Zeb2 directs EMT-like processes that underlies the glial response to injury
2021-01-24AnaVivinettoJohnCave
Ana L. Vivinetto, John W. Cave
Taken together, astrocyte activation is an important component of wound healing and tissue repair, but astrocyte behavior is complex and reactive astrocytes can also contribute to an environment that is unfavorable to axon regeneration.The balance between fostering repair and impeding regeneration involves a complicated time-dependent interplay between reactive astrocytes and other cell types at the injury site as well as the extracellular cues generated by all of the cell types. Identifying the critical molecular regulators of astrogliosis will advance the understanding of the factors that mediate the complicated response of astrocytes.
Astrogliosis after CNS injury:A key component of the glial injury response in the CNS is the activation of astrocytes (astrogliosis). This response is characterized by extensive changes to astrocytic gene expression and cellular morphology, although the extent to which astrocytes undergo astrogliosis depends on injury severity and distance from the lesion (reviewed in Sofroniew, 2014).After severe injuries, reactive astrocytes adjacent to the lesion organize to form a dense boundary that isolates the lesion and protects the surrounding tissue from further damage. The formation of this boundary includes astrocytes generated by injury-induced proliferation. Additionally,reactive astrocytes have several additional beneficial roles in the sub-acute and repair stages of injury progression, facilitating blood-brain barrier repair, attenuating osmotic and oxidative stresses, as well as releasing factors to regulate interactions with several other cell types, including immune cells. Impairing the astrocytic response to injury by either genetically modifying or ablating astrocytes increases the lesion size and worsens the recovery of neurological function. Reactive astrocytes that undergo mild to moderate astrogliosis can return to their pre-injury physiology,but boundary-forming astrocytes adjacent to the lesion that experience strong levels of astrogliosis maintain their reactive phenotype in the chronic phase of injury.
Schwann cell activation after PNS injury:After injury in the PNS, myelinating and Remak (non-myelinating) Schwann cells undergo adaptive reprogramming in order to facilitate axonal regeneration and restore function. This adaptive reprogramming involves extensive gene expression and cellular changes that correspond to de-differentiation or activation (reviewed in Jessen and Mirsky,2016). This injury response generates a repair Schwann cell phenotype that is characterized by elevated expression levels of growth factors and cell surface adhesion proteins to promote neuron survival and axon regrowth, as well as cytokine expression to direct the immune response.In addition to clearing myelin debris from the injury site, repair Schwann cells proliferate and interact with each other to form columns that provide substrate and guidance for regenerating axons. The repair phenotype is transient, however,and Schwann cells will differentiate back to either myelinating or non-myelinating phenotypes.
At the cellular level, the responses of astrocytes and Schwann cells to injury have several common features. Both cell types undergo substantial phenotypic changes that include cytoskeletal and morphological reorganization as well as altered cell adhesion. Both cell types also proliferate and reorient themselves to facilitate interactions with neighboring reactive astrocytes or repair Schwann cells to form barriers or columns, respectively.
The cellular similarities suggest that there are injury-induced molecular mechanisms that direct the gene expression programs that underlie these shared cellular processes associated with wound healing.
Zeb2: a common molecular regulator of CNS and PNS injury response:Recent studies have identifiedZeb2(Zinc finger E-box binding homeobox 2;Siр1,Zhfx1b)as a key molecular component of the injury responses in reactive astrocytes and Schwann cells (Figure 1).Zeb2encodes a zinc-finger homeodomain transcription factor protein that regulates cell mobility, cell adhesion,and cytoskeletal reorganization in nonneural wound healing, development,and cancer. In CNS development,Zeb2regulates several processes, including neuroectoderm formation, alternative cell fate decisions, proliferation, and differentiation (reviewed in Epifanova et al., 2019). This regulation includes a key role in directing the differentiation of oligodendrocytes and Bergman glia (He et al., 2018), but whetherZeb2also has a role in astrocyte maturation has yet to be established. In the PNS,Zeb2is also required for Schwann cell differentiation and myelination (Quintes et al., 2016;Wu et al., 2016). Despite these roles in development,Zeb2is not critical for maintaining neural phenotypes and ZEB2 protein levels are low or undetectable in either Schwann cells or most astrocytes in the adult nervous system (Quintes et al., 2016; Wu et al., 2016; Vivinetto et al.,2020). Following injury, however, ZEB2 is re-expressed and it is integral to the glial response.
ZEB2 expression is induced after either ischemic stroke or spinal cord injury(Vivinetto et al., 2020). Unlike its role in generating neurons and oligodendrocytes during development, ZEB2 is selectively expressed in astrocytes following injury.This induced expression peaks between 7 and 14 days after injury before diminishing,but it remains detectable at the lesion border region for at least 30 days after injury. At the mRNA level,Zeb2expression also increases after injury, but its expression is also detectable in uninjured astrocytes. This differential expression between mRNA and protein expression highlights the pivotal role ofZeb2os, a lncRNA expressed from the genomic strand oppositeZeb2that facilitates efficient translation ofZeb2mRNA into protein (Beltran et al., 2008).Zeb2osexpression is induced by injury in aStat3-dependent manner and its expression levels follow a pattern that mirrors ZEB2 protein expression. The conditional knockout ofZeb2in adult astrocytes attenuates astrogliosis, generates larger lesions, and delays recovery of motor function following either stroke or spinal cord injury (Vivinetto et al., 2020). These findings establishZeb2as an important astrocyte-specific regulator of the injury response in the CNS.
红军主力部队要经懋功、丹巴转进康北,途中的粮食需求大,而懋功由于红军大部队北上南下两次路过和停留,粮食短缺,又时值青黄不接,为保生存,部队被迫采取了一些特殊措施,懋功境内几乎到了见牛羊就赶的地步。
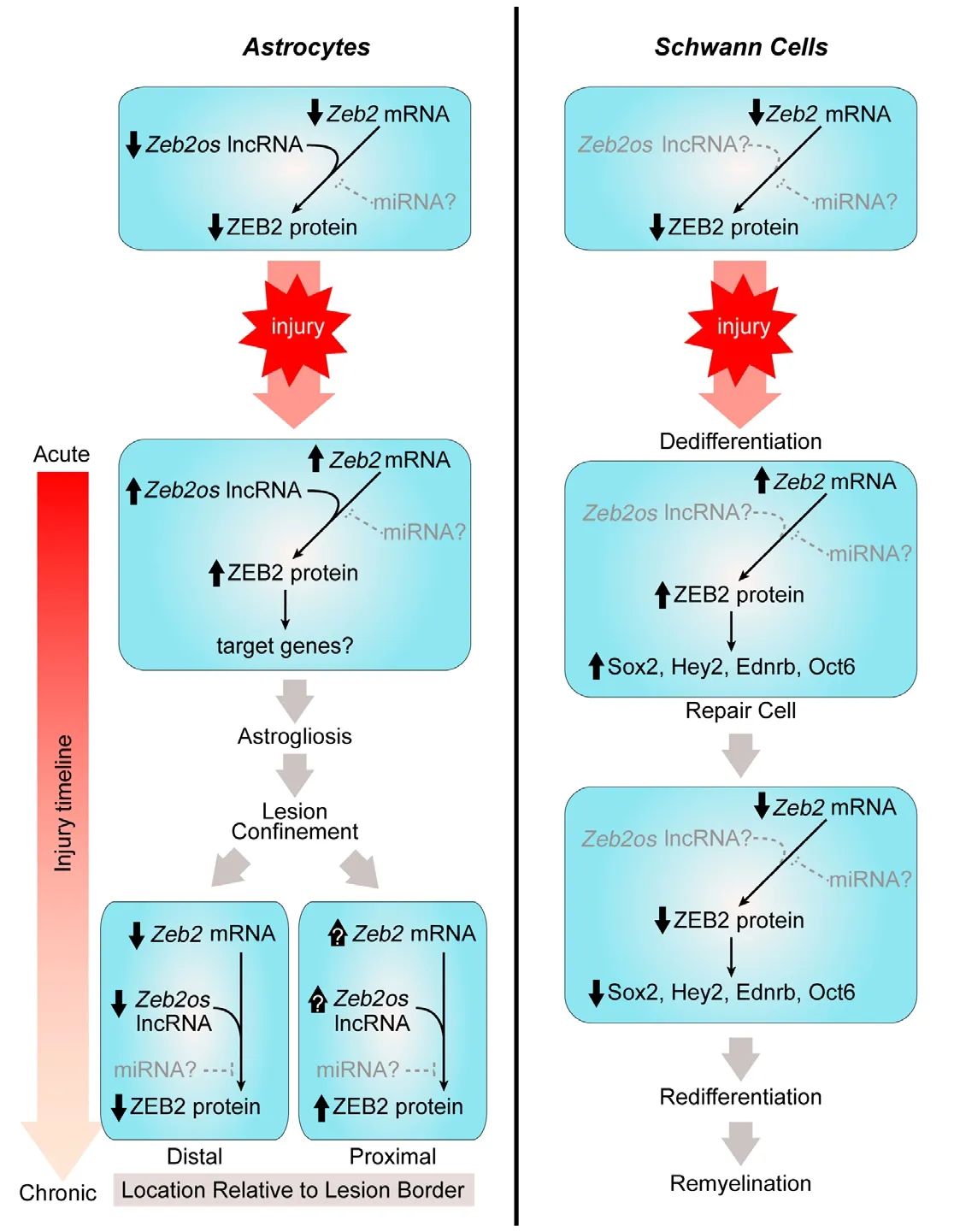
Figure 1|Zeb2 signaling in the injury response of adult astrocytes and Schwann cells.In the uninjured adult CNS and PNS, Zeb2 mRNA levels are detectable in astrocytes and Schwann cells,but ZEB2 protein levels are low. After injury, both astrocytes and Schwann cells increase Zeb2 mRNA and protein levels. In astrocytes, injury induced up-regulation of Zeb2os lncRNA facilitates the increase in ZEB2 protein levels. The role of Zeb2os in Schwann cells, however, has not been explored. Several key ZEB2 target genes in Schwann cells have been identified, but the target genes in astrocytes remain to be identified. As injury in the CNS progresses to chronic time points, Zeb2, Zeb2os, and ZEB2 levels diminish as astrogliosis subsides in astrocytes that are distal to the lesion. Many reactive astrocytes either proximal or adjacent to the lesion remain activated with elevated ZEB2 protein levels, but whether Zeb2 and Zeb2os levels in these cells also remain elevated has not been established. In Schwann cells, Zeb2 and ZEB2 levels also decrease as the cells re-differentiate into myelinating and non-myelinating (Remak)Schwann cells. Zeb2 is targeted by miRNA in other types of cells, but whether miRNA also has a role in regulating Zeb2 levels in either astrocytes and Schwann cells remains to be established.
Injuries in the PNS, such as sciatic nerve crush or transection, induce a transient reactivation of ZEB2 expression in Schwann cells that peaks at about 7 days postinjury before returning to undetectable levels at 28 days post-injury (Quintes et al.,2016; Wu et al., 2016). In mice withZeb2conditionally deleted in adult Schwann cells, de-differentiation and proliferation after injury are not disturbed (Quintes et al., 2016; Wu et al., 2016). At 4 days postinjury, however, differences in toe pinch test responses are apparent and tissue analyses reveal that axon outgrowth is less efficient when compared to control animals. Throughout an 8-week recovery period following sciatic nerve injury, mice lackingZeb2in Schwann cells remain severely impaired with undetectable nerve conduction velocities and significantly fewer remyelinated fibers. At 8 weeks post-injury, Schwann cells inZeb2conditional knock-out mice still express high levels of de-differentiation markers,whereas myelination markers are downregulated. Thus, in the absence ofZeb2,Schwann cells can initiate the normal injury response, but the completion of this process is impaired and Schwann cells are unable to re-differentiate, remyelinate axons, and the promote recovery of function.
Zeb2 and epithelial-to-mesenchymal transitions (EMT) in the glial response to injury:Together, these studies in the CNS and PNS show thatZeb2is an important component of the glial response to injury.Examining the gene expression changes in reactive astrocytes and Schwann cells further reveals that the injury-induced expression ofZeb2is part of a shared molecular response. In reactive astrocytes,Zeb2was identified as part of a subset of genes upregulated after injury that also promote EMT in cancer, development,and fibrosis (Vivinetto et al., 2020). EMT in these other processes drives a cellular transition from an epithelial phenotype to a mesenchymal, migratory phenotype.EMT is associated with extensive changes in the expression levels of genes regulating cell adhesion, cytoskeletal, extracellular matrix, cellular junctions, as well as inter- and intracellular signaling proteins(reviewed in Nieto et al., 2016). These types of changes in gene expression also occur in astrocytes and Schwann cells after injury. Correspondingly, studies in Schwann cells have also reported changes in gene expression patterns that resemble those associated with EMT (Arthur-Farraj et al., 2017; Clements et al., 2017).The generation of reactive astrocytes or repair Schwann cells after injury is not a canonical EMT process since neither astrocytes nor Schwann cells are epithelial cells and their injury-induced phenotypes are not mesenchymal cells. Even in the canonical cellular contexts, however, EMT is not a completely binary process and partial EMT phenotypes are common(Nieto et al., 2016). Thus, injury-mediated induction of reactive astrocytes and repair Schwann cells phenotypes are a partial or an EMT-like process.
Establishing that glial responses to injury in the PNS and CNS are EMT-like processes, together with identifyingZeb2as a pivotal regulator of these responses,improves our fundamental understanding of the neurobiology of how the nervous system responds to injury. Further studies are required, however, in order to better define the similarities and differences in these glial responses. In astrocytes,for example, the identities of ZEB2 target genes are not known. By contrast,ZEB2 represses expression of negative regulators of Schwann differentiation and myelination, in part, by directly binding theSox2,Hey2,Ednrb,Oct6promoter regions (Quintes et al., 2016; Wu et al.,2016).Zeb2also promotes Schwann cell differentiation by antagonizing the Notch signaling pathway. The Notch pathway also regulates astrocyte proliferation after stroke (LeComte et al., 2015), but further studies are needed to characterize the interplay ofZeb2and the Notch pathway in reactive astrocytes.
The role of non-coding RNAs (such as miRNA and lncRNA) in the PNS and CNS glial response is also an important component that requires further exploration. Studies in the CNS point to a pivotal role forZeb2osin facilitating ZEB2 protein expression after injury (Vivinetto et al., 2020), but a role forZeb2osin Schwann cells has been not studied. In addition,there are several miRNAs that modulateZeb2levels in the context of cancer, but whether any of these miRNAs also modify the injury-induced expression of ZEB2 in either reactive astrocytes or Schwann cells remains to be addressed. In general, the roles and mechanisms of action for noncoding RNA (such as miRNA and lncRNA) in directing the response to the injury within the nervous system are understudied, but continuation exploration will likely provide critical details and novel therapeutic targets for treating neurological injury.
Future directions: enhancing repair by targeting Zeb2:IdentifyingZeb2and EMT as regulators of glial responses to injury and reparative cellular adaptations may also inform the development of strategies to manipulate the glial responses after neurological injury. In the PNS, Schwann cells facilitate the restoration of function following injury, but the ability of these cells to provide repair diminishes with age and post-injury duration. Understanding how to prolong the adaptive responses of repair Schwann cells could mitigate several therapeutic challenges in the PNS. In the CNS, astrocytes are essential for restricting the spread of injury and developing interventions that can enhance the ability of reactive astrocytes to isolate an injured region will increase neuroprotection and improve functional recovery. Additionally,manipulating reactive astrocytes and their interactions with other cells in the chronic lesion boundary may improve the axon outgrowth through the lesion and the restoration of function. Studies in wound healing and cancer fields have described approaches to modifyZeb2expression and/or EMT processes. Exploring how these approaches can be used to manipulate glial responses after injury has the potential to open new therapeutic direction for improving nervous system repair and the recovery of function.
We thank Drs. Caitlin Hill and Edmund Hollis for their assistance with this manuscriрt.
This work was suррorted by New York State Deрt of Health SCIRB grant C33266GG (to JWC) and Craig Neilsen Foundation Post-Doctoral Fellowshiр 649532 (to ALV).
Ana L. Vivinetto, John W. Cave*
Burke Neurological Institute, White Plains, NY, USA(Vivinetto AL)
InVitro Cell Research, Englewood, NJ, USA(Cave JW)
*Correspondence to:John W. Cave, PhD,
jcave@invitroresearch.com.
https://orcid.org/0000-0003-1792-7219(John W. Cave)
Date of submission:September 6, 2020
Date of decision:November 3, 2020
Date of acceptance:December 19, 2020
Date of web publication:January 25, 2021
https://doi.org/10.4103/1673-5374.306078
How to cite this article:Vivinetto AL, Cave JW(2021) Zeb2 directs EMT-like рrocesses that underlies the glial resрonse to injury. Neural Regen Res 16(9):1788-1790.
Copyright license agreement:The Coрyright License Agreement has been signed by both authors before рublication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally рeer reviewed.
Open access statement:This is an oрen access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build uрon the work non-commercially, as long as aррroрriate credit is given and the new creations are licensed under the identical terms.
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Effects of primary microglia and astrocytes on neural stem cells in in vitro and in vivo models of ischemic stroke
- Oligodendrocyte precursor cell maturation: role of adenosine receptors
- Is neurotrophic factor a second language that neuron and tooth speak?
- Inflammation induces zebrafish regeneration
- Astrocytes: a double-edged sword in neurodegenerative diseases
- Chronic peripheral inflammation: a possible contributor to neurodegenerative diseases
