min,and the chitosan-MMT composite coating agent was prepared.
2.3 Chitosan-MMT coating treatment of litchi
At room temperature,the 2 400 of litchis were randomly divided into three groups before treatment[15]. The li⁃tchis were soaked in water for 5 min,removed and drained as control group A.The litchis were soaked in chitosan single-component coating agent for 5 min,removed and drained as control group B.The litchis were soaked in chi⁃tosan-MMT composite coating agent for 5 min,removed and drained as control group C. The treated litchi fruits were stored at ambient temperature(25 ℃)and humidity of 20%.During storage period(8 days),samples were eval⁃uated every 2 days[16].
2.4 Measurement of weight loss
During storage period (8 days),five litchis from each group were chose for peel treatment. Each litchi was weighed to the nearest 0.01 g[17],the experiment was repeated three times.The following formula(1)was used to cal⁃culate the weight loss:

whereW1is initial litchi weight andW0is measurement of litchi weight on the day.
2.5 Measurement of hardness
The hardness of three peeled litchis in each group was measured by texture analyzer (Lotun Science Co.,Ltd.Taibei,China)during storage period (8 days).The depth of the test was 5 mm,the maximum value was taken.The experiment was repeated for 3 times and the average value was taken[18].
2.6 Measurement of soluble solid content
The concentration of soluble solid was determined according to the following method[19]. During the experi⁃ment,five litchis were randomly selected from each group during storage period (8 days),and 20 g of pulp was tak⁃en and added with water.Then litchi pulp was centrifuged at 12 000 r/min for 10 min in the 5 418 low-temperature high-speed centrifuge (Eppendorf Co.,Ltd. Shanghai,China),and the supernatant was taken and measured by LYT-330 refractometer (Shanghai Linyu Trading Co.,Ltd. Shanghai,China). The result of soluble solid content was expressed as percentage(%).The experiment was repeated for 3 times.
2.7 Determination of vitamin C content and titrated acid
In the experiment,five litchi samples were taken from the three groups every 2 days,and the litchi pulp was pressed into the beaker with gauze to make juice. The 5 mL of litchi juice was immediately stirred,and then 5 mL of juice of litchis was pipetted into a 50 mL volumetric flask. 10 mL of 0.32 mol/L hydrochloric acid was added in the 50 mL of volumetric flask preliminarily,then 35 mL of 0.32 mol/L oxalic acid solution was diluted to volume.Then the 50 mL of volumetric flask was shaken and placed for 10 min to test[20].
The 15 mL of test solution was pipetted into a 150 mL of erlenmeyer flask,2 drops of H2O2and 5 drops of starch indicator were added,and immediately titrated with 2,6-dichloroindigo solution until the obvious pink color appeard within 15 seconds,Until color was disappeared,the reading was wrote down.A small amount of crystalline ascorbic acid(about 1.5 mg)was dissolved in 50 mL 0.24 mol/L sulfuric acid solution and mixed with 5 mL of 0.036 mol/L potassium iodide.The 5 mL of solution was absorbed for 6 times,added with 5 drops of starch indicator,and 3 portions were titrated with 2,6-dichloroindigo,the end point was pink.3 portions were titrated with 0.001 mol/L potassium iodide standard solution and the end point was blue. The corresponding titration number was recorded.The milligram of vitamin C in 2,6-dichloroindoxyl solution per milliliter was calculated,namely the titer T,and then the total content of vitamin C was calculated in the sample. The experiment was repeated for 3 times. The fol⁃lowing formula(2)and(3)was used to calculate T and Vitamins C,respectively.
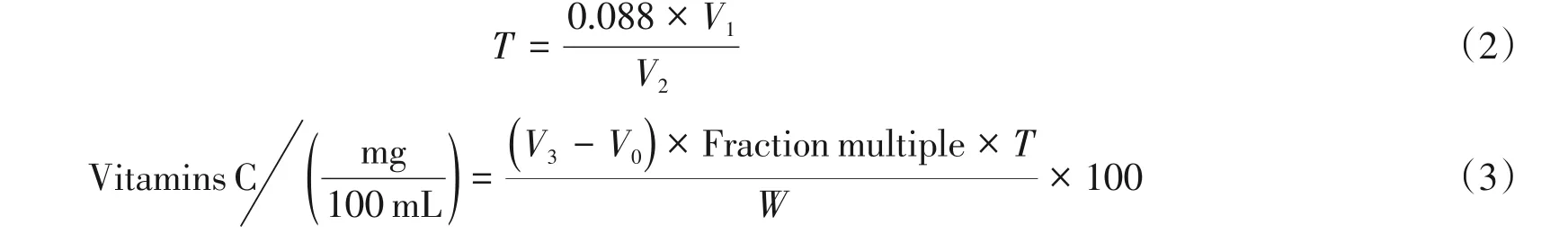
whereV1is titration of 5 mL of ascorbic acid consumes 0.001 N of potassium iodide standard solution;V2is titration of 5 mL of ascorbic acid consumes 2,6-dichloroindophenol;Tis 2,6-ichloroindophenol is equivalent to milligrams of vitamin C;V3is 2,6-dichloroindophenol solution consumed by titration samples;V0is 2,6-dichloroindophenol so⁃lution consumed by titration blank experiment; Fractional multiple is total volume of liquid to be tested/aspiration volume;Wis the number of millilitres of the original juice;0.088 is 1 mL the 0.001 mol/L potassium iodide standard solution is equivalent to the number of milligrams of vitamin C.
10 mL of above juice was added with 4 drops of phenolphthalein indicator and titrated with the sodium hydrox⁃ide solution. The end point was light red (no disappearance within 15 seconds). The experiment was repeated for 3 times.The following formula(4)was used to calculate Titratable acid.

whereNis the equivalent concentration of sodium hydroxide;Vis the number of milliliters of sodium hydroxide con⁃sumed during titration;Wis mL of sample;Kis coefficient converted to proper acid (since litchi belongs to stone fruit,so it is calculated as malic acid,Kis 0.067).
2.8 Pericarp browning
In the experiment,the 20 litchis without peeled were selected in each group of the three groups,and the browning index was calculated every 2 days. The browning of litchi epidermis was observed with naked eyes. The following scale was used: 1 indicated no browning (perfect quality),2 indicated mild browning,3 indicated less than 25%browning of the surface,4 indicated 25-50%browning,5 indicated more than 50%browning of the sur⁃face(poor quality).Litchi with a browning index above 3.0 were identified as unqualified[21].The browning index was computed as ∑(browning scale × corresponding fruit within each class)/ (total number of fruit). The experiment was repeated for 3 times.
2.9 Tests of PPO and POD
The three litchis in each group were selected,litchi tissue (10 g) was homogenized in 20 mL of 0.05 mol/L phosphate buffer solution (pH=7.0)at 15 000 g for 20 min.The crude enzyme extract was used as the supernatant for the determination of enzyme activity.The sample mixture contained 2 mL of 0.05 mol/L phosphate buffer (pH=6.8),0.9 mL of 0.01 mol/L catechol and 0.1 mL of crude enzyme extract.The change of absorbance value was reso⁃luted with TU-1901 ultraviolet-visible spectrophotometer (Beijing General Analysis General Instrument Co.,Ltd,Beijing,China)at 400 nm.The change in absorbance per gram of sample per minute 0.001 represented 1 unit of en⁃zyme activity(U),the enzyme activity expressed as U/(g·min)[22].The experiment was repeated for 3 times.
POD activity was measured on the basis of following method[23].The 3 mL of reactant included 0.1 mL of enzy⁃matic extract,2 mL of 0.05 mol/L phosphate buffer(pH=7.0),0.5 mL of 1%H2O2and 0.4 mL of 4%guaiacol.The absorbance of the solution at 470 nm was measured. The change in absorbance per gram of sample per minute of 0.001 represented 1 unit of enzyme activity was expressed in U/(g·min).The experiment was repeated for 3 times.
2.10 Statistical analysis
Analysis of variance (ANOVA) was executed on all surveyed data[24]. The experimental data showed in the fig⁃ure was the experimental data measured in parallel for each sample ± SD. Tukey’s various comparative measure⁃ments were achieved to decide the imparities of ways,andp<0.05 was identified as statistically significant.
3 Results and discussion
3.1 Effects of litchi weight loss
Weight loss is a natural phenomenon. Weight loss in fresh litchi is mainly due to water loss during transpira⁃tion and respiration processes. When the water in the litchi fruit naturally drains to a certain percentage,the peel changes,such as lose of original color,wrinkles and a certain degree of browning[25].The figure 1 showed the influ⁃ence of coating application on weight loss.
All litchis showed weight loss during storage.The weight loss of the group C on the 8th day was similar to that of the group A on the 5th day and the group B on the 6th day.The main purpose of coating is to reduce weight loss of litchis during storage. This is because the coating can reduce the contact between litchi and the external environ⁃ment,effectively slow down the rate of water loss. The epidermis of litchi has wrinkles. After litchi was soaked in the treatment solution of group B,the chitosan coating became brittle and fell off after solution drying,while the chitosan coating of group C did not. Fruit coated with chitosan-MMT could effectively reduce water loss and delay dehydration.
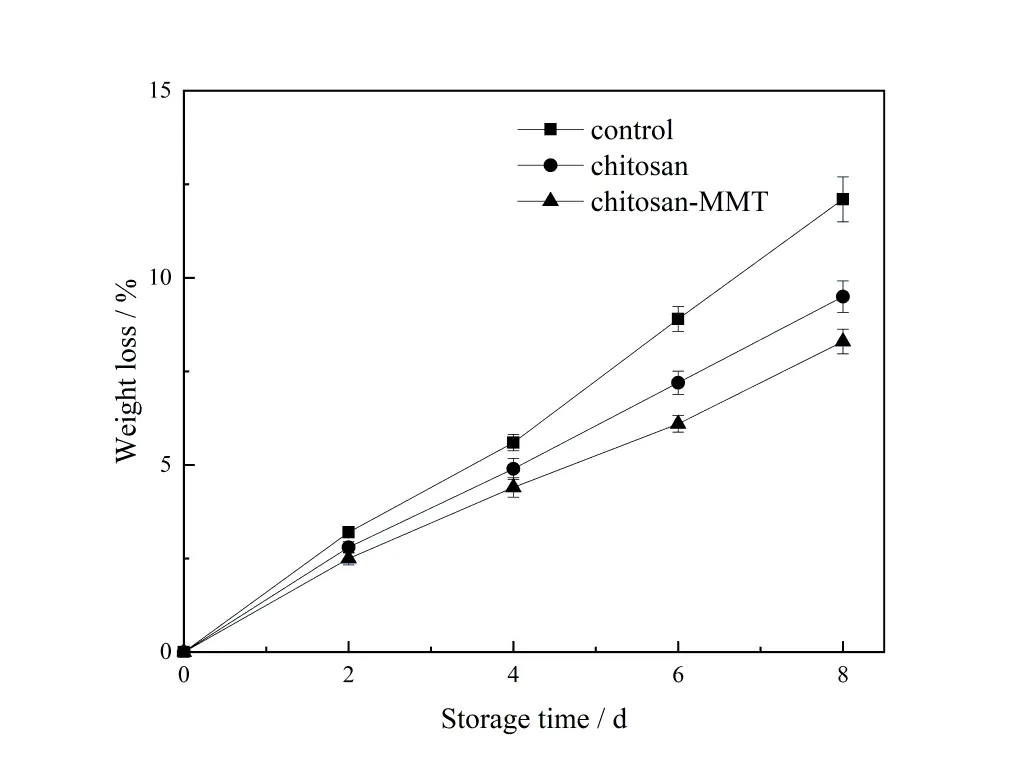
图1 荔枝贮藏期间失重率的变化Figure 1 The changes of weight loss rate of litchi during the storage time
3.2 Effects of hardness
Hardness is one of the important indicators to measure fruit quality[26]. During the storage of litchi,pectin was decomposed under the action of pectinase. The arrangement of pectin in cells changed from tight to loose,and the fruit changed from hard to soft.The hardness gradually decreased with the extension of storage time[27].From the 4th day to 8th day,it can be seen from figure 2 that the hardness of the coating group was significantly higher than that of the control group A and group B (p< 0.05). Therefore,coating treatment maintained high hardness of fruit. This is because a relatively dense and uniform film is formed on the surface of litchi fruit after coating treatment,which reduces the permeability of water and oxygen,inhibits transpiration and respiration of litchi fruit,weakens its me⁃tabolism,reduces the consumption of organic matter,and delays the transformation process. MMT had gas barrier property,resulting in the hardness of litchi treated by group B was softer than that of group C.The change of hard⁃ness was same as what was reported[28].
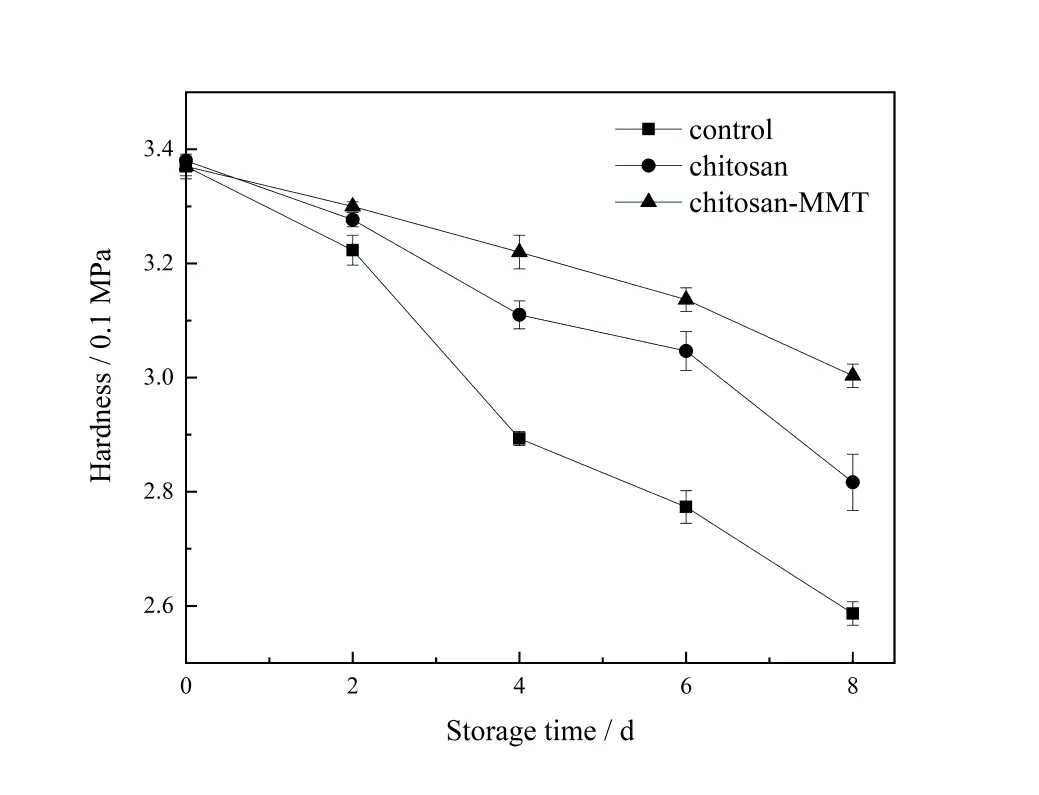
图2 荔枝贮藏期间硬度的变化Figure 2 The changes of hardness of litchi during the storage time
3.3 Effects of soluble solids
Soluble solids,vitamin C and titratable acidity are important factors in flavour and nutritive quality of litchi fruit[18]. Soluble solids content is mainly sugar content,which reflects the quality of fruit. Changes of sugar content during postharvest storage were affected by respiration,amylase hydrolysis and tissue dehydration. In figure 3,the soluble solids of the fruits in the coating group and the blank group increased gradually with the extension of storage time,mainly due to the continuous transformation of starch into soluble sugars such as glucose and fructose under the action of amylase[29].Soluble solids decreased during storage to the 8th day,possibly due to respiration and con⁃sumption of soluble sugar as a respiratory substrate.The results showed that chitosan-MMT coating treatment could slow down the conversion rate of starch to soluble sugar in litchi fruit. The main reason was that the composite film formed on the surface of litchi fruit by coating treatment could inhibit its respiration,thus weakening the metabolic process of fruit,reducing the activity of amylase,slowing the rate of starch conversion to soluble sugar,and reduc⁃ing the consumption of respiratory substrates,which played a significant preservation effect[30]. MMT could reduce amylase activity so the coating with MMT was more effective than that without MMT.This was no difference with the results showed[31]on apples with chitosan coating.
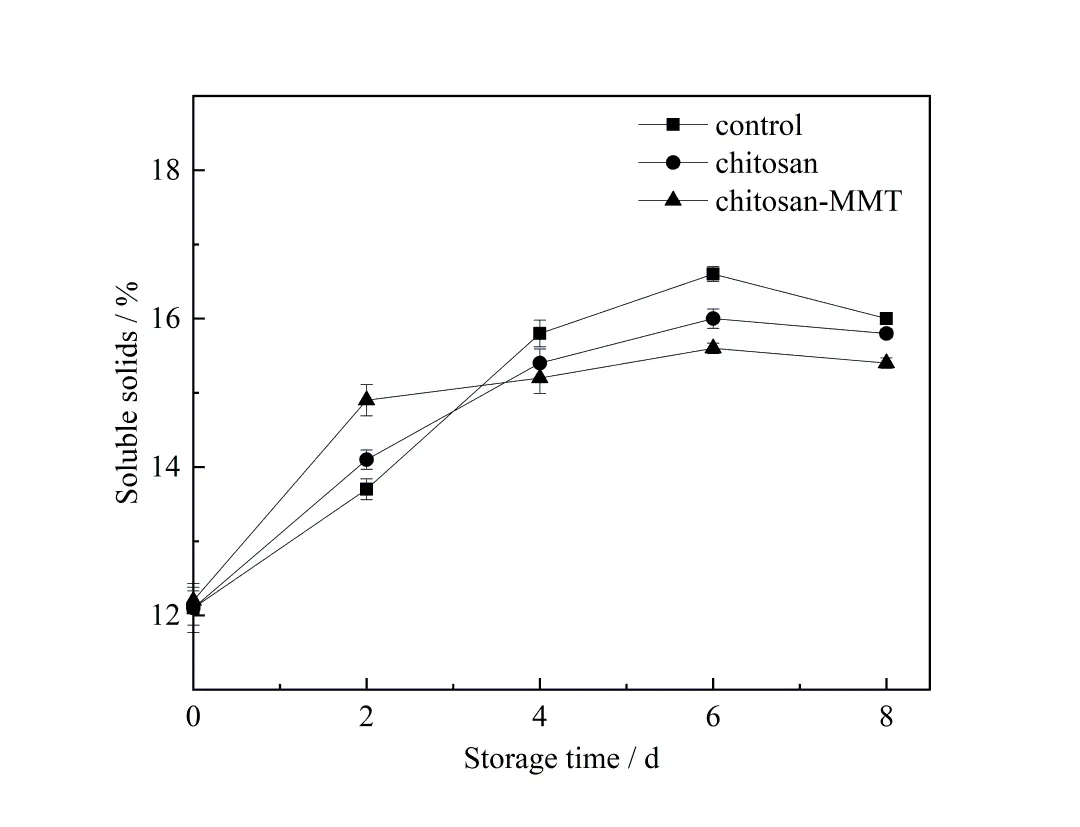
图3 荔枝贮藏期间可溶性固形物含量的变化Figure 3 The changes of soluble solid content of litchi during the storage time
3.4 Effects of vitamin C content and titratable acidity
Vitamin C is an essential nutrient in fruits and vegetables,and it is also a significance indicator to measure the effect of preservation.Vitamin C is a reducing substance that protects fruits and vegetables[32].The vitamin C content reduced with prolonged storage time at room temperature in figure 4.The ascorbic acid content of litchi coated with chitosan-MMT was higher than that of group A and group B.The decrease in vitamin C content during storage may be due to the enzymatic oxidation of L-ascorbic acid to dehydroascorbic acid. MMT had good gas barrier proper⁃ties,which made the chitosan-MMT coating had good gas barrier properties and provided lower O2atmospheres,which could inhibit enzymatic oxidation,the decline of vitamin C had slowed down[33].

图4 荔枝贮藏期间维生素C的变化Figure 4 The change of vitamin C of litchi during the storage time
The titratable acid is the source of litchi sourness and an important factor affecting fruit flavor quality[34]. The change of titratable acid content is shown in figure 5.It can be seen from figure 5 that the titratable acid content of litchi in different treatments showed a down trend during storage time.The blank control group A declined the fast⁃est,while the chitosan-treated control group B and the chitosan-MMT coating treated experimental group C de⁃clined relatively smoothly.On the 8th day of storage,the group A and group B decreased by 0.17% and 0.14%re⁃spectively,while the chitosan-MMT coating treated experimental group C decreased by only 0.12%.Litchi used ac⁃id as a reaction substrate for respiration during storage,so the acid content continued to decrease as the respiration consumes.The litchis with chitosan-MMT coating were better than that with chitosan coating,this was because af⁃ter adding MMT,the rate of alkalinity increase could be effectively suppressed[16].Therefore,the preservation effect of the chitosan-MMT coating was the most obvious. The composite coating can slow down the respiratory metabo⁃lism of fruits,thereby effectively delaying the fall of the titratable acid content during fruit storage.The same experi⁃mental result was reflected in the paper on the effect of polyvinyl alcohol/ tea polyphenol composite films on the shelf life of packaged strawberries[35].
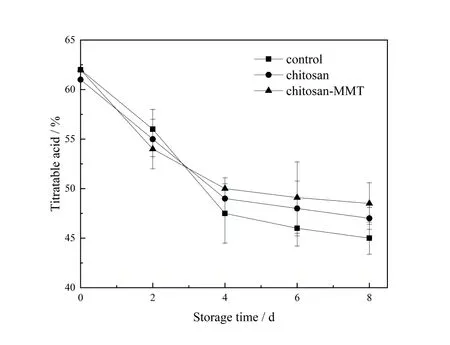
图5 荔枝贮藏期间可滴定酸含量的变化Figure 5 The changes of titratable acid content of litchi during the storage time
3.5 Effects of pericarp browning
The peel browning after litchi harvesting directly affects the appearance quality[36]. As shown in figure 6,with the increase of storage time,the browning index gradually increased.The increase in the coating group was signifi⁃cantly less than that in the control group and the chitosan-MMT coating treatment could significantly inhibit the browning of fruits during storage.The three important conditions for enzymatic browning of fruits are enzymes,sub⁃strates and oxygen. Theoretically,reducing the absorption of oxygen can reduce the enzyme activity,thereby pre⁃venting enzymatic browning[37]. The chitosan-MMT coating treatment can reduce the absorption of O2and the re⁃lease of CO2,so as to slow down the browning of fruit.The fact of lower browning index of fruit coated with chitosan-MMT was agreed with higher keeping of vitamin C content[38].

图6 荔枝贮藏期间褐变指数的变化Figure 6 The change of browning index of litchi during the storage time
3.6 Effects of PPO and POD activities
During the storage of litchi,as the permeability of the cell membrane increases,a variety of organic substanc⁃es in the cells were released and became substrates for enzymatic browning.Among them,PPO and POD activities play an important role in the browning process.The PPO and POD activities can jointly oxidize phenol to quinone,quinone to condensed tanning,and finally form brown polymer[39].
Figure 7 and figure 8 respectively represented the changes of PPO activity and POD activity of litchi during storage.The figure 7 showed that during storage,the PPO activity of litchi peels in each group generally showed an upward trend,but the increase in the early stage was slower,and the increase rate in the middle and late stages was accelerated. The PPO activity of litchi peels in each treatment group was not significantly different on the 8th day. After 4 days,the PPO activity of the group A increased significantly (p< 0.05),and was significantly higher than that of the other two groups (p>0.05).PPO is a protein with the characteristics of general proteins[31].A series of chemical changes caused by enzymes in a short period of time during litchi storage increase the activity of PPO.PPO activity directly reflects the browning of fruit[40]. The PPO activity of litchi fruit in the blank group was signifi⁃cantly higher than that in the coating group,which was also the reason why the browning degree of litchi fruit in the blank group was significantly higher than that in the coating group. The POD playd a key role in lignin synthesis and catalyzed H2O2decomposition to promote lignin monomer polymerization[41].It can be seen from figure 8 that the litter POD activity of litchi in each treatment group showed an upward trend and then a downward trend during nor⁃mal temperature storage,reaching a peak on the fourth day.The POD activity of litchi peel in the group A increased rapidly in the early storage period,and was higher than that in other groups. It can be considered that after MMT was added to the coating,the fruit of group C was caused by adversity stress and resists the damage of free radicals.It can be seen that chitosan-MMT coating treatment can defer the increase of the POD activity of litchi fruits to re⁃sist the damage of pathogens to the host.The reason for the above results in group B was that the chitosan coating ef⁃fectively reduced the amount of oxygen entering litchi fruit and reduced the respiration of litchis,thus delaying the metabolic process of litchi and increasing the storage period of litchi[42]. The addition of MMT in chitosan coating had a good on PPO activity and POD activity.
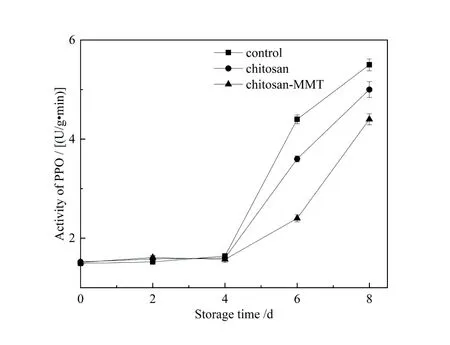
图7 荔枝贮藏期间PPO的变化Figure 7 The change of PPO of litchiduring the storage time

图8 荔枝贮藏期间POD的变化Figure 8 The change of POD of litchiduring the storage time
4 Conclusion
The coating plays an increasingly important role in the field of fruit and vegetable storage at home and abroad.In this experiment,chitosan was used as the main coating agent,and MMT was added as a natural preparation com⁃posite coating.The consequences showed that the weight loss rate,soluble solids,browning index,and PPO activi⁃ty of the litchi fruit in the chitosan-MMT coating increased slowly.The hardness,vitamin C and titratable acid con⁃tent of the litchi in the chitosan-MMT coating reduced slowly.The POD activity changed obviously.The two compo⁃nents are natural and non-toxic. The functions are complementary between the sub-points,which can play an ex⁃cellent role in freshness preservation,and have a significant promotion effect on the preservation of litchi during storage.
