Circulating tumor DNA: Where are we now? A mini review of the literature
2021-01-14GliceidaMariaGalarzaFortunaKathrinDvir
Gliceida Maria Galarza Fortuna, Kathrin Dvir
Gliceida Maria Galarza Fortuna, Kathrin Dvir, Internal Medicine, Mount Sinai Medical Center,Miami Beach, FL 33140, United States
Abstract For many years tissue biopsy has been the primary procedure to establish cancer diagnosis and determine further treatment and prognosis. However, this method has multiple drawbacks, including, to mention some, being an invasive procedure carrying significant risk for fragile patients and allowing only for a “snapshot” of the tumor biology in time. The process of liquid biopsy allows for a minimally invasive procedure that provides molecular information about underlying cancer by analyzing circulating tumor DNA (ctDNA) via next-generation sequencing technology and circulating tumor cells. This paper focuses on describing the basis of ctDNA and its current utilities.
Key Words: Circulating tumor DNA; Liquid biopsy; Molecular profiling; Cancer diagnosis; Cancer screening; Cancer treatment
INTRODUCTION
Understanding tumor genetic make-up is more important now than ever given the vast array of available targeted therapies. Traditionally, a biopsy was the only existing approach to understanding tissue histological composition and its genetic environment. However, this approach allows a merely static analysis of a tumor at a given time and a given location, while cancer is a rather dynamic entity undergoing continual alterations. The concept that a tumor can harvest different genetic material,which can be identified by next-generation sequencing (NGS), has been extensively studied and validated; this discordance can occur both within the primary tumor and between primary and metastatic lesions[1]and is partly because a tumor is comprised of different cell clones[2]. For example, Geyeret al[3]demonstrated the presence of intratumor heterogeneity in breast cancer, as evidenced by the presence of overexpressedHER2mutation only in some regions of a primary tumor. This lack of uniformity within a tumor’s genetic environment and spatial heterogeneity is a therapeutic challenge. A single biopsy would not signify an accurate and complete assessment of a tumor’s genetic composition. Hence, a need has risen for more comprehensive techniques, which would yield a better characterization of tumor composition and its driver mutations.
HOW IS CIRCULATING TUMOR DNA DETECTED?
Circulating tumor cells (CTCs) have been observed in patients’ bloodstream. CTCs are believed to reach a patient’s plasma by migration from the principal or metastatic tumor site secondary to tumor invasion, shedding or after the tumor site experiences mechanical stress after surgery[4]. Analysis of both CTC and circulating tumor DNA(ctDNA) is the backbone of the development of liquid biopsy.
In 1948, a group of French scientists detected free DNA fragments circulating in the plasma[5]. Several successive studies have been conducted to pursue the mechanism in which DNA fragments are released into the serum from the cells in their healthy,inflamed, or diseased states. To date, the consensus hypothesis is that the DNA enters the circulation through passive and active mechanisms[6]. The passive release of DNA fragments into the circulation is thought to be secondary to cell death, both through the apoptosis and necrosis pathways. In contrast, the active secretion of DNA fragments into the bloodstream has yet to be understood entirely[2]. Some studies propose that tumor cells release micro-vesicles (exons) containing fragments of double-stranded DNA (ctDNA); however, this theory is still not universally accepted[7]. Even though circulating cell-free DNA (cfDNA) is detectable in healthy individuals, its concentration is significantly increased in cancer patients[8]. ctDNA is also released to the bloodstream by the above-described mechanisms from primary and metastatic tumor sites.
Two main approaches are utilized for the detection of ctDNA - a targeted, and an untargeted approach. The targeted approach can detect previously determined genetic mutations, such as specific driver mutations that frequently occur in individual tumors and toward which targeted therapy has been developed[9]. On the other hand, the untargeted approach does not need any prior knowledge of the genetic mutations associated with the tumor under study.
PCR techniques are the backbone of all strategies for the detection of ctDNA in the targeted approach. Several PCR techniques such as digital PCR (dPCR), emulsion PCR(ePCR), and BEAMing (beads, emulsion, amplification, and magnetics) – which is a high-throughput droplet-based dPCR, have been developed for the detection of determining DNA mutations[10]. Nuneset al[11]used quantitative methylation-specific PCR to determineAPC,HOXA9,RARB2, andRASSF1Apromoter methylation levels.Their study was able to detect highHOXA9methylation levels in patients with squamous cell lung cancer with 82.2% accuracy. Correspondingly, Klotenet al[12]identifiedKRASmutation in patients with colorectal cancer using Intplex allelespecific PCR (Intplex PCR). In this study, ctDNA analysis using the Intplex technique had a 70% specificity and 50% sensitivity when comparing serum samples with the tissue sample. Furthermore, the concordance with their match tissue sample was 66%.
NGS, on the other hand, is capable of analyzing millions of DNA sequences and then compare it with a pre-determined genome or produce ade novosequence assembly[13]. Thompsonet al[14]detected approximately 275 alterations in 45 genes on patients, of which 86% were previously diagnosed with lung cancer.Epidermal growth factor receptor(EGFR) mutation was the most common mutation in their study. When compared to tissue DNA analysis, their concordance was 79%. Another important finding of this study was that patients who had failed treatment, as evidenced by disease progression while on therapy, ctDNA analysis showed newly emerged resistance mutations when tissue analysis was not feasible.
Given the presence of both circulating cell-free DNA from healthy tissue and ctDNA, the isolation of ctDNA continues to be a diagnostic challenge, as only approximately 0.01% of all circulating DNA is tumor-derived[15]. This limitation has been overcome by the recent development of “ultra-sensitive” assays that allow differentiating ctDNA from cfDNA, which are being used not only for the detection of genetic mutations but also for the early detection of disease recurrence and monitoring for therapy response[7]. One example of an ultrasensitive assay is the Cancer Personalized Profiling by Deep Sequencing (CAPP-Seq); this technology consists of a capture-based ctDNA detection method which can detect most of the main types of mutations: Copy number alterations, rearrangement, indels, and single nucleotide variants, by the evaluation of large segments of the genome utilizing enriched genomic regions that have been selected before sequencing[16,17]. This method allows for the detection of various mutations, increasing the sensitivity of the test, compared to other NGS based assays, and aids the evaluation of intratumor heterogeneity[17]. This technological advancement has led to the development of liquid biopsy, which provides a genetic characterization of tumors from blood, bronchial alveolar lavage samples, or colony-stimulating factor samples. This technology brings many clinical utilities, especially in patients with solid tumors that are not amenable to repeat biopsies, including the measurement of disease burden and detection of emerging mutations, among others.
LIQUID BIOPSY AS A PROGNOSTIC MARKER
As already mentioned, cfDNA levels are higher in the disease state when compared to healthy individuals[8]. Hence, several studies have tried to establish a correlation between cfDNA and CTC levels and disease prognosis. Leeet al[18]studied the relationship between CTC in patients with epithelial ovarian cancer and disease prognosis; they found that newly diagnosed patients with CTC > 3 had a significantly shorter progression-free survival. This marker was also determined to be a poor prognostic factor in multivariate Analysis (HR = 1.3; 95%CI: 3.08-32.149).
Likewise, Itoet al[19]studied a group of patients with metastatic breast cancer treated with the microtubule-depolymerizing agent eribulin and determined the presence of CTC. They further classified the CTC into mesenchymal and epithelial depending on their vimentin or pan-cytokeratin positivity. Patients with a high number of CTC (≥ 3)had a significantly shorter overall survival (P= 0.037). No difference was observed on the sub-analysis of mesenchymal and epithelial CTC.
Furthermore, some genetic alterations detected through ctDNA analysis are associated with increased survival. Chenget al[20]established that the detection ofERBB2exon 17 mutation andK-Ras G12Vmutation in a cohort of patients with metastatic pancreatic cancer was associated with a statistically significant increase in overall survival (Pvalues = 0.016 and 0.015; respectively). Another important finding of this research is an increased observed rate ofBRCA2mutations in patients with metastatic pancreatic cancer; prior data showed a 5% mutation rate in these patients;this study showed an 11.7% mutation rate.BRCA2mutations in pancreatic cancer have been associated with an improved response to cisplatin-base chemotherapy. These observations have led to a new possible pharmacological approach to a disease that often carries a dismal survival.
Correspondingly, Xuet al[21]developed and validated a combined prognosis score(cp-score) using eight methylation markers found on ctDNA in addition to clinical,demographic, and the American Joint Committee on Cancer (AJCC) stage. In their research among 377 hepatocellular carcinomas (HCC) samples, a cp-score ≤ 0.24 was determined to be a low risk while a cp-score > 0.24 was classified as high risk, with a statistically significant median survival (P< 0.0001). This research showed that cpscore, in combination with Tumor-Node-Metastasis staging, increased the prognostic prediction accuracy for patients with HCC.
LIQUID BIOPSY AND THERAPEUTIC GUIDANCE
Therapy selection is among the most clinically relevant current utilization of liquid biopsies; this holds especially true for tumors that are difficult to biopsy or for patients that are too fragile to undergo surgical exploration[22]. Moreover, this approach also provides the means to monitor tumor evolution and the development of therapy resistance.
Shuet al[23]studied ctDNA by targeted NGS-based gene mutation profiling in a total of 605 cancer patients with 29 different tumor types. In their study, the most frequently observed mutated tumor suppressor genes wereTP53,APCandDNMT3A, while the most commonly mutated oncogenes wereEGFR,KRAS, andPIK3CA; 35.3% of the detected mutations were clinically-actionable, and 66% of those mutated genes have FDA approved targeted therapy or have therapy undergoing current clinical trials.This fact makes ctDNA evaluation a helpful tool to identify molecular mutations to which targeted therapies are available and thus guide management, which would,after that, influences survival.
Correspondingly, microsatellite instability (MSI) is a biomarker used to predict response to immune checkpoint blockade for cervical, cholangiocarcinoma, colorectal,endometrial, esophageal, esophagogastric, gastric, ovarian, pancreatic and prostate cancer[24]. Williset al[24]validated MSI detection using a plasma-based genotyping panel, Guardant360. This method was able to detect MSI with an accuracy of 98.4%and a positive predictive value of 95% compared to tissue-based testing. Moreover, in their study they were able to follow up 16 patients with metastatic gastric cancer treated with either pembrolizumab or nivolumab after not achieving remission with the standard chemotherapy regimen; 10 of the 16 patients achieved complete (3/16) or partial response (7/16) while three patients were reported to have stable disease,resulting in an objective response rate of 63% and a disease control rate of 81%.Immune checkpoint inhibitors are gaining FDA approval rapidly in advanced cancer,and the detection of MSI provides the clinical oncologist with a novel, fast and noninvasive tool to predict response to therapy that is especially beneficial in this patient population given their poor clinical and performance status, which limits the possibility of more traditional testing such as biopsies.
MONITORING TREATMENT RESISTANCE WITH CTDNA
In lung cancer, namely non-small cell lung cancer, the presence of a somatic mutation in theEGFR-L858Rand exon 19 deletion- has been noted to predict the response to EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib, gefitinib, and afatinib.Therefore, several PCR based platforms have been used to detect this mutation in plasma[25,26]. However, this response is often limited to the first 10-12 mo of treatment;when this is lost given the development of acquired resistance mutations[27].
Tumor resistance to either targeted therapy or chemotherapy follows one of two pathways, a pharmacological or biological resistance. Pharmacological resistance infers the progression of disease in the setting of inadequate drug exposure against a targeted protein. On the other hand, biological resistance consists of changes in the drug target, such as the development of a secondaryEGFRmutation such asT790M,D761Y, andL747Smutations. This biological mechanism, in part, depends on the initial biological heterogeneity of the tumor. Directed therapy to specific oncogenes leads to increase gene copies of the sub-clones present in the tumor as a response to the selection pressure. The selected sub-clones often lead to the emergence of a structural change of the targeted protein – alterations in the drug target – or leads to the development of a bypass track that feeds the tumor even in the presence of the inhibition on the initial target[26].
Therefore, monitoring ctDNA to detect secondary mutations’ emergence could provide a tool to adjust therapy before the development of clinical signs of disease progression. The JP-CLEAR trial analyzed the plasma of 121 patients with advance or postoperative recurrent non-small cell lung cancer on first or second-generation TKI therapy without known disease progression. Their plasma was monitor every 1-2 mo with a PCR based method developed to detect sensitizingEGFRmutations and theT790Mresistance mutation. In their study, 33.3% of the patients developedT790Mmutation and disease progression while on first-line therapy with TKIs, proving ctDNA monitoring to be a useful tool to assess the emergence of secondary mutations associated with treatment resistance[28].
Similarly, colorectal cancer with wild typeRASandBRAFgenes is known for responding to cetuximab treatment, whileHER2amplification has been associated with treatment resistance[29]. Liuet al[29]used a ddPCR based method to detectHER2amplification on a total of 36 patients with wild typeRAS/BRAFmetastatic colorectal cancer undergoing therapy with cetuximab. TheirHER2status was determined at the time of disease progression. Of the 36 patients with documented disease progression while on cetuximab treatment, five were found to haveHER2amplification at the time of progression. During the study, plasma ctDNA forHER2status was carried out, andHER2levels were able to predict radiological progression with a lead time of 2 mo.This idea again proves that monitoring plasma ctDNA while on therapy can be an essential tool in detecting disease progression before any apparent changes in radiographic studies.
CTDNA AS A SCREENING TOOL
To date, ctDNA has not yet made its way onto becoming a screening tool. This technology holds significant potential to become a vital screening strategy, leading to earlier diagnosis. However, ctDNA must overcome many barriers before being applied as a screening strategy. One of the most critical limiting factors is the low concentration of ctDNA in asymptomatic patients[30]. The level of ctDNA in the healthy population has been determined to range from 1-10 ng/mL[31]. Given its low concentration, approximately 150-300 mL of blood would be required to reach a 95%sensitivity of a screening test for breast cancer[30,32]. Moreover, given that healthy cells contribute to cfDNA in plasma, introducing ctDNA as a screening tool could lead to an increased rate of false positives[30].
DISCUSSION
The introduction of liquid biopsy into clinical practice provided a novel tool for the detection, monitoring, and characterization of malignancy. It allows the detection of tumor circulating DNAviaperipheral blood sampling, analyzing its genomic compositing and specific targetable genomic alterations, thereby enhancing the delivery of personalized medicineviatargeted treatments. Liquid biopsy can be utilized to monitor the response to therapy and act as a surveillance modality in detecting disease progression. Moreover, its non-invasive nature renders a feasible solution for patients who are not candidates for surgical intervention or tissue sampling. Despite its many advantages, widely acknowledged by the scientific community, liquid biopsy has essential disadvantages, namely test sensitivity, which reaches 85% in certain malignancies[33]and high cost.
Since its introduction, liquid biopsy has been the focus of research and has been incorporated into many clinical trials’ protocols worldwide. Many of the currently registered trials researching liquid biopsy and ctDNA in clinicaltrials.gov (Table 1) are aiming to characterize the following three topics: (1) The detection of targetable genetic alterations in patients who are not amenable to surgical biopsies; (2)Monitoring response to therapy and detecting minimal residual disease; and (3)Identifying the development ofde-novomutations after administration of specific treatments. Commonly used modalities to detect circulating tumor DNA extractedvialiquid biopsy are NGS, BEAMing, and PCR. While these modalities can identify mutant alleles in as little as 2% frequency[6], all three have significant limitations. NGS requires high-quality DNA fragments for the analysis, which is often difficult to detect through liquid biopsy; it necessitates trained bioinformaticians to analyze the data,and it is associated with a high cost with variable insurance coverage[6]. The BEAMing modality provides an even higher mutation detection ability while carrying a lower cost when compared to PCR and NGS. However, it requires a previously established DNA template to target the genomic area of interest, therefore limiting its mutations detection repertoire[34]. Similarly, the different PCR modalities (i.e., dPCR and ePCR),also require pre-established genomic templates, thereby limiting its array of detection to specific targetable mutations rather than allowing a broad screening for alternated tumor genome[35].
In summary, liquid biopsy is useful in detecting ctDNA in most types of cancer at both early and advanced stages of cancer. Studies have shown ctDNA often precedes the radiological and clinical signs of disease by as early as six months[36]. Its ability to detect tumor genomevianon-invasive technique holds an immense potential to be utilized as a screening method for cancer detection on appropriate individuals.
CONCLUSION
Multiple commercial ctDNA analysis platforms are currently available and are widelyused in clinical oncology. This mini-review aims to describe the fundamental technical aspects of liquid biopsies and their clinical implications. Since its introduction, liquid biopsies have been incorporated into clinical protocols and clinical trials worldwide.The current registered active phase 2-4 clinical trials have been summarized (Table 1).Most of these studies aim to utilize liquid biopsy for: (1) The detection of known mutations that have targeted therapy in patients who are not amenable for surgical biopsies; (2) Monitoring disease progression and therapeutic response – minimal residual disease; and (3) Detection the development ofde novomutations after specific therapies. The introduction of liquid biopsy into clinical practice provides a novel tool for the diagnosis, future development of personalized/targeted treatments, and monitoring of multiple malignancies. Furthermore, it gives the clinician with a new understanding of tumors’ biology by providing a more comprehensive map of the genetic origin of cancerous processes. With the increased use of liquid biopsies, new associations between driver mutations and specific tumors will become more apparent, which could lead to the development of more targeted therapies and even the utilization of this method for screening purposes before the development of diseased states.
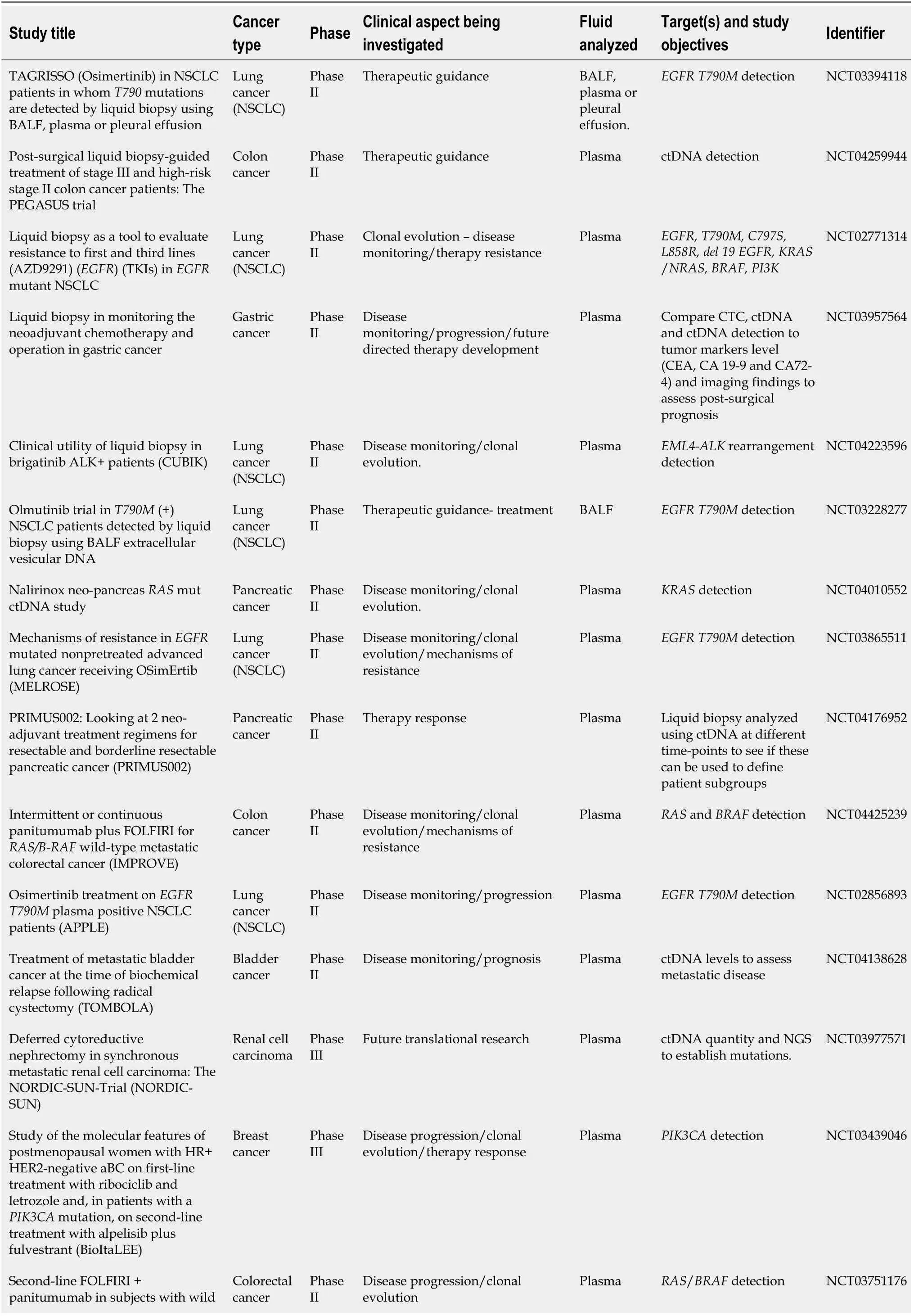
Table 1 Current active and completed phase II-III clinical trials utilizing liquid biopsy technology

NSCLC: Non-small cell lung cancers; BALF: Bronchoalveolar lavage fluid; EGFR: Epidermal growth factor receptor; TKIs: Tyrosine kinase inhibitors;ctDNA: Circulating tumor DNA; CEA: Carcinoembryonic antigen; CTC: Circulating tumor cells; NGS: Next-generation sequencing.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. Mike Cusnir, for his contribution and support in this project.
杂志排行
World Journal of Clinical Oncology的其它文章
- Comparison of efficacy between adjuvant chemotherapy and chemoradiation therapy for pancreatic cancer: AJCC stage-based approach
- Diacerein treatment prevents colitis-associated cancer in mice
- Healthcare delivery interventions to reduce cancer disparities worldwide
- Powerful quantifiers for cancer transcriptomics
