Distribution and geochemical significance of dibenzofurans,phenyldibenzofurans and benzo[b]naphthofurans in source rock extracts from Niger Delta basin, Nigeria
2021-01-09AbiodunOgbesejanaOluwasesanBello
Abiodun B. Ogbesejana · Oluwasesan M. Bello
Abstract The distribution and geochemical significance of dibenzofurans, phenyldibenzofurans and benzo[b]napthofurans in source rocks from Niger Delta basin, Nigeria,were investigated by Rock–Eval pyrolysis and gas chromatography-mass spectrometry (GC–MS). The data obtained from the source rocks evaluation showed that the rock samples contained type II/III kerogen capable of generating oil and gas and were within immature to early mature stage. The relative abundance of the C0-, C1- and C2-dibenzofurans range from 1.75%to 29.82%,27.60%to 40.52% and 29.66% to 68.89%, respectively. The dibenzofurans were dominated by C2-dibenzofurans.Among the C1-dibenzofurans, 2-+3-methyldibenzofuran was the most abundant in the rock samples while 1-methyldibenzofuran appeared to be the least. The relative abundance of benzo[b]naphtho[1,2-d]furan ([1,2]BNF), benzo[b]naphtha[2,1-d]furan ([2,1]BNF) and benzo[b]naphtha[2,3-d]furan ([2,3]BNF) in the rock extracts range from 12.01% to 52.58%, 32.61% to 75.21% and 10.27% to 52.43%. The wide range of values recorded for the three isomers of benzo[b]napthofurans in the samples suggest source rocks formed from mixed organic matter. Among the phenyldibenzofuran isomers, 4-phenyldibenzofuran was the most abundant while 1-phenyldibenzofuran was the least. Phenyldibenzofuran ratio-1 (PhFR-1) and phenyldibenzofuran ratio-2 (PhFR-2) values range from 0.13 to 1.20 and 0.11 to 2.11, respectively. The results showed that the relative abundance of PhFR-1 and PhFR-2 increase gradually with increasing burial depth and maturity (VR0 ≤0.77%, MPI-1 ≤0.62, Tmax ≤443 °C),and have a good correlations with calculated vitrinite reflectance, MPI-1 and maximum Temperature (Tmax).This range of values suggested immature to early mature source rocks. The source rocks were found to have shale and coal lithologies and deposited in a lacustrine/fluvial/deltaic settings within immature to early mature stages based on the distribution of dibenzofurans, phenyldibenzofurans and benzo[b]naphthofurans in the source rocks.This study showed that dibenzofurans, phenyldibenzofurans and benzo[b]naphthofurans were effective in determining the origin, depositional environment and thermal maturity of source rocks in Niger Delta basin, Nigeria.
Keywords Dibenzofurans · Benzo[b]napthofurans ·Source rocks · Niger delta · Gas chromatography-mass spectrometry
1 Introduction
Dibenzofuran (DBF) and its derivatives (alkylated homologues and benzo[b]naphthofurans (BNFs) have been discovered in a range of geological products, such as crude oils (Williams et al. 1986; Li and Ellis 2015), source rock extracts (Radke et al. 2000; Li and Ellis 2015), coal(Hayatsu et al.1975,1977;White and Lee 1980;Armstroff 2004) and coal tar (Borwitzky and Schomburg 1979).Several years ago,Radke et al.(2000)and recently,Li and Ellis (2015) performed a systematic investigation into the occurrence and distribution of dibenzofuran, alkyldibenzofurans and benzo[b]napthofurans. Radke et al. (2000),observed the preferential release of dibenzofurans at vitrinite reflectance of approximately 0.77%. The concentrations of alkyldibenzofurans are higher in organic matter from terrestrial sources compared to alkyldibenzothiophenes. Higher plant material deposition in lacustrine settings is attributed to their predominance(Radke et al.2000;Sephton et al. 1999; Li et al. 2013a, b, c).
The occurrence and distribution of DBFs rely significantly on the type and/or depositional setting of the source rock (Fan et al. 1990, 1991; Radke et al. 2000). For instance, DBFs appear to be abundant in the freshwater source rocks, terrigenous oils and coals (Fan et al.1990, 1991; Li et al. 2011, 2013a, b, c, 2014) while In marine shales and carbonates, sulfur-heterocyclic compounds like dibenzothiophenes (DBTs) are more abundant(Li et al. 2013a, b, c; Hughes 1984; Hughes et al. 1995).The relative abundance of alkyl-dibenzothiophene(ADBT)relative to alkyl-dibenzofuran was proposed to differentiate depositional conditions (Radke et al. 2000; Sephton et al.1999; Kruge 2000). Li et al. (2011) also observed that secondary migration processes also influence the absolute concentrations of DBFs in crude oils.The petroleum DBFs are therefore potential molecular markers indicating the distances of oil migration and the filling pathway(Li et al.2011, 2018; Ogbesejana et al. 2018b).
An immediate biological precursor for alkyl dibenzofurans is uncertain. Previous studies have proposed that tannins suggesting a polycondensed phenolic structure may be biological precursors (Born et al. 1989) or may derive from condensed and dehydrated polysaccharides (Sephton et al. 1999) by means of thermally controlled responses to aldol, retro-alderol or Diels–alder. Elevated abundances of DBF, DBT, and biphenyl in Permian rocks (East Greenland) are likely to be derived from woody plant phenolic lignin compounds, according to Fenton et al. (2007). Furthermore, the majority of natural products related to dibenzofuran are metabolites of lichens, or higher fungi.The lichen dibenzofurans tend to be formed by carbon–carbon oxidative coupling of orsellinic acid and its counterparts, according to Sargent and Stransky (1984).Therefore, Radke et al. (2000) proposed the prospective biomarkers for lichens being DBFs in crude oils and sediment extracts. However, according to Asif (2010), simulation studies and geological observations have shown that dibenzofuran can be formed by biphenyl and oxygen.Methyl-substituted biphenyls can also react to yield the correct methylated DBFs. Biphenyl-derivatives are often used in the laboratory as reactants for synthesizing dibenzofurans (Sargent and Stransky 1984).

Fig. 1 Niger Delta depobelts and sample locations (after Tuttle et al. 1999)
The phenyl derivatives of DBF were found in marine sedimentary rocks and hydrothermal petroleum (Marynowski et al. 2002), while sediment extracts and crude oils formed from lacustrine shale, marine shale, marine carbonate, and terrestrial (fluvial/deltaic/freshwater) mudstone were investigated for their occurrence and potential implications in petroleum organic geochemistry (Yang et al. 2017). Diagenetic/catagenetic oxidation of sedimentary organic matter in submerged sedimentary rocks at the redox interface and oxidizing pyrolysis of coal and coalderived materials are major sources of phenyl-substituted polycyclic aromatic hydrocarbons (PAHs) (Marynowski et al. 2002; Meyer zu Reckendorf 1997, 2000; Rospondek et al.2007,2009).Based on comparative experiments with ionic versus free-radical phenylation, it was proposed that free-radical PAH phenylation reactions must have formed these compounds (Rospondek et al. 2009). PhDBFs and phenyldibenzothiophenes(PhDBTs)usually occur together in sedimentary rocks and it is estimated that these two classes of compounds form under similar conditions(Marynowski et al. 2002). The distribution of PhDBTs has been reported to be influenced primarily by the maturity of organic matter. The geochemical applications of oxygenheterocyclic aromatic compounds, however, are far less than their sulfur counterparts (Li et al.2008,2012,2013a,b,c,2014;Fang et al.2016;Yang et al.2016).
Benzo[b]naphtho[d]furans were identified by authentic standards in fluids and source rocks and were the most abundant in coal and coal shales (Li and Ellis 2015). Such compounds have also been found in northern (Poland) and southern (Argentina) hemispheres bitumen from fluvialdeltaic siltstone and charcoal from Jurassic records of wildfires (Marynowski and Simoneit 2009; Marynowski et al. 2011). Currently, there is no specific underlying factors influencing the isomerisation of benzo[b]naphtho[d]furans,and the source of these compounds is still not clear. Nevertheless, these oxygenated compounds aresupposed to originate from terrestrial organic matter,which would explain their especially high occurrence in coal and coaly shales (Li and Ellis 2015). Li and Ellis (2015)identified three isomers of benzo[b]naphthofurans in crude oils and rock source extracts from Beibuwan and Tarim basins, China and suggested that the relative abundance of benzo[b]naphthofuran isomers, i.e. the benzo[b]naphtho[2,1-d]furan/{benzo[b]naphtho[2,1-d]fu-ran + benzo[b]naphtho[1,2-d]furan} ratio, could be a potential geochemical parameter for indicating oil migration pathways and distances. Ogbesejana et al. (2018b)reported dibenzofurans and Bezo[b]naphthofurans in the crude oils from Niger Delta and found that the concentrations of DBFs and BNFs in the oils were not affected by source facies, depositional environments and thermal maturity in most of the samples which showed that the concentrations must have been affected by other factors such as migration other than source facies and thermal maturity. However, in Niger Delta source rock extracts,dibenzofuran and its derivatives have not been reported.In the present study, the distribution and geochemical significance of dibenzofurans, phenyldibenzofurans and benzo[b]naphthofurans were investigated for the first time in the Niger Delta source rock extracts by gas chromatography-mass spectrometry (GC–MS).

Table 1 Summary of the Rock–Eval pyrolysis data for rock samples from Niger Delta

Fig. 2 Plot of S2 versus total organic carbon (TOC) for the source rocks from Niger Delta basin (modified after Peters and Cassa 1994)

Fig. 3 Plots of HI vs OI of rock samples from Niger Delta, Nigeria(After Van Krevelen et al. 1961)
2 Geological and stratigraphic setting
Niger delta is a sedimentary basin located in the Gulf of Guinea, West Africa re-entrant. Niger Delta’s sub-aerial part includes about 75,000 km2and extends from apex to mouth at about 200 km.The complete sedimentary prism is 140,000 km2, with a maximum stratigraphic thickness of approximately 12 km (Whiteman 1982). The stratigraphy of the thick sedimentary sequence is split into three lithostratigraphic units, namely the Akata, Agbada and Benin Formations (Short and Stauble 1967).
The uppermost unit, the Benin Formation ranging from oligocene to latest era, consists of continental/fluviatile deposits of up to 2500 m thick sands, gravels, and backswamps. The Agbada Formation of paralic, brackish to marine, coastal and fluvio-marine deposits underlies these.These are mostly interbedded sandstones and shale divided into coarsening upward ‘offlap’ cycles with minor lignite.Underlying this unit is the Akata Formation, which ranges from Paleocene to Miocene in age which primarily consists of overpressure shales deposited under full marine circumstances.
The deposits are divided into 6–7 east–west bound blocks corresponding to separate phases of developmental deltas from the oldest in the north to the youngest offshore in the south (Doust and Omatsola 1990). Each depobelt is thought to be a more or less independent unit in terms of sedimentation, structural deformation and the generation and accumulation of hydrocarbons (Evamy et al. 1978).There are available source rocks in the basin primarily in the reduced paralytic sequence (Agbada Formation) and upper strata of the ongoing marine shale(Akata Formation;Evamy et al. 1978; Ekweozor and Daukoru 1994). The Niger Delta’s hydrocarbon habitat is mostly the Agbada Formation’s sandstone reservoir where oil and gas are generally trapped in growth-related rollover anticlines.

Fig. 4 Summed mass fragmentograms of the m/z 168, 182, 196 and 218 showing the distributions of (a) dibenzofuran and methyldibenzofurans, (b) dimethyldibenzofurans (c) benzo[b]naphthofurans and(d) phenyldibenzofurans in representative source rocks from Niger Delta basin
3 Experimental
3.1 Samples
Twenty one rock samples were collected at depth ranging from 2079 to 2909 m from three wells in three oilfields located in the offshore Niger Delta basin, Nigeria. The sample locations are shown in Fig. 1.
3.2 Rock-Eval analyses
Rock–Eval analyzes were carried out using the Rock–Eval 6 analyzer. Using a unique temperature program, 100 mg of powdered rock sample was gradually heated to 850 °C.During the heating process, four distinctive peaks were achieved.S1which is the first peak,is hydrocarbon already present in the sample, which is mostly removed at about300 °C temperatures. The second peak, S2reflects hydrocarbons produced at temperatures between 300 and 650 °C by thermal cracking of kerogen, while the S3peak represents the CO2produced from the kerogen at the same moment that the hydrocarbons of S2are produced. The fourth peak, S4, shows the quantity of CO2generated at a temperature of about 850 °C by oxidation during combustion.OPTKIN software has been used to acquire kinetic parameters of pyrolysis. The parameters are S1, S2, S3,hydrogen index (HI), oxygen index (OI), S2/S3, manufacturing index(PI),PC percent,and Tmaxfor rock quality and maturation evaluation. Standard was run between the analyzes to guarantee that the produced information was reproducible and accurate.

Table 2 Peak identification of dibenzofuran compounds in the Niger Delta source rocks
3.3 Extraction
Rock samples were crushed into powder <100 mesh and extracted for 72 h in batches in a Soxhlet device with 400 ml dichloromethane and methanol (93:7, v:v). Rock samples have been separated into fractions of saturated and aromatic hydrocarbons using columns of silica gel/alumina chromatography eluted with n-hexane and dichloromethane:n-hexane(2:1,v:v),respectively(Li et al.2012).
3.4 GC-MS analysis
An Agilent 5975i gas chromatography(GC)equipped with an HP-5MS (5% phenylmethylpolysiloxane) fused silica capillary column (60 m × 0.25 mm i.d., × 0.25 μm film thickness) combined with an Agilent 5975i mass spectrometry (MS) were applied in the analyses of the saturate and aromatic fractions of the rock extracts. The operating conditions of the GC are as follows:the temperature of the oven was held isothermally at 80 °C for 1 min, ramped at 310 °C for 3 °C/min, and kept isothermal for 16 min (Li et al. 2012). Helium was used with a steady flow rate of 1.2 mL/min as carrier gas. The MS was operated at 70 eV in the mode of electron impact (EI), 250 °C ion source temperature and 285 °C injection temperature. In the respective mass chromatograms,the order of detection and elution of dibenzofurans, phenyldibenzofurans and benzo[b]naphthofurans were determined by comparing their mass spectra and comparative retention times with those reported in literature (Armstroff 2004; Radke et al.2000;Li and Ellis 2015;Yang et al.2017;Ogbesejana et al.2018b). The relative abundance was calculated in the corresponding ion chromatograms from integrated peak regions.
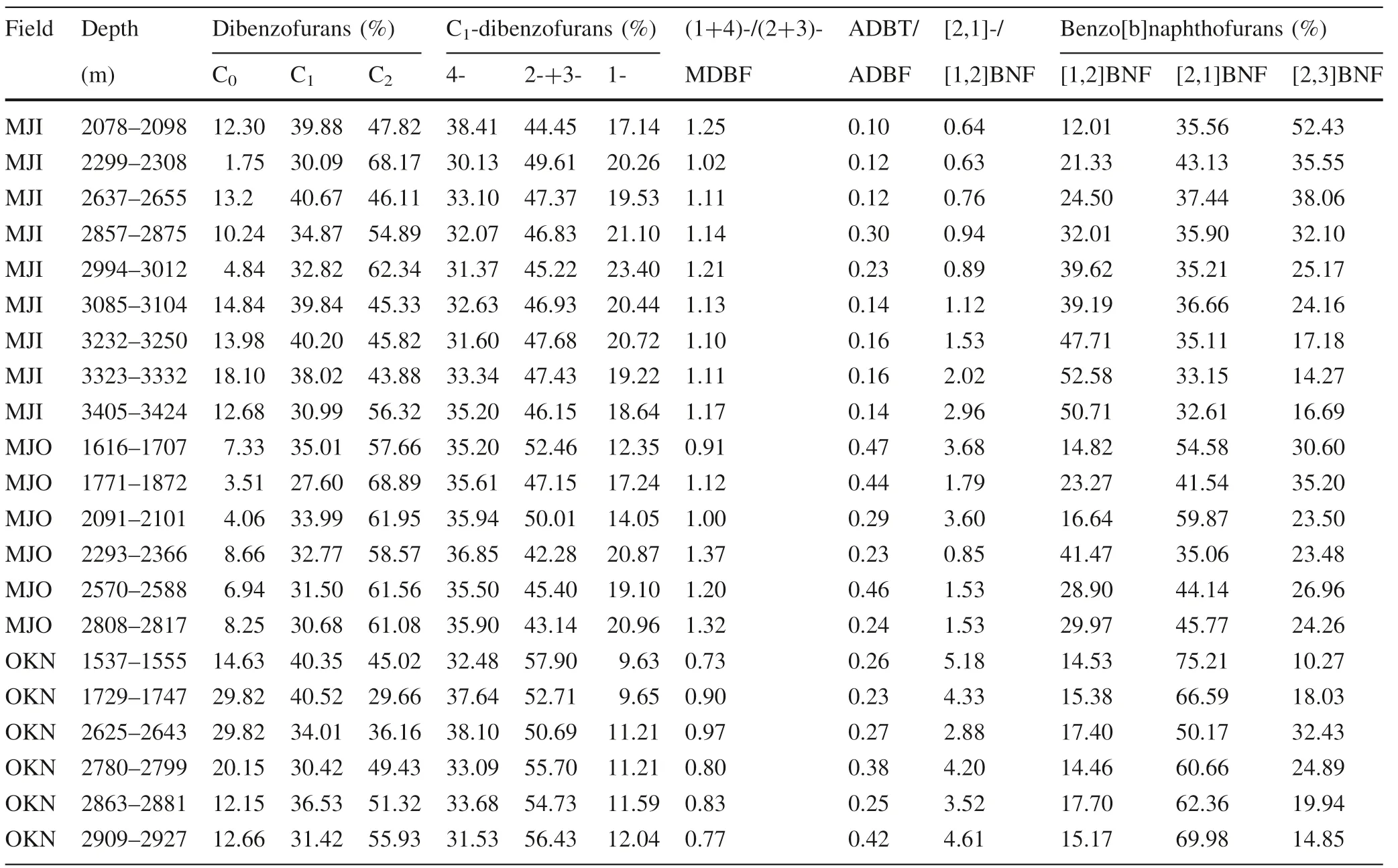
Table 3 Relative abundance of dibenzofurans and Benzo[b]naphthofurans and related parameters in rock samples from Niger Delta
4 Results and discussion
4.1 Summary of the source rock evaluation
Based on the Rock–Eval pyrolysis, the source rocks’hydrocarbon potential was evaluated. The data for the Rock–Eval evaluation are presented in Table 1. The Total organic carbon (TOC) and genetic potential (GP) values range from 0.63 to 3.77 wt% and 1.80 to 25.65 mg/g for the rock samples (Table 1). The TOC and GP values for potential petroleum source rocks exceeded the minimum limit values of 0.5 wt% and 2.0 mg/g (Tissot and Welte 1984; Killops and Killops 1993, 2005; Hunt 1996; Peters et al. 2005). The S2plot against TOC values classifies the source rocks as fair to very good source rocks (Peters and Cassa 1994) (Fig. 2). The hydrogen index (HI) values for MJI and MJO rock samples range from 38 to 140 mg/g TOC, whereas the HI values for OKN samples range from 389 to 560 mg/g TOC (Table 1), showing that MJI and MJO rock samples contained type III kerogen while OKN rock samples contained type II kerogen (Killops and Killops 1993;Peters et al.2005).OKN samples fall within the kerogen area of type II while MJI and MJO rock samples fall within type III kerogen on the hydrogen index (HI)versus oxygen index (OI) plots in Fig. 3. This shows that both oil and gas can be generated by the source rocks.The Tmaxand production index (PI) values range from 383 to 443 °C and 0.05 to 0.37, respectively (Table 1). These range of values indicate immature to early mature source rocks (Killops and Killops 1993; Peters et al. 2005).
4.2 Occurrence and distribution of dibenzofurans,phenyldibenzofurans and benzo[b]naphthofurans

Fig. 5 Cross plots of a ADBT/ADBF versus Pr/Ph (Radke et al. 2000), b (1+4)-/(2+3)-MDBF versus Pr/Ph (Yang et al. 2017)
The m/z 168,182,196,218 and 244 mass fragmentograms showing the distribution of dibenzofuran, methyldibenzofurans,dimethyldibenzofurans,benzo[b]naphthofurans and phenyldibenzofurans of representative rock sample from Niger Delta are shown in Fig. 4. The peak identities and relative abundances of identified compounds are listed in Tables 2,3 and 4,respectively.The relative abundances of dibenzofurans, methyldibenzofurans (C1-dibenzofurans)and dimethyldibenzofurans (C2-dibenzofurans) in rock samples vary from 1.75% to 29.82%, 27.60% to 40.52%and 29.66% to 68.89% respectively (Tables 3 and 4). C2-dibenzofurans dominate the dibenzofurans (Table 3,Fig. 4). Among C1-dibenzofurans, 2-+3-methyldibenzofuran is the most abundant in rock samples, whereas 1-methyldibenzofuran is often the least (Table 3). This distribution pattern was observed by Armstroff (2004)in a paleozoic coals from Pennine Basin, Central and Northern England and crude oils from Niger Delta basin, Nigeria(Ogbesejana et al. 2018b). This observation, however, is distinct from what was reported in Sakoa Basin, Germany(Radke et al. 2000) and Beibuwan Basin South China Sea(Li and Ellis 2015), whereby 4-methyldibenzofuran was the dominant compound among C1-dibenzofurans. Among C2-dibenzofurans, greater quantities of ethyldibenzofuran-1 (EDBF-1), dimethyldibenzofuran-3 (DMDBF-3) and dimethyldibenzofuran-6 (DMDBF-6) were identified(Table 4), while very small quantities ofdimethyldibenzofuran-1 (DMDBF-1) were present. In the source rocks benzo[b]naphthofurans were detected in elevated abundance (Fig. 4c). The relative abundance of[1,2]BNF, [2,1]BNF and [2,3]BNF in the rock extracts range from 12.01% to 52.58%, 32.61% to 75.21% and 10.27% to 52.43% (Table 3). The wide range of values recorded for the three isomers of benzo[b]napthofurans in the samples suggest source rocks formed from mixed organic matter (Cesar and Grice 2017; Ogbesejana et al.2018b). The relatively high values recorded for [1,2]BNF isomer suggest fluvial/deltaic depositional environment while elevated values observed in [2,1]BNF isomer indicate source rocks derived from marine depositional settings(Cesar and Grice 2017; Ogbesejana et al. 2018b). The abundance of [2,3]BNF isomer is high in most of the samples(Fig. 4,Table 3).It has been recently reported that the abundance of [2,3]BNF is usually higher in the source rocks compared to crude oils (Cesar and Grice 2017).However, Ogbesejana et al. (2018b) attributed the unusual high value of[2,3]BNF in one oil sample from Niger Delta Basin, Nigeria to high thermal maturity of the sample.
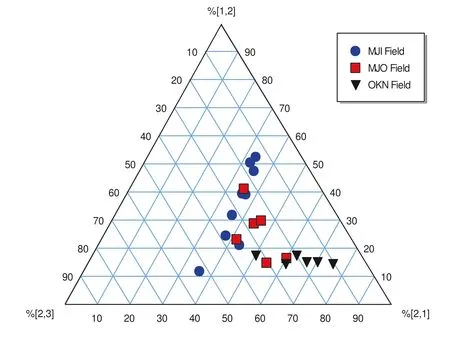
Fig. 6 ternary plots of benzonaphthofurans in Niger Delta source rocks (Cesar and Grice 2017)

Fig. 7 Plots of a phenyldibenzofuran ratio-1 (PhFR-1) and b phenyldibenzofuran ratio-2 (PhFR-2) against burial depths in Niger Delta source rocks

Fig. 8 Cross plots of PhFR-1 and PhFR-2 against MPI-1 in Niger Delta source rocks
Four phenyldibenzofuran (PhDBF) isomers, namely 1-,4-, 2-, and 3-PhDBF were identified in the m/z 244 mass chromatograms of the aromatic fractions of all the rock extracts. Among the four isomers of PhDBF, 4-PhDBF is the most abundant while 1-PhDBF occur as the least abundant (Fig. 4). This pattern of distribution has been reported in crude oils from Beibuwan, Tarim and Liaohe Basins in China and Termit Basin, Niger (Yang et al.2017).
4.3 Dibenzofurans and benzo[b]naphthofurans as source facies and depositional environments indicators
Source input and depositional environments can influence the distribution and abundance of dibenzofuran and its derivatives in geological materials (Fan et al. 1990, 1991;Radke et al. 2000; Yang et al. 2017; Li et al. 2018;Ogbesejana et al.2018b).Studies have shown,for instance,that oils from terrestrial/freshwater lacustrine settings contained more dibenzofurans than oils from marine/saline or brackish lacustrine settings. (Fan et al. 1990, 1991;Radke et al.2000;Chang et al.2011;Li et al.2013b).The values of alkyldibenzothiophenes/alkyldibenzofurans(ADBT/ADBF) and (1+4)-/(2+3)-MDBF computed for the rock extracts range from 0.10 to 0.47 and 0.73 to 1.37,respectively (Table 3). According to Yang et al. (2017),(1+4)-/(2+3)-MDBF values ≤1.4, ≤2.2 and >2.2 suggest lacustrine shale, fluvial/deltaic/lacustrine shale and marine carbonate, respectively while values ranging from 1.8 to 2.2 and 1.4 to 2.2 indicate marine shale and marine carbonate, respectively. Therefore, the values of (1+4)-/(2+3)-MDBF obtained for rock samples indicate source rocks formed from mixed origin and depositional environments (Radke et al. 2000; Yang et al. 2017). This finding is consistent with the results obtained on the cross plots of HI against OI in Fig. 3.The cross plots of ADBT/ADBF and (1+4)-/(2+3)-MDBF against pristane(Pr)/phytane (Ph) are shown in Fig. 5. These plots have been successfully applied to delineate crude oils and source rocks into different depositional environments and lithologies (Radke et al. 2000; Yang et al. 2017; Ogbesejana et al.2018b).The rock samples plotted in zones 3 and 4; and zones 2 and 3 on the plots of ADBT/ADBF and(1+4)-/(2+3)-MDBF against Pr/Ph, indicating shale and carbonaceous shale and coal lithologies for the rock samples and lacustrine/fluvial/deltaic/freshwater depositional settings(Fig. 5).This further support that the rock samples studied have mixed origin and deposited in lacustrine/fluvial/deltaic environment.
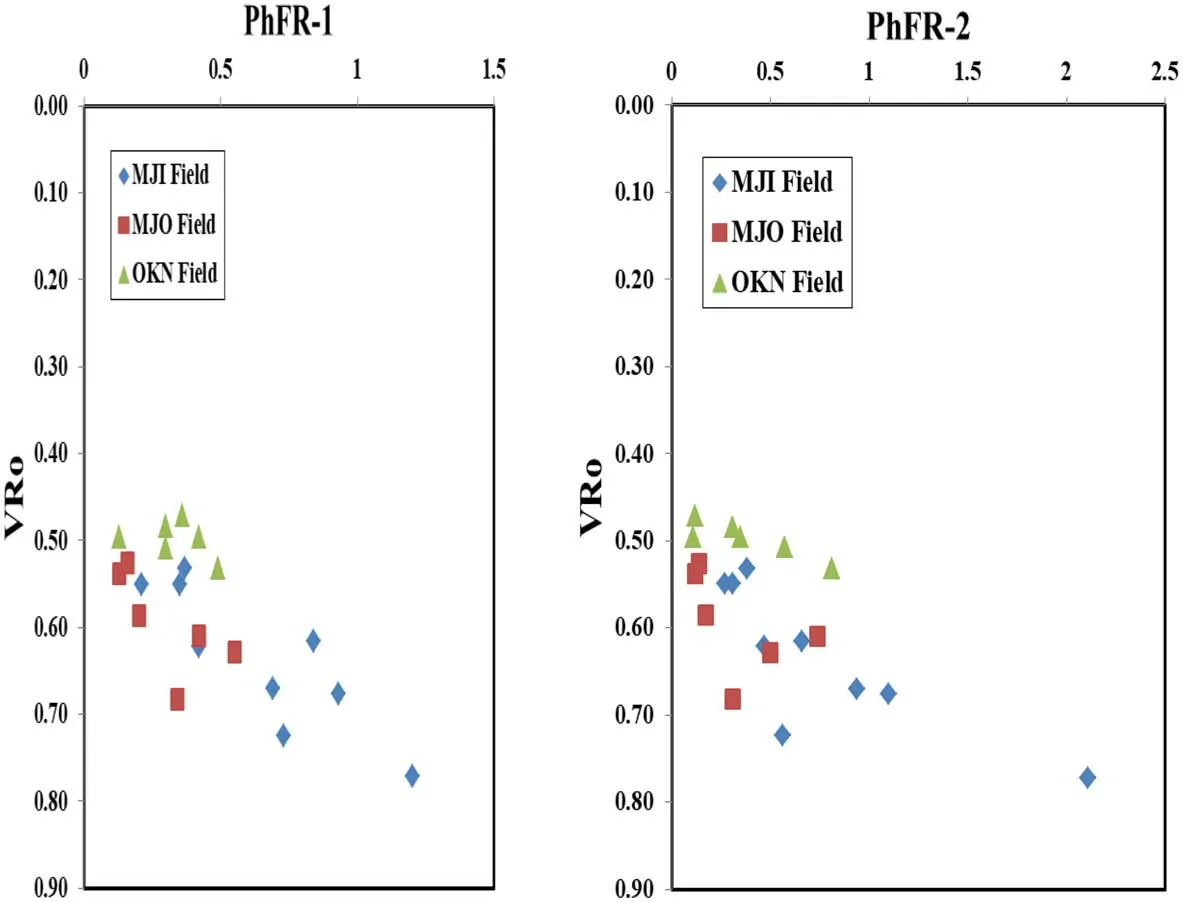
Fig. 9 Cross plots of PhFR-1 and PhFR-2 against VRo in Niger Delta source rocks
BNFs are potential indicators for source rock type.Previous studies have shown that the formation of[1,2]BNF is mainly controlled by clay catalysis; therefore the ratio[2,1]-/[1,2]BNF can be used to describe lithofacies(Cesar and Grice 2017). The [2,1]-/[1,2]BNF values that range from 0.9 to 1.5, 1.6 to 2.1 and 2.1 to 3.8 suggest fluvial/deltaic/claystone,Fluvial-deltaic to marginal marine silty claystone and lacustrine shale, respectively while values ≤7,>3.8 and ≤0.8 indicate fluvial/deltaic shale,marine carbonate and marine shale,respectively(Cesar and Grice 2017).In this study,the[2,1]-/[1,2]BNF values in the rock extracts range from 0.63 to 5.18 (Table 3). This relatively wide range of values recorded for the samples suggest source rocks of mixed origin (terrestrial and marine) deposited under marine/fluvial/deltaic environment.The ternary plots of BNF isomers (Cesar and Grice 2017)is shown in Fig. 6. The plot clearly support mixed origin and depositional environment for the rock samples already inferred from the ADBT/ADBF and[2,1]-/[1,2]BNF ratios in the samples. This is also consistent with the results obtained from the rock–eval pyrolysis (Fig. 3).
4.4 Phenyldibenzofurans as maturity indicators

Fig. 10 Cross plots of PhFR-1 and PhFR-2 against Tmax (°C) in Niger Delta source rocks
Thermal maturity is one of the secondary processes that affect the distribution and abundance of phenyldibenzofurans in geological materials.For instance,the abundance of 2-PhDBF and 3-PhDBF are relatively higher with significant predominance of 2-PhDBF over 4-PhDBF in source rocks with lower maturity (Yang et al. 2017), but with increasing maturity, report showed that the abundance of 2-PhDBF and 3-PhDBF relative to 4-PhDBF exhibited a general decrease (Yang et al. 2017). The thermodynamic stability of PhDBF homologues was evaluated by calculating their formation energies (ΔEf) and it was discovered that 4-PhDBF had the highest formation energies and that a substituent at the 4-position of PhDBF should be thermodynamically more stable than those at the 2-,3-,and 1-positions, respectively (Yang et al. 2017), and that the potential of their use as maturity indicators can be explored(Yang et al. 2017). Yang et al. (2017) identified four isomers (1-, 4-, 2- and 3-PhDBF) phenyldibenzofurans in crude oils and source rock extracts from Liaohe,Tarim and Beibuwan Basins in china and Termit Basin in Niger and observed that the ratios 4-PhDBF/2-PhDBF defined as PhFR-1 (phenyldibenzofuran ratio-1) and 4-PhDBF/(2-PhDBF+3-PhDBF) designated as PhFR-2 (phenyldibenzofuran ratio-2) increased with burial depths and maturity parameter(vitrinite reflectance)at the stage of oil window.Based on these observations,the authors proposed PhFR-1 and PhFR-2 as potential indicators of thermal maturity for mature sediments(Yang et al.2017).In this study,PhFR-1 and PhFR-2 values range from 0.13 to 1.20 and 0.11 to 2.11, respectively (Table 4). This range of values suggest immature to early mature source rocks. This result is consistent with previous reports on Niger Delta source rocks (Ogbesejana et al. 2018a, 2019). Figure 7 show the plots of these parameters against burial depths. It is observed that PhFR-1 and PhFR-2 show a general increase with increasing burial depths in the source rocks studied.In order to be firmly sure whether these parameters are affected by maturity, PhFR-1 and PhFR-2 were plotted against a well established maturity parameter, MPI-1, in Fig. 8. The results showed that the relative abundance of PhFR-1 and PhFR-2 increase gradually with increasing burial depth and maturity (VR0≤0.77%, MPI-1 ≤0.62,Tmax≤443 °C), and have a good correlation with calculated vitrinite reflectance, MPI-1 and maximum Temperature (Tmax) (Figs. 8, 9, 10), an indication that the parameters are influenced by thermal maturity. These two ratios,defined as phenyldibenzofuran ratio-1 and-2(PhFR-1 and PhFR-2), may be potential maturity indicators for sediments within immature to early mature stage in Niger Delta Basin (Figs. 9, 10).
5 Conclusion
The distribution and significance of dibenzofurans,phenyldibenzofurans and benzo[b]naphthofurans were investigated in the Niger Delta source rocks by Rock–Eval pyrolysis and gas chromatography-mass spectrometry.The source rocks in the Niger Delta basin were found to have potential to generate oil and gas based on the quality and quantity of organic matter. The maturity parameters computed from the rock–eval pyrolysis indicated that the source rocks have immaure to oil window maturity status.C2-dibenzofurans dominated the dibenzofurans. Among C1-dibenzofurans, 2-+3-methyldibenzofuran was the most abundant in rock samples, whereas 1-methyldibenzofuran was the least.The benzo[b]naphthofurans were detected in elevated abundance in the source rocks. Among the phenyldibenzofuran isomers, 4-phenyldibenzofuran was the most abundant while 1-phenyldibenzofuran was the least. The source rocks were found to be formed from mixed origin and have shale and coal lithologies deposited in a lacustrine/fluvial/deltaic settings within immature to early mature stages based on the distribution of dibenzofurans, phenyldibenzofurans and benzo[b]naphthofurans.This study showed that dibenzofurans, phenyldibenzofurans and benzo[b]naphthofurans were effective in determining the origin, depositional environment and thermal maturity of source rocks in Niger Delta Basin, Nigeria.
AcknowledgementsThe authors thank the Department of Petroelum Resources of Nigeria and Chevron Nigeria Limited for providing the crude oils and rock samples.A.B.Ogbesejana appreciate Cheng Quan and Zhao Jiang for their assistance in the laboratory works.This paper benefitted immensely from the constructive criticism of Prof. Meijun Li of the State Key Laboratory of Petroleum Resources and Prospecting, China University of Petroleum Beijing, China.
FundingThe authors gratefully acknowledge the State Key Laboratory of Petroleum Resources and Prospecting, College of Geoscience, China University of Petroleum, Beijing, China for granting A. B. Ogbesejana an international visiting research fellowship towards this research work.
Compliance with ethical standards
Conflict of interestThe authors declare that they have no conflict of interest.
杂志排行
Acta Geochimica的其它文章
- Germanium/silica ratio and trace element composition of Early Cambrian siliceous rocks in Keping:implications for the siliceous rocks’ formation and paleoenvironment interpretations
- In situ LA-ICP-MS analyses of mica and wolframite from the Maoping tungsten deposit, southern Jiangxi, China
- Mineralogy and geochemistry of pozzolans from the Tombel Plain, Bamileke Plateau, and Noun Plain monogenetic volcanoes in the central part of the Cameroon Volcanic Line
- Zircon U-Pb geochronology, whole-rock geochemical,and Sr-Nd-Pb isotopic constraints on the timing and origin of Permian and Triassic mafic dykes from eastern North China Craton
- Geotectonic significance of the Neoproterozoic ophiolitic metagabbros of Muiswirab area, South Eastern Desert, Egypt:constraints from their mineralogical and geochemical characteristics
- Contribution of Asian dust to soils in Southeast China estimated with Nd and Pb isotopic compositions
