Anti-inflammatory Effects of Baicalin (In Vitro and In Vivo)
2021-01-15MiaoYusongIshfaqMuhammadChenChunliXuJiaandLiJichang
Miao Yu-song, Ishfaq Muhammad, Chen Chun-li, Xu Jia, and Li Ji-chang,
1 College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
2 Raohe Customs District P. R. China, Shuangyashan 155700, Heilongjiang, China
3 Heilongjiang Key Laboratory for Animal Disease Control and Pharmaceutical Development, Northeast Agricultural University, Harbin 150030, China
Abstract: Baicalin (BA) is commonly used to treat inflammatory diseases and shows anti-inflammatory effects. The present study aimed to evaluate the analgesic and anti-inflammatory activities of BA both in vitro and in vivo. In animal models, acetic acid-induced writhing was used to assess analgesic activity. In addition, a variety of tests including xylene-induced ear edema test, minimum inhibitory concentration (MIC) assays and acetic acid-induced peritoneal capillary hyperpermeability test were used to evaluate antiinflammatory activity of BA. BA at 0.2 and 0.1 mg · mL-1 doses expressed analgesic as well as anti-inflammatory activities in mice.In acetic acid induced writhing test, BA applying three times, twice and once a day significantly inhibited the acetic acid-induced writhing response within 15 min by 7.80% (*p<0.05), 7.50% (**p<0.01) and 6.25% (**p<0.01). In xylene-induced ear edema test,BA at 0.2, 0.1 and 0.05 mg · mL-1 decreased ear edema by 45.50% (**p<0.01), 15.20% (*p<0.05) and 9.10% (*p<0.05). In acetic acidinduced peritoneal capillary hyperpermeability test, BA exhibited significant inhibition (*p<0.05 versus control) of inflammation.In MIC assays, the MIC values of baicalin for S. aureus and Escherichia coli were 125 mg · mL-1, and the MIC values for the control bacteria ATCC25922 and ATCC25923 were 62.5 mg · mL-1. These findings suggested baicalin might contain analgesic and antiinflammatory agents which supported its use in traditional medicine.
Key words: baicalin, analgesic activity, anti-inflammatory activity
Introduction
Baicalin (BA, 7-glucuronic acid, 5, 6-dihydroxyflavone) is a flavonoid compound whose chemical structure has been verified and extracted from a rhizome ofAstragalus membranaceusbelonging to the family Lamiaceae (Oliveiraet al., 2015). Baicalin can be used in the treatment of inflammatory diseases,such as chronic hepatitis, bronchitis and atopic dermatitis (Lin and Shieh, 1996; Chiet al., 2003). It is reported that BA and its metabolite baicalein have anti-inflammatory, anti-bacterial and anti-diarrheal effects (Amagayaet al., 1989; Gaoet al., 1999; Shuklaet al., 2010). Studies have shown that it has multiple biological properties, including anti-oxidant (Gaoet al., 2001), anti-tumor (Ikezoeet al., 2001) and antiischemic properies. Furthermore, baicalin can induce autophagy in cancer (Zhanget al., 2012) causing subsequent autophagic tumor cell death.
Baicalin is commonly used to treat various inflammatory diseases, such as bronchitis, nephritis,hepatitis, asthma and atopic dermatitis (Kuboet al.,1984). The anti-inflammatory activities of these flavonoids have been attributed to their anti-oxidant ability and their capability to restrain LPS-induced NO production and nitric oxide synthase (inducible nitric oxide synthase, iNOS) gene, as well as the increase in TNF-αlevels (Chiuet al., 2002). Previous studies have shown that BA can inhibit T cell proliferation caused by toxin stimulation, and can also inhibit the production of several inflammatory mediators by monocytes (Krakaueret al., 2001); BA can limit its biological function by binding to various chemokines(Liet al., 2000); BA inhibits leukocyte activation by preventing leukocyte adhesion and down-regulating the production of reactive oxygen intermediates (Shenet al., 2003). The previous studies have shown that BA inhibits the production of IFN-g.
Therefore, studying the effects of BA on macrophage activation is important for understanding its anti-inflammatory effects. It has been reported that BA and structural flavonoids baicalein have an inhibitory effect on iNOS expression in RAW264.7 cells (Chenet al., 2001).
The anti-inflammatory activity of baicalin has been associated with NF-κB, as shown in various acute and chronic inflammation models (Kimet al., 2006), most of which are induced by LPS. Recent studies have shown that baicalin greatly inhibits lipopolysaccharide(LPS)-induced IL-8, IL-6 and TNF-αproductions by down-regulating phospho-IκB kinase (IKK)α/βand phospho-nuclear factor (NF)-κBp65 deliverance in HBE16 airway epithelial cells (Donget al., 2015).Baicalin also activates the toll-like receptor (TLR) 4 signaling pathway in the peripheral blood mononuclear cells in a rat model of LPS-induced fever (Yeet al.,2015) and is shown to regulate the immune response and mitigate ulcerative colitis-induced inflammation by promoting proliferation of CD4+CD29+ cells and modulating immunosuppressive pathways in humans(Yuet al., 2014). Besides, baicalin weakened LPS-induced inflammation and apoptosis of cow mammary epithelial cells by regulating NF-κB and heat shock protein 72 (HSP72) (Yanget al., 2016). In addition to thein vitrostudies, baicalin is found to relieve joint inflammation in collagen-induced arthritis rats through the NF-κB pathway (Wanget al., 2014). Moreover,baicalin attenuated inflammation in mice with ovalbumin (OVA)-induced asthma by restraining NF-κB(Liuet al., 2016).
After calving, most cattle (80%-90%) uteri are contaminated with genital tract bacteria, most of which areFusobacterium necrophorum,Escherichia coli(E. coli),Fusobacterium nucleatum,Prevotella spp.andArcanobacterium pyogenes(Croninet al., 2012;Werneret al., 2012). Endometritis, including clinical and subclinical endometritis, refers to the inflammation of the endometrium. On average, 20% of cattle develop clinical endometritis, which is characterized by purulent uterine secreta in the vagina 3 weeks after calving. Roughly 30% of cattle experience subclinical endometritis, leading to subfertility and infertility in the absence of systematic symptoms (Sheldonet al.,2009; Swangchan-Uthaiet al., 2012; Gilbertet al.,2005). Exploration of new analgesic and anti-inflammatory drugs are till now an arduous project and research is crucial to discover various alternatives.Plants based medicines may fulfill this requirement by providing nontoxic, more potent, efficacious and safe drugs to treat pain and inflammation. The objective of the present study was to investigate whether BA influence the production of inflammatory mediators and to evaluate the analgesic and anti-inflammatory activities of BA bothin vitroandin vivo.
Materials and Methods
Drugs, chemicals and apparatus
Baicalin (Xuancheng Baicao Plant Industry and Trade Co., Ltd., content≥98%), Xylene was purchased from Xinda Chemical Factory (Heilongjiang Province,China, batch No.161122) and acetic acid was purchased from Guangfu Fine Chemical Research Institute (Tianjin, China). Compound dexamethasone acetate ointment (CDAO) was provided by China Resource Sanjiu Pharmaceutical Co., Ltd (Batch No.1604001, specification: 30 g: 22.5 mg). Evans Blue (Shanghai Biaozhuo Biotechnology Co., Ltd.),sodium sulfide (Hubei Huahan Chemical Co., Ltd.),conduit oxytetracycline injection (Qilu Animal Health Products Co., Ltd. Approval number: veterinary drug word (2014) 150252787, specification: 50 mL:10 g), peptone (Biochemical Reagent) lot number:20151201 (Beijing Aoboxing Biotechnology Co.,Ltd.), beef cream (Biochemical Reagent) lot number:20150609 (Beijing Aoboxing Biotechnology Co.,Ltd.), agar powder (biochemical reagent) lot number:20150907 (Beijing Soleil Bao Company), sodium chloride (analytical grade) lot number: 20140424(Tianjin Dongtianzheng Fine Chemical Reagent Factory), 752UV spectrophotometer (Shanghai Optical Instrument Factory), ZDY-11 turbidity comparator(Shanghai Fosun Hao Biotechnology Co., Ltd.), BC-5000 Vet automatic animal blood cell analyzer (Shenzhen Mindray Biomedical Electronics Co., Ltd.).
Bacterial isolates
Escherichia coliK99 andStaphylococcus aureusk88 were isolated from cattle with endometritis and identified by biochemical and PCR methods by the Institute of Pharmacology and Toxicology, College of Veterinary Medicine, Northeast Agricultural University. Quality controlE. coli ATCC25922 andStaphylococcus aureus ATCC25923 were purchased from China National Institute for the Control of Pharmaceutical and Biological Products.
Laboratory animals
All the laboratory animals, including female and male Kunming (KM) mice weighing 20-25 g and female Japanese rabbits with healthy white hair, no pregnancy,no reproductive system disease weighing 2-2.5 kg(Certificate No. SCXK, Heilongjiang, 2013-001),were obtained from Department of Laboratory Animal Science, Harbin Medical University (Heilongjiang,China). All the animals were spontaneously given a standard pellet diet and water, the animals were acclimated to the environment for 7 days, and then free water ingress was banned 12 h prior to the experiment.All the procedures involving animals were complied with China National Institutes of Healthy Guidelines for the Care and Use of Laboratory Animals.
Acetic acid-induced abdominal writhing in mice
The acute inflammatory activity of baicalin was evaluated using xylene-induced ear edema in mice as previously described. The mice were randomly divided into five treatment groups of 10 animals in each group including control (smudge substrate,10 mg · mL-1), positive control (compound dexamethasone acetate ointment, 10 mg · g-1) and three doses of baicalin (dosing concentration 0.2 mg · mL-1, dosage 15 μL · g-1, applied three times, twice and once a day).The mice in each group were depilated with 8%sodium sulfide solution, and the skin was washed with physiological saline solution after depilation. These treatments were applied for five consecutive days.
On the 6th day after 1 h of administration, the mice were induced to reverse with 0.6% acetic acid solution, and abnormal saline was intraperitoneally injected at a dose of 10 mL · kg-1. The writhing frequency (abdominal contraction, pelvic rotation and hind limb extension) was recorded for 15 min and the first (recorded as latency) writhing after acetic acid injection was recorded. The following formula:inhibition(%)=[numbers of writhes (control)-numbers of writhes (test)]/numbers of writhes (control)×100%was used to calculate percentage analgesic activity.
Evaluation by method of xylene-induced ear edema in mice
As described above, the acute inflammatory activity of baicalin was evaluated using xylene-induced mouse ear edema. The mice were divided into the following five treatment groups including control (smudge substrate, 1 mg · g-1), positive control (compound dexamethasone acetate ointment, 5 mg · g-1) and three doses of baicalin (concentration 0.2, 0.1 and 0.05 mg · mL-1,dosage 5 μL · g-1). Dosed the right and left sides of the right ear of each administration group for three consecutive days. On the 4th day after 1 h of admini-stration, the drug was diluted with distilled water,and the left ear was used as a control, and 30 μL of xylene was applied to both surfaces of the right ear to induce edema. Mice were sacrificed 1 h after xylene treatment by cervical dislocation. The right and left ears of the mice were removed with an 8 mm diameter cork drill and the ears were weighed. The difference in edema weight between the right ear and the left ear of the same animal was measured. The percentage of suppression compared with the control group was computed using the following formula: inhibition (%)=[(weight of edema (control) - weight of edema (test)]/weight of edema (control)×100%.
Evaluation by method of acetic acid-induced peritoneal capillary hyperpermeability in mice
Took 0.05g Evans blue and diluted to 500 mL with physiological saline to prepare 100 μg · mL-1mother liquor. Took 5 mL of mother liquor, diluted to 50 mL,prepared 10 μg · mL-1working solution, and then double-diluted to prepare a series of concentrations of Evans blue standard solution. The OD value was measured at 590 nm with an ultraviolet spectrophotometer, and then an Evans blue standard curve was prepared.
The mice were randomly divided into the following five medicine treatment groups of 10 animals each group: control (smudge substrate, 10 mg · g-1), positive control (compound dexamethasone acetate ointment,10 mg · g-1) and three doses of baicalin (dosing concentration 0.2 mg · mL-1, dosage 15 μL · g-1, applied three times, twice and once a day). The mice in each group were depilated with 8% sodium sulfide solution,and the skin was washed with physiological saline solution after depilation. These medical treatments were applied for five subsequent days. On the 6th day at 1 h after management, the mice were induced to writh with 0.6% acetic acid solution in normal saline squirted intraperitoneally at a dose of 10 mL · kg-1.After 30 min, the mice were injected with 1% Evans blue dye solution in normal brine (10 μL · g-1). 20 min later, the mice were sacrificed and peritoneal exudates were collected by washing cavities with 10 mL of normal brine and by centrifuging at 3 000 r · min-1for 15 min. The absorbance of the supernatant was read at 590 nm. The amount of dye infiltrated into the abdominal cavity of each mouse was defined using a standard curve, and thet-test between groups was performed.
Minimum inhibitory concentration (MIC) assays
MICs of baicalin were determined by the tube double dilution method using baicalin over a range of dilutions from 0.98 to 500 mg · mL-1or using Gentamicin over a range of dilutions from 0.03 to 500 μg · mL-1.Briefly, took 10 numbered sterilized test tubes, added 1 mL of nutrient broth in 1 to 10 tubes, then pipetted 5 mL of traditional Chinese medicine solution (the concentration of crude drug was 1 g · mL-1) into No.1 tube. After mixing, removed 5 mL of the mixed solution and transfered to No. 2 tube. The test tube was operated to No.10 tube in turn, and No.10 tube was discarded to 5 mL. The final volume of each tube was 5 mL, so that the liquid was diluted into 0.98, 1.96, 3.91, 7.82, 15.63, 31.25, 62.5, 125, 250 and 500 μg · mL-1for baicalin. Then, eachE. coliandStaphylococcus aureusstrain was re-suspended from trays in MH broth to a concentration of 0.5 McFarland criterion and the concentration of the bacterial solution was approximately 1×108CFU · mL-1and then distributed into test tube at a concentration of 100 μL.MICs were defined as the lowest antibiotic densities for which no visible growth ofE. coliandStaphylococcus aureuswas detected after 16-18 h at 37℃. At the same time, set nutrient broth as a negative control (No.11 tube) and bacterial suspension without any drug was positive growth controls (No.12 tube).E. coli ATCC25922 andStaphylococcus aureus ATCC25923 strains were used as the quality control strains.
Results
The analgesic activities of BA on acetic acid-induced ventral writhing in mice are shown in Fig. 1. BA applying three times, twice and once significantly restrained the acetic acid-induced writhing response within 15 min by 7.80% (*p<0.05), 7.50% (**p<0.01)and 6.25% (**p<0.01), separately. The positive control CDAO (10 mg · g-1) also strongly restrained the writhing response by 10.31% (**p<0.01).

Fig. 1 Effect of BA on acetic acid-induced ventral writhing in miceRate of recurrence of writhing within 15 min after intraperitoneal syringe of acetic acid. Blank control mice are administered with smudge substrate and CDAO (10 mg · g-1) is used as positive control. Results are expressed as mean±SEM (n=10). *p<0.05 and **p<0.01 are considered significant when compared with blank control (smudge substrate) after management.
The dose dependant affects of BA against acute inflammation in xylene-induce ear edema in mice are shown in Fig. 2. Compared with the control treatment,BA at 0.2, 0.1 and 0.05 mg · mL-1decreased ear edema by 45.50% (**p<0.01), 15.20% (*p<0.05) and 9.10%(*p<0.05), respectively. Meanwhile, the positive control CDAO (5 mg · g-1) inhibited ear edema by 60.60%(**p<0.01).
In the experiment the effects of acetic acid-induced peritoneal capillary permeability in mice, the OD values of the series of Evans blue standard solutions were determined to be 0.5945, 0.3143, 0.1847,0.1138, 0.0773 and 0.0579, respectively. The standard curve wasy=0.055x+0.0431 (R2=0.9998), as shown in Fig. 3.
The dose dependent effects of BA against acute inflammation in peritoneal capillary permeability in mice are shown in Fig. 4. The control group OD value was 0.0725±0.0020. BA dose group OD values were 0.0669±0.0436, 0.0687±0.0044 and 0.0694±0.0039. The positive control group OD value was 0.0351±0.0280. Compared with the control treatment,BA applying three times, twice and once decreased peritoneal capillary permeability subsequently. The positive control CDAO (10 mg · g-1) significantly inhibited peritoneal capillary permeability.

Fig. 2 Effect of BA on xylene-induced ear edema in miceMice are applied with BA three consecutive days before xylene(30 μL · ear-1) is applied to right ears of mice. Blank control mice are applied with smudge substrate and CDAO (5 mg · g-1) is used as positive control. Results are expressed as mean±SEM (n=10). *p<0.05 and **p<0.01 are considered significant, when compared with blank control (smudge substrate) after management. *p<0.05 is considered significants when compared with positive control (CDAO) after management.

Fig. 3 Evans blue standard curve
As shown in Table 1, MIC values of baicalin forS. aureusandEscherichia coliwere 125 mg · mL-1, and MIC values for the control bacteriaATCC25922 andATCC25923 were 62.5 mg · mL-1. As shown in Table 2,MIC values of the control drug gentamicin againstEscherichia coliandStaphylococcus aureuswere 0.5 mg · mL-1, and MIC values of the control bacteriaATCC25922 andATCC25923 were 0.49 μg · mL-1(the normal antibacterial range was 0-2.5 μg · mL-1).

Fig. 4 Effect of BA on capillary permeability of mouse peritoneal cavity30 min after HAC injection, mice are injected with 0.5% Evans blue saline in tail vein. Amount of dye infiltrates into peritoneal cavity of each mouse,OD value is found on standard curve. Vehicle control mice are applied with smudge substrate and CDAO (10 mg· g-1) is used as positive control. Results are expressed as mean±SEM (n=10). *p<0.05 and **p<0.01 are considered significant when compared with blank control (smudge substrate) after management.

Table 1 MIC value determination results of baicalin
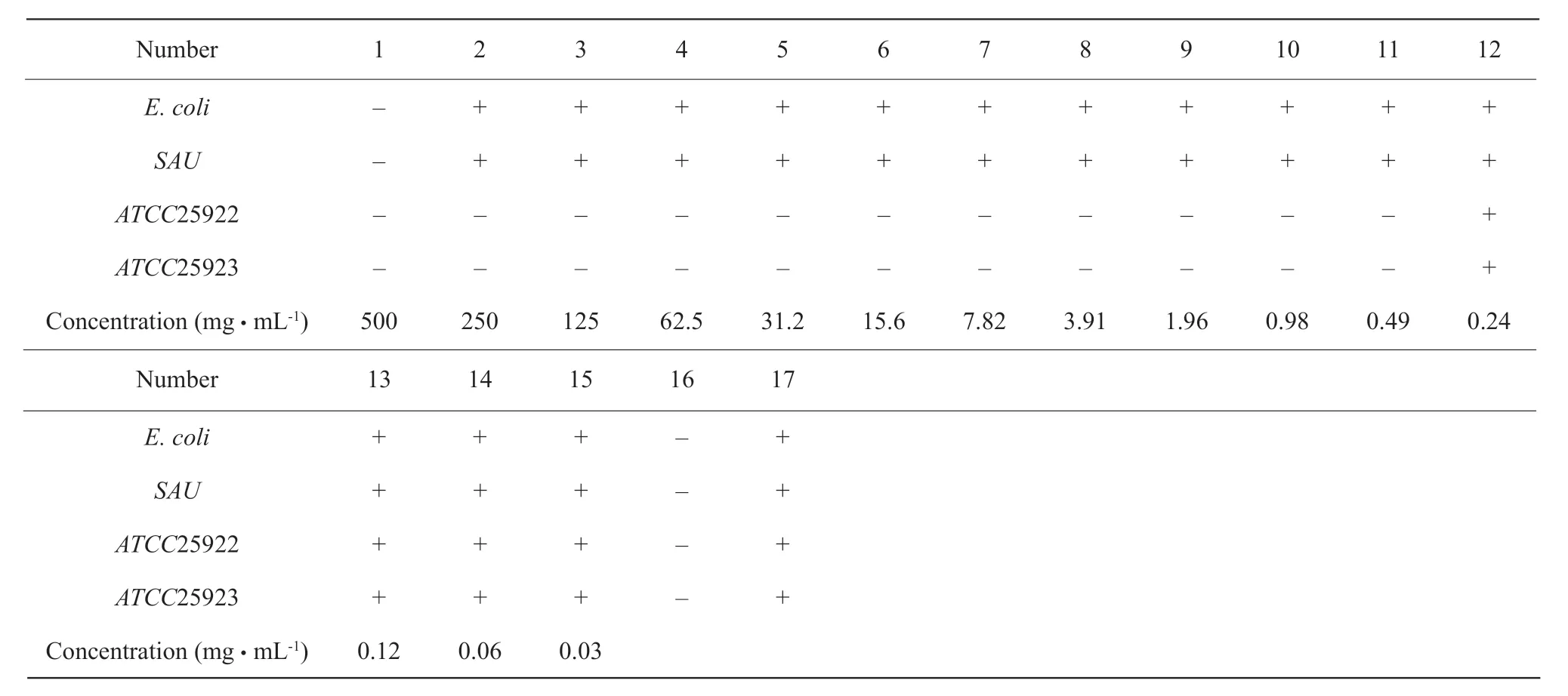
Table 2 MIC value determination results of gentamicin
Discussion
The abdominal contraction response induced by acetic acid was a very sensitive process which was used to screen the peripheral analgesic effect (Adeyemiet al.,2011) and such abdominal contraction response was supposed to involve local peritoneal receptors (Bentleyet al., 1983). In mice, acetic acid had been attributed to the release of arachidonic acid and it also resulted in the compound of prostaglandin through the enzyme called cyclooxygenase (Davieset al., 1984). Acetic acid produced an abdominal writhing response due to peripheral nociceptive sensitization by prostaglandin.This method was also related to increase levels of prostanoids in general, for example, prostaglandin E2 (PGE2) and prostaglandin F2α(PGF2α) as well as lipoxygenase productsin peritoneal fluids (Levineet al., 1984; Dharaet al., 2000; Yaduet al., 2010;Ghuleet al., 2011). Inflammation due to intraperitoneal injection of acetic acid also caused the release of endogenous substances like bradykinin,serotonin and histamine, which stimulated the central nociceptive neurons (Vermaet al., 2005; Rathiet al.,2003). Different models of chemical pain in animals were used to assess the analgesic activities and anti-inflammatory of BA. The acetic acid-induced abdominal writhing test was commonly used to evaluate peripheral activity models (Tjolsen and Hole, 1997; Khanet al., 2009). Localirritation produced by an intraperitoneal injection of acetic acid triggered the emancipation pro-inflammatory cytokines, such as TNF-α, IL-8, IL-6 and IL-1. These analgesic models suggested that the analgesic affect of BA might be mediated by inhibiting the compound and emancipation of prostaglandins and other proinflammatory cell factors, such as TNF-α, IL-8, IL-1 and IL-6 (Deraedtet al., 1980; Habib and Waheed,2013).
Wooet al. (2006) established a lipopolysaccharide(LPS)-induced RAW264.7 cell inflammatory model to study the anti-inflammatory mechanism of baicalin,and found that baicalin could inhibit the expression of cyclooxygenaseCOX-2 gene to prevent transcription factor C/EBPβ. Baicalin regulated estrogen activity and inhibited lipopolysaccharide (LPS)-induced inflammation through the NF-κB pathway (Fanet al.,2013). The anti-inflammatory principle of baicalin was to inhibit NF-κB and p-p38, and to reduce TNF-α,IL-6 and IL-1βexpression (Guoet al., 2013). Baicalin blocked the activity of NF-κB and inhibited the utterance of IL-6 and interleukin-8, IL-8 mRNA (Nakamuraet al., 2003). Baicalin could inhibit the inflammatory mediators IL-6, TNF-αand PGE2, NO release, block nitric oxide synthase iNOS, IL-6, TNF-α,COX-2 gene expression (Yanget al., 2013). In the present study, acute models of inflammations were adopted to assess the anti-inflammatory affects of BA. The model of xylene-induced ear edema in mice was linked to neurogenous edema release of inflammatory mediators, such as histamine, kinin and fibrinolysin.It was reported that phenolicacids and flavonoids armamentarium had high anti-inflammatory activities.The current study confirmed the anti-inflammatory and analgesic properties of BA through several animal tests. However, further studies were warranted to elucidate the exact mechanism of action and confirm the chemical compounds responsible for the antiinflammatory and analgesic effects of BA.
Chinese herbal medicine had antibacterial, antiviral and anti-inflammatory effects. A variety of active ingredients in traditional Chinese medicine, such as alkaloids, glycosides, acids, ketones, aldehydes,phenols and volatile oils, had antibacterial and antiviral effects. These active ingredients all directly inhibited or killed bacteria, fungi and viruses. It was mainly involved in inhibiting the reproduction of bacteria,fungi and viruses, participating in the biochemical processes of bacteria and molds, and changing its functions, such as enzymes and cell membranes.Baicalin had significant antibacterial effect and could effectively inhibit the growth of various bacteria, such asListeria monocytogenes,Salmonella,Escherichia coli,StaphylococcusaureusandBacillus cereus,etc(Shanet al., 2007). Baicalin inhibited the growth of bacteria, which might be related to its inhibition of ATP synthase, formation of microbial envelope and inhibition of expression of certain proteins (Chinnamet al., 2010; Daiet al., 2009). The combination of baicalin with gentamicin, fluconazole andβ-lactam antibiotics (Changet al., 2007; Huanget al., 2008;Janget al., 2014) would produce a synergistic effect and enhance the antibacterial effect.
Conclusions
In conclusion, it has been suggested from the present results that baicalin possessed analgesic and antiinflammatory activities. The results showed the baicalin exhibited significantly analgesic effects and anti-inflammatory activities by inhibiting capillary permeability, xylene-induced ear edema and relieving acetic acid-induced writhing in mice. The study provided a scientific foundation for the traditional Chinese medicine i.e. baicalin in relieving pain and curing inflammatory disease.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Application of Hyperspectral Imaging Technology in Rapid Detection of Preservative in Milk
- Length-weight and Length-length Relationships for Nine Freshwater Species from Huaihongxinhe River, Huaihe River Basin, East China
- Effect of Chromium Propionate Substituting 25% Rumen-protected Choline on Production Performance and Blood Indicators of Perinatal Dairy Cows
- Effects of Crop Rotation and Microbial Fertilizer on Nutrient Absorption and Beneficial Bacterium Abundance in Rhizosphere of Continuous Cropped Eggplant
- Effects of Two Chelating Agents on Availability of Calcium and Phosphorus in Black Soil of Vegetable Fields
- A Bicycle Tourism Based Study on Planning and Designing of Urbanrural Recreational Greenway
—— A Case Study of Harbin City
