Chemical compositions of sulfides in the porphyry Cu ores,Yangla Cu deposit, Yunnan, China: implication for ore genesis
2021-01-09XinfuWangBoLiZuopengXiangYanYueGuoTang
Xinfu Wang · Bo Li· Zuopeng Xiang · Yan Yue · Guo Tang,2
Abstract The Yangla Cu deposit is the largest ore deposit in the Jinshajiang polymetallic metallogenic belt, northwest Yunnan, China. There is no consensus on the genesis of the ore deposit owing to the limited studies on the chemical compositions of sulfides. This study used an electron probe micro-analyzer to constrain the chemical compositions of pyrite, chalcopyrite, molybdenite, and sphalerite in the porphyry Cu ore of the Yangla Cu deposit and compared them with the chemical compositions of sulfides in the skarn Cu ore.The trace element contents and their occurrences were used to estimate the metallogenic temperature and infer the genesis of the Yangla deposit.The results show that the sulfides in the porphyry Cu ores have variations of ore element concentrations relative to their theoretical values.Pyrite is depleted in S but elevated in Fe; chalcopyrite is depleted in Cu, Fe, and S; and molybdenite and sphalerite are enriched in S whilst depleted in Mo and Zn. The concentrations of the main metallogenic elements Cu, Fe, Mo, Zn, and S in the porphyry are generally lower than those in skarn, suggesting that the porphyry ore was formed in a moderate to moderate-high temperature metallogenic environment. The formation time may also be slightly later than that of the skarn Cu ore. Elements such as As, Co, Cu, Pb, Zn, Mo,Cd, and Ni mainly exist as isomorphic replacements and mineral inclusions in the sulfides of both porphyry and skarn Cu ores.The trace element features of sulfides in the two ore bodies show that the Yangla Cu deposit may be a composite super imposed ore deposit,and its formation has undergone the process of exhalative-sedimentary to skarnporphyry mineralization.
Keywords Sulfides · Trace elements · Chemical composition · Yangla Cu deposit · Yunnan
1 Introduction
The Yangla Cu deposit (YCD) is located in the Yangla Township, Deqin County, Yunnan Province of southwestern China(lat.28°52′–28°59′N,long.99°04′–99°07′E)(Du et al. 2017). Its tectonic location is in the central part of the Jinshajiang tectonic belt, between the Zhongzan-Zhongdian and the Changdu-Simao block. It is one of the most important copper deposits in the ‘‘Sanjiang’’ region(Jinshajiang, Lancangjian, and Nujiang), with an average copper grade of ~1% and prospective reserves of 150 million tons of ore (Zhu et al. 2015). It has attracted the interest of many scholars for its large Cu reserves,superior geological conditions, and great prospect potentials. Since the 1960s, many production and scientific research institutions have carried out a large number of geological exploration and scientific research work on it and achieved fruitful research in the ore deposit geology (Zhu et al.2009;Zhu 2011),ore-bearing strata(Zhu 2011,2012),orecontrolling structures (Wei et al. 2000; Yang et al. 2012a;Li et al. 2014a; Zeng et al. 2015; Chen et al. 2017), magmatic rocks (Lu et al. 2000; Wang et al. 2010; Yang et al.2011,2012b; Zhu et al. 2011a, b; Yang et al. 2013; Zeng et al.2018;Li et al.2020),fluid inclusions(Lu et al.1998;Yang 2012, 2014b; Chen et al. 2013a; Du 2017; Li et al.2018), isotope (carbon-hydrogen–oxygen-sulfur-lead)geochemistry(Pan et al.2000a;Yang et al.2012c,d;Chen et al. 2013b; Chen 2013; Du et al. 2017; Xie 2018; Wang et al.2020),and genesis of ores(Pan et al.2001;Zhu 2011;Yang 2012;Liu 2014;Zhu et al.2015;Hu 2015;Du 2017).However, there remains controversy in the metallogenic mechanism and genetic type of the ore deposit. This controversy of the genesis of the deposit is mainly concentrated on the SEDEX-type(Pan et al.2000b;2001)and the skarn-type (Chen et al.2013a, b; Chen 2013; Yang et al.2014a,b;Meng 2016,Du et al.2017,2019),porphyry-type(Chen et al.1999;Wei et al.2000;Liu 2014;Hu 2015),and composite superposition-type (Qu et al. 2004; Zeng et al.2015; Li et al. 2018). In addition, the previous determination of the metallogenic temperature was determined mainly by the gangue minerals, such as garnet, quartz,epidote, and diopside fluid inclusions (Chen et al. 2013a;Yang et al. 2014b; Du 2017; Du et al. 2019), and not directly determined by the sulfides geochemical characteristics. The occurrences of the trace elements in sulfides of Cu ore are also unclear. Now, the mineralized porphyry plutons and porphyry type Cu ore bodies have been discovered during the prospecting process,which has enriched the understanding of the metallogenic theory of the YCD.It can provide a favorable opportunity to study porphyry and skarn mineralization in YCD.Studies have shown that metal sulfides can be formed throughout the entire mineralization process of metal sulfides deposits,and directly or indirectly record the information of mineralization physicochemical conditions (Yang et al. 2014a). Some trace element characteristics can indicate mineralization temperature, the forms occurrences of the trace elements,and the genetic type of ore deposits (Yang et al. 2014a),and then the process of mineralization can be reversed to determine the genesis of the ore deposit.
In this paper, we used the electronic probe micro analysis (EPMA) to determine and analyze the trace element contents of sulfides (pyrite, chalcopyrite,molybdenite,and sphalerite)in porphyry Cu ore of YCD,and compared them to previously published data of the trace element contents of the sulfides (pyrite, chalcopyrite, molybdenite, and sphalerite) in skarn Cu ore (Zhu 2011; Yang 2012; Chen 2013). These new data further constrains the metallogenic temperature environment, the form occurrence of trace element, and the genetic type of the YCD.
2 Geological background
2.1 General background
The ‘‘Sanjiang’’ metallogenic belt (Sanjiang: Jinshajiang,Langcanjiang, and Nujiang), in the southeast Tibetan Plateau and northwest Yunnan, forms part of the Tethyan tectonic belt and represents one of the most important polymetallic belts in China, hosting various types of deposit, especially, porphyry-skarn Cu ± Au ± Pb–Zn deposits (Jia et al. 2020). The YCD is located in the Jinshajiang tectonic belt of the central Sanjiang region(Fig. 1A), sandwiched between the regional Jinshajiang and Yangla Faults, which have an approximately NS orientation (Fig. 1B). The Yangla Cu mineralization is associated with Triassic granodiorite plutons (~230 Ma) that intruded during this post-subduction stage(Jia et al.2020).In this region (Jinshajiang-Ailaoshan Suture), the base of the Yangtze continental plate has experienced multiple geologic transformations due to extension, rift-sag, subduction,and collision,which have resulted in the formation of multiple arc-basins (Zhu 2011; Chen 2013). It has created a complex structural skeleton system in the region,which provides favorable space conditions for the magmatic emplacement, migration, and enrichment of oreforming hydrothermal fluids. There are two dominant stages of intermediate to felsic intrusive activity and associated mineralization occurs in the Jinshajiang-Ailaoshan suture zones in responses to geological events during the evolution of the Jinshajiang-Ailaoshan Tethys Ocean and left-lateral movement of the Ailaoshan-Red River shear zone (Hou et al. 2007). The first stage of intrusions was associated with ca.250–260 Ma subductionrelated magmatism and post-subduction magmatism(Deng et al. 2014). The second episode of intrusions and related mineralization in the Jinshajiang-Ailaoshan suture zones is related to the Eocene–Oligocene (~35 Ma) alkali-rich porphyry belt along the Jinshajiang-Ailaoshan strike-slip fault (Mao et al. 2017). The outcropping strata in the Jinshajiang suture zones mainly include the Upper Proterozoic(Pt3), Paleozoic Paleozoic (Silurian, Devonian, Carboniferous, and Permian), Mesozoic (Triassic), and Cenozoic(Ternary and Quaternary) and the exposed lithology is mainly marble,sandy slate,quartzite,and schist(Zhu 2011;Yang 2012; Chen et al. 2013a; Chen 2013). The Devonian is an important ore-bearing horizon of the YCD,which has mineralized with Cu, Pb, Zn, Au, and Ag in the contact zone and near the medium-acid intrusive plutons (Gao et al. 2010; Li et al. 2010). The structure was mainly dominated by a series of NS orientation linear folds and faults, which control the sedimentary construction, metamorphism, magmatism, and related minerals in the region,and the secondary and derived ‘‘λ’’ type faults control the spatial orientation and output morphology of ore bodies.Both intrusive rocks and volcanic rocks are exposed in the area, and the intrusive rocks are mainly granodiorite,masanophyre, and granite porphyry, which are distributed laterally along the deep and major fault zones and belong to Jiaren granite belt.The volcanic rocks are mainly basalt,hornhill andesite,and diorite,and the rocks are enriched in amphibole and mainly distributed between Yangla and Jinshajiang Fault. The intrusive rocks and volcanic rocks constitute the magmatic rock belts distributed in the near NS-direction, which control the spatial distribution of Cu polymetallic minerals in the Jinshajiang suture zones.

Fig. 1 A Tectonic map of the Sanjiang region(SW China),showing the major geological terranes,suture zones,volcanic belts,and locations of the major ore deposits (modified from Zhu et al. 2015); B Geologic map of the Yangla Cu deposit showing the distribution of the major orerelated intrusion, faults, ore block,and orebodies (modified from Li et al. 2014a). 1. Gajinxueshan Group; 2. Silurian; 3. Devonian; 4.Carboniferous Beiwu Formation; 5. Tertiary; 6. Granodiorit; 7. Granitic Porphyry; 8. Monzonite granite; 9. Quartz—diorite; 10. Hercynian(Ultrabasic rock); 11. Orebodies; 12. Faults; 13. Geological Boundary; 14. Ore block
2.2 Geology of the Yangla Ore Deposit
The Jinshajiang suture zone is the northernmost segment of the Jinshajiang-Ailaoshan suture and is located to the east of Simao terrane and the west of the Zhongza block(Fig. 1A).With aresource of 150 Mt@1 %Cu,Yangla is the largest Paleozoic (~230 Ma) copper deposit in the central part of the Jinshajiang suture zone (Meng et al.2016; Jia et al. 2020).The YCD is bounded by the Jinshajiang Fault to the east and the Yangla Fault to the west (Fig. 1B) (Du et al. 2017). There are seven main ore blocks(Beiwu,Nilv,Linong,Jiangbian,Lunong,Tongjige,and Jiaren),with the Linongore block being the largest one and accounting for 90 % of the Yangla total Cu metal reserve (Du et al. 2017; Wang et al. 2020). The exposed strata of the districtmainly consist of Silurian quartzite,marble with schist, Devonian marble, quartzite, sericite sandy slate, and Carboniferous basalt. The ore-bearing strata are mainly composed of the Devonian Jiangbian Formation (D1j) and the Linong Formation (D2+3l), while the ore-bearing lithology is dominated by diopside garnet skarns, followed by marble, quartzite, sericite sandy slate,granodiorite,and granitic porphyry.The YCD is controlled by structure/fracture.In addition to the Jinshajiang and the Yangla Faults, the F4 faults run in the NE-trending and a large number of NS-trending interlayer fracture zones and secondary structural fractures have also developed (Zhu 2011;Li et al.2014a).The contact zones/fractures between the magmatic intrusions and the surrounding wall rocks(marble, quartzite, and sericite sandy slate) controlled the morphology of skarn ore bodies (Wang et al. 2020).
Magmatic rocks are widely distributed within the Yangla mining district, which nearly NS-trending in the western part of the Jinshajiang tectonic zone. Extrusive rocks, intrusive rocks, and dyke rocks all have developed.Extrusive rocks mainly consist of andesites and basalts(362 and 296 Ma)and appear to have little association with ore formation(Lu et al.2000).Intrusive rocks were mainly formed during the Indosinian Period (208–239 Ma, and it is concentrated between 227 and 238 Ma, with an average of 230 Ma) are mainly granodiorites intrusion and are spatially related to the ore deposits (Wang et al. 2020).From north to south,the intrusive rocks can be divided into the Beiwu, Linong, Lunong, and Jiaren plutons. Each granodiorite pluton intrudes into the overlying Devonian marble, quartzite, and sericite sandy slate. Four plutons along the west side of Jinshajiang assume a linear distribution, forming the NS-oriented granitic belt (Zhu et al.2011a). The Linong granodiorites pluton, which is located in the central part,is the most fertile pluton in the YCD.It extends 2 km along the S–N direction and1.5 km along the E-W direction, is exposed over about 2.64 km2and forms an elliptical shape (Chen et al. 2013a, b). Dikes mainly consist of diabase dikes (~222 Ma) (Wang et al. 2010)and fine-grained granitic dikes,appearing as irregular dikes and stockworks filled along joints and fractures(Zhu 2011;Chen et al.2013a,b;Du et al.2017;Wang et al.2020).The wall-rock alteration mainly consists of skarnization, hornfels,silicifcation,carbonatization,and supergene alteration(Du et al. 2017, 2019; Jia et al. 2020).
For the ore genesis, Even though many studies over the past two decades arguing that the Yangla deposit belongs to a Triassic intrusion-related Cu skarn system (Zhu et al.2015; Meng et al. 2016; Du et al. 2017, 2019), the relationship between the granitic magma and the Cu mineralization and ore genesis remain controversial.Some authors argued that the deposit is of a sedimentary exhalative(SEDEX)-type based on fluid inclusion,geochemistry,and C-O isotope studies (Lu et al. 1999; Pan et al.2000a, b, 2001). But more scholars consider it as a skarntype Cu deposit based on skarn, ore-related granitic intrusion, fluid inclusions, C–H–O–S–Pb isotope geochemistry,hydrothermal alteration, and corresponding mineral assemblages (Zhu et al. 2015; Meng et al. 2016; Du et al.2017, 2019; Xie 2018; Jia et al. 2020). Previous studies have shown that the hypogene ore minerals are dominated by chalcopyrite, pyrite,and pyrrhotite, with small amounts of bornite, galena,sphalerite, molybdenite, and magnetite(Chen et al. 2013a; Yang et al. 2014a; Du et al. 2019; Jia et al. 2020). The gangue minerals are garnet, diopside,epidote,actinolite,chlorite,quartz,and calcite(Yang et al.2014a; Du et al. 2019; Jia et al. 2020). The hydrothermal alteration mainly consists of skarnization, silicifcation,carbonatization,and supergene alteration.The ore-forming fluids show an evolutionary trend from high temperaturesalinity-capture pressure to low temperature-salinity-capture pressure corresponding with the early skarn stage to the late calcite-sulfides stage (Chen et al. 2013a; Yang et al. 2014b; Du 2017; Du et al. 2019). Therefore, they suggested that the Yangla deposit is an ore-related granitic intrusion typical skarn-type copper deposit in the Jinshajiang suture zones. However, with the deepening of prospecting and exploration work in recent years, some scholars realized that there may still be porphyry mineralization in the Yangla mining district (Chen et al. 1999;Hu 2015; Li et al. 2020; Wang et al. 2020). Wang et al.(2020) further confirmed that the Yangla deposit has obvious porphyry mineralization, which is significantly different from the skarn mineralization (such as fluid inclusions,H–O–S–Pb isotope geochemistry,hydrothermal alteration, mineral assemblages and metallogenic mechanism). However, considering the characteristics of the porphyry pluton which are currently revealed, the Yangla deposit lacks typical porphyry alteration zones. The minerals are mainly chlorite,sericite,kaolinite,quartz,calcite,pyrite, and chalcopyrite, which is different from skarn mineralization (such as garnet, epidote, diopside, and magnetite) (Li et al. 2020).

Fig. 2 The no. 25 prospecting line profile map in the Linong ore block, Yangla Cu deposit, Yunnan, China (Li et al. 2020)
The stratiform,stratoid,or lenticular skarn Cu orebodies are distributed in the skarn zones, within the contact between the granitoid and host rocks, or within distal interformational fracture zones (Fig. 2). The molybdenite Re–Os isochron age is 233–230 Ma(avg.230 Ma)of skarn Cu orebodies (Yang et al. 2011; 2013; Zhu et al. 2015).The major Linong ore block comprising orebodies KT2,KT4, and KT5 contains predominantly skarn ores (avg.1.03 % Cu; Zhu et al. 2015; Du et al. 2017, 2019). The skarn Cu orebodies are generally dipping 5°to 45°to the northwest in metasomatic contact with marble, and are featured by sulfide veinlets and minorskarn fragments.The wall-rock alteration consists predominantly of skarnization,hornfels, silicifcation, carbonatization, and supergene alteration(Du et al.2017;Jia et al.2020).The hypogene ore minerals mainly dominated by chalcopyrite, pyrite, pyrrhotite, and magnetite, with small amounts of bornite,galena,sphalerite,and molybdenite.Some of the hypogene ore minerals are altered tomalachite, limonite, and azurite,and occurred mainly at the surface or in the shallow subsurface (Du et al. 2017, 2019;Jia et al. 2020). The gangue minerals are quartz, calcite, garnet (andradite/-grossular),pyroxene (diopside/hedenbergite), and epidote (Du et al.2019; Jia et al. 2020; Bian et al. 2020).
The porphyry type Cu ore bodies of the YCD mainly occur as vein-like distributions at depths of 3250 m within the Linong ore block (Fig. 3). The host rocks are granite porphyry and the ore body exhibit widths of 1–5 m and lengths of 20–300 m. The porphyry-type Cu ore bodies occur primarily in the NE-trending fault zone as granitic intrusions, with a general strike of 60° towards NE, a NW dip of ~40°, and with Cu-bearing grades of 0.2–1.2%(Table 1). The alterations in the ore-related porphyry intrusion mainly dominated by silicification, carbonatization, and metal mineralization, and the corresponding minerals are mainly quartz, calcite, pyrite, chalcopyrite,sphalerite, and molybdenite. The wall rocks constitute of the Devonian Liong Formation comprising quartzite,marble, and sericite sandy-slate, with the contact zone exhibiting strong silicification,sericitization,chloritization,carbonation, and argillization.
The porphyry ores are grayish to off-white in color,with euhedral-anhedral granular, residual porphyritic–porphyritic, cataclastic, and interstitial textures, and massive dense structures, which can also be disseminated, stockwork, banded, and nodular (Fig. 4A–C). The ore minerals mainly include pyrites, chalcopyrites, molybdenites, and sphalerites (Fig. 4D–O), while the gangue minerals are chiefly quartz and calcite (Fig. 4J–L). Both types of minerals show fine veins with stockwork and disseminated structures, while a large number of fine veins, scattereddisseminated chalcopyrite and pyrite, and a small amount of sphalerite have developed in the quartz vein. The chalcopyrite cuts across and replaces the pyrite, which is then intersected and replaced by sphalerite (Fig. 4M–O).Accordingly, the quartz-sulfide veins are replaced by the calcite vein (Fig. 4L).
According to their crosscutting relationship, the sequence of mineral formation in the porphyry type Cu oremay be indicated as molybdenite + pyrite + quartz→pyrite + chalcopyrite + quartz →sphalerite + quartz+ calcite. The characteristic features of each metal sulfide are as follows:

Fig. 3 The underground tunnel 3250 m section geology map in the Linong ore block, Yangla Cu deposit, Yunnan, China
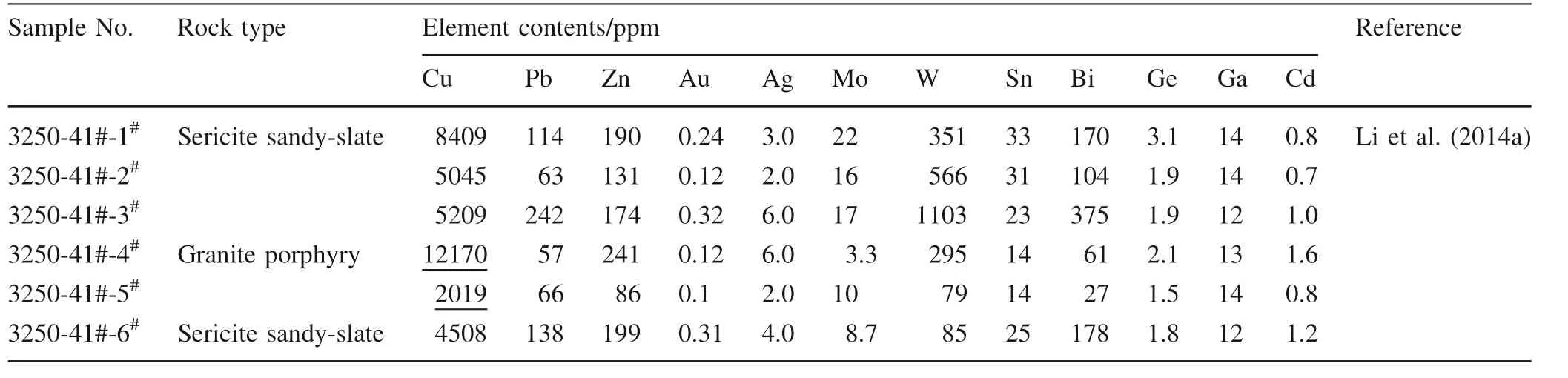
Table 1 Statistical of the mineralization elements in granite porphyry and wall rock, in the undergroundstope 41#, tunnel 3250 m section,Yangla Cu deposit, Yunnan, China
Molybdenite: light to dark gray-colored, developed within the quartz veins in the form of schistose-scaly,strips, radial, acicular, and irregular textures, partially coexisting with pyrite (Fig. 4D–F).
Pyrite: light yellow or yellowish-white sulfides, which filled the cracks in the early skarn minerals or as fine-veins and scattered-disseminated grains within the quartz veins,have cubic forms, and pentagonal dodecahedrons and euhedral-anhedral granular, irregular, and cataclastic textures (Fig. 4D, G, H, J–O). This sulfide shows multi-stage growth.
Chalcopyrite: brassy-yellow colored mineral, occurring as fine-grained disseminated, fine-veined, bays, anhedral granular, cataclastic, and irregular textures, and fill the cracks in the early minerals or coexist with the quartzsulfides veins that replace the early minerals (such as pyrite) (Fig. 4G–O).
Sphalerite: gray to dark gray, shows fine-grained,anhedral granular, fine-veins, and irregular textures, and develops within the quartz veins occurring outside pyrite and chalcopyrite. Sphalerite replaces the pyrite and chalcopyrite (Fig. 4M–O).
Based on the mineral assemblages and the interstitial or crosscutting relationships between the different minerals,and the various mineral formation sequence in skarn ore bodies reported by previous researches (Chen 2013; Du et al. 2019), we divided the metallogenic process of the YCD into three stages: (i) the skarn-porphyry, (ii)quartzsulfides, and (iii)the supergene (Table 2). The skarn-porphyry stage can further be divided into three stages: the anhydrous prograde skarn, the hydrous retrograde skarn,and the porphyry stages. Accordingly, during the first stage,a large number of anhydrous silicate minerals(garnet and diopside) were formed, while large formations of hydrous silicate minerals(actinolite,epidote,and chlorite),magnetite, pyrite, chalcopyrite, and molybdenite signify the hydrous retrograde skarn stage.The porphyry stage can again be separated into early, middle, and late stages. The early-stage was characterized by the presence of small amounts of molybdenite, pyrite, and quartz; the middle by pyrite, chalcopyrite, bornite, and quartz; and the late by sphalerite, quartz, and calcite. The quartz-sulfides period can be distinguished into early and late stages(Chen 2013),wherein the early stage represents the main metallogenic stage of the YCD, marked by the formation of a large number of sulfides such as chalcopyrite, pyrrhotite, pyrite,and quartz. Alternately, galena, sphalerite, calcite, and other gangue minerals were formed during the late stage.Finally,in the supergene stage,limonite and secondary Cu minerals like malachite and covellite formations replaced the primary pyrite and chalcopyrite.

Fig. 4 The porphyry Cu ores and minerals photos of Yangla Cu deposit, Yunnan, China. Mt-molybdenite; Py-pyrite; Ccp-chalcopyrite; Spsphalerite; Qz-Quartz; Cc-Calcite

Table 2 The metallogenic period and sequence diagram of minerals, Yangla Cu deposit, Yunnan, China (After Chen et al. 2013a)
3 Samples and analytical methods
The porphyry Cu ore samples were collected from the underground tunnel at 3500 meters section of the Linong ore block,YCD.At the same time,the data of the electron probe micro-analyzer(EPMA)of sulfides in skarn-type Cu ore were collected (Zhu 2011; Yang 2012; Chen 2013;Yang et al. 2012e, 2014a), and the trace element geochemistry of sulfides in both types of Cu ore bodies was compared.
The sulfides were analyzed by EPMA using a Shimadzu EPMA-1720H (Shimadzu Corporation, Japan) housed at the School of Geosciences and Info-physics(SGI),Central South University (CSU), Changsha, China. The standard operating conditions of the electron microprobe wereas follows: 15-kV accelerating voltage, 60-nA beam current,1-μm diameter electron beam, 18 °C ambient room temperature, and 10 % humidity. The peak and background count time of the elements were set at the 20 s and 10 s,respectively. The ten elements measured for pyrite, chalcopyrite, and molybdenite are Fe, Co, Ni, Cu, Zn, Pb, Sb,S, As, and Mo. Similarly, 14 elements were measured in sphalerite: Fe, Ga, Ge, Cu, Zn, Pb, Au, S, Sn, Sb, As, In,Mo, and Cd. The mineral and metal standards used for elemental calibrations included pyrite (S and Fe), chalcopyrite (S, Fe, and Cu), gallium arsenide (As), sphalerite(Zn), gallium arsenide (Ga), greenockite (Cd), indium antimonide(In),and herzenbergite(Sn),cobalt(Co),nickel(Ni), gold (Au), galena (Pb), molybdenite (Mo), chromite(Ge) and stibnite (Sb) (Liu et al. 2017; Shao et al. 2018).The resulting data as corrected by the atomic number (Z),absorption(A),and fluorescence(F)effects(ZAF)-method using proprietary Shimadzu software(Liu et al.2017).The detection limits are 0.01 wt% for Ni, 0.02 wt% for Cu, In,As, and S, 0.03 wt% for Zn, Fe, Cd, Co, Sb, Mo and Ga,0.04 wt% for Pb, and Ge, 0.06 wt% for Au and 0.07 wt%for Sn (Liu et al. 2017; Shao et al. 2018). The analytical errors are <1 % for Fe and S, and <2 % for As, Au, Co,Ni, Cu, Pb, Zn, Cd, Ga, Ge, Sb, Sn, and Mo.
4 Results
Tables 3 and 4 list the representative trace element concentrations in pyrite, chalcopyrite, and molybdenite of the porphyry and skarn Cu ore bodies, respectively. Table 5 lists the representative trace element concentrations in sphalerite of the porphyry and skarn Cu ore bodies. Furthermore, the data of pyrrhotite in skarn Cu ores are also collected and listed in Table 6 (Zhu 2011; Yang et al.2012e; Yang et al. 2014a).
4.1 Pyrite
The S and Fe concentrations in the pyrite of the porphyry Cu ore (Pyp) range from 51.55 to 52.97 wt% (avg. 52.47 wt%) and 45.73 to 47.27 wt% (avg. 46.66 wt%), respectively (Table 3). Comparable ranges were obtained in the pyrite of the skarn Cu ore(Pys)at 51.66 to 53.67 wt%(avg.53.01 wt%)for S and 44.87 to 47.71 wt%(avg. 46.31 wt%)for Fe (Table 4). Arsenic and Mo concentrations(0.17–1.42 wt% and 0.55–0.70 wt%, respectively) were found to be higher in Pypthan those in Pys(As, 0.01–0.06 wt% and Mo, 0.02–0.71 wt%). On the other hand, from Tables 3 and 4, it can be observed that the concentrations of Co, Cu, Pb, Zn, and other elements in pyrite are similar in both porphyry and skarn Cu ores.When compared to the theoretical elemental concentrations of S and Fe in pyrite(where S = 53.45 and Fe = 46.55, Fe/S = 0.875 wt%) (Li et al. 2008), the S content is lower in the Pypand Pys,whereas Fe content is higher in porphyry, but similar in skarn. The avg. Fe/S ratio at 0.889 (0.887 to 0.894) in Pypis greater than the theoretical ratio, as opposed to the avg.ratio of 0.874 in Pys(0.856 to 0.913). Thus, the pyrite in the former shows a deficit S and rich Fe content, but the opposite relationship in the latter. The molecular formula of pyrite in Pypand Pysvary as Fe1.030S2to Fe1.032S2and Fe0.980S2to Fe1.049S2, respectively.
4.2 Chalcopyrite
The EPMA results for trace elements in chalcopyrite of the porphyry Cu ore (Ccpp) showed S, Fe, and Cu concentrations in the respective ranges of 33.59–34.46 wt% (avg.34.05 wt%), 29.95–30.57 wt% (avg. 30.30 wt%), and 33.53–34.30 wt% (avg. 33.84 wt%) (Table 3). These concentrations were found to be lower than their theoretical values in chalcopyrite (S = 34.56 wt%, Fe = 30.52 wt%,and Cu = 34.92 wt%)(Li et al.2008),thereby indicating a deficit. In the skarn Cu ore(Ccps),S varied between 33.96 and 35.64 wt% (avg. 34.78 wt%); Fe, 29.98–31.22 wt%(avg. 30.77 wt%); and Cu, 33.25–35.39 wt% (avg. 34.43 wt%) (Table 4). This ore body revealed deficit S and Fe content but is enriched in Cu. Higher concentrations of As and Mo occur in the Ccpp(0.15–0.18 wt% and 0.41–0.54 wt%, respectively) as compared to that of Ccps(0.01–0.2 wt% and 0.28–0.44 wt%, respectively). Both types of ore bodies exhibit similar Co, Pb, and Zn contents (0.01–0.06 wt%, 0.03–0.14 wt%, and 0.03–0.17 wt%, respectively in porphyry and 0.02–0.08 wt%, 0.02–0.16 wt%, and 0.05–0.19 wt%,respectively in skarn)in their chalcopyrite.The molecular formula for Ccppand Ccpsvaries asCu0.982Fe0.998S2to Cu1.010Fe1.029S2and Cu0.958Fe1.015S2to Cu1.009Fe1.030S2, respectively.
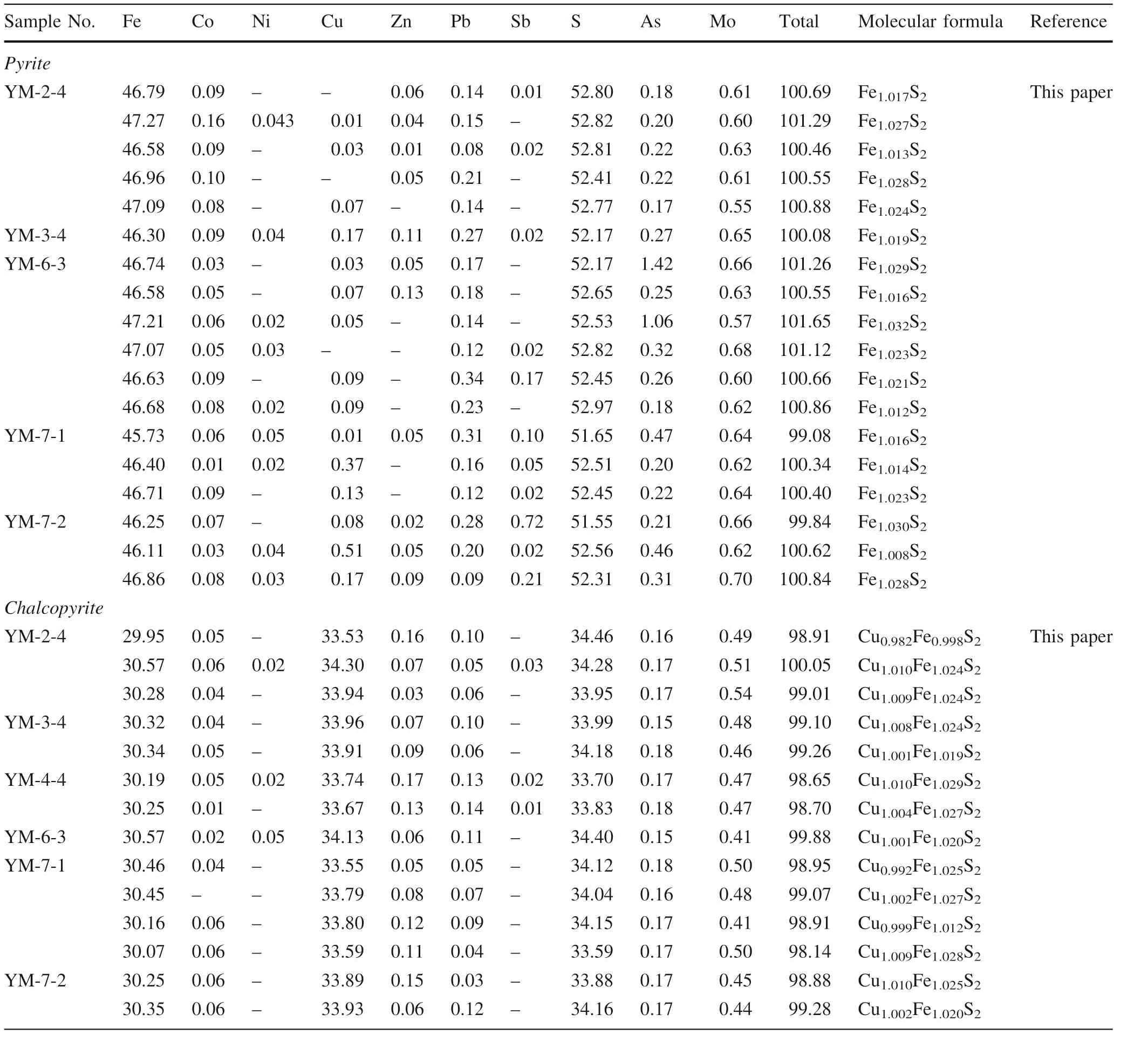
Table 3 The results of electron probe micro-analyzer (EPMA) (wt%) of sulfides, porphyry Cu orebodies, Yangla Cu deposit
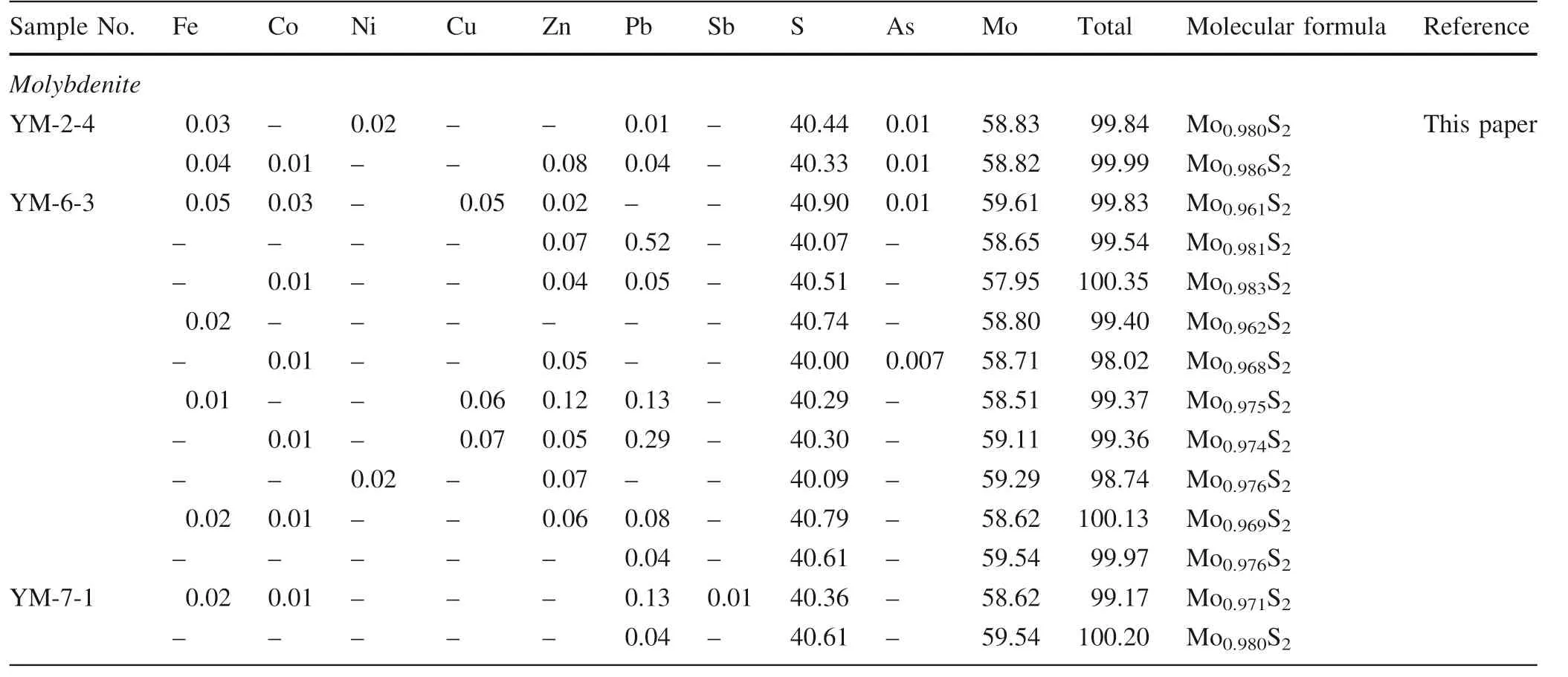
Table 3 continued
4.3 Molybdenite
Molybdenites in the porphyry Cu ore (Mtp) contain 40–40.79 wt%S(with an avg.concentration of 40.43 wt%)and 57.95–59.61 wt% Mo (avg. 58.95 wt%) (Table 3),while the skarn Cu ore (Mts) displayed ranges of 40.10–40.84 wt% S (avg. 40.47 wt%) and 59.63–60.28 wt% Mo (avg. 59.96 wt%) (Table 4). The theoretical elemental weight percentages of S and Mo in molybdenite are 40.06 and 49.94, respectively (Li et al. 2008). In comparison, the higher average concentration of S in the Mtpsignifies its enrichment, while the depleted Mo content characterizes a deficit. With respect to the Mts, S shows similar enrichment as in porphyry,whereas the Mo content is identical to its theoretical value.The As,Fe,Co,Ni,Sb,and Cu contents are relatively lower in Mtp. However, the Pb and Zn concentrations are higher at 0.01–0.52 wt%and 0.02–0.12 wt%,respectively,as opposed to 0.06–0.07 wt%in Mts. Based on the obtained compositions, the molecular formulae of Mtpand Mtsmay thus vary as Mo0.961S2to Mo0.986S2, and Mo0.976S2to Mo1.005S2, respectively.
4.4 Sphalerite
The Zn, S, and Fe concentrations in sphalerite of the porphyry Cu ore(Spp)varied as 42.37–61.78 wt%(avg.55.29 wt%),32.68–33.44 wt%(avg.33.01 wt%),and 2.57–10.31 wt%, (avg. 5.17 wt%), respectively. Likewise, the Cu content was obtained as 2.32–11.64 wt% (avg. 5.63 wt%);Pb, 0.01–0.10 wt% (avg. 0.04 wt%); Sn, 0.01–0.24 wt%(avg.0.13 wt%);Cd,0.28–0.45 wt%(avg.0.36 wt%);Mo,0.44–0.47 wt%(avg.0.45 wt%);Ga,0.05–0.11 wt%,(avg.0.08 wt%); and Sb, 0.03–0.38 wt% (avg. 0.13 wt%)(Table 5). In comparison, the sphalerite in the skarn ore(Sps) displays these trace element concentrations as: Zn,52.74–56.10 wt% (avg. 54.42 wt%); S, 32.91–34.37 wt%(avg.33.64 wt%);Fe 7.53–7.85 wt% (avg.7.69 wt%); Cu,0.28–3.21 wt% (avg. 1.75 wt%); Au,(0.24–0.59 wt% (avg.0.42 wt%); and Cd, 0.50–0.69 wt% (avg. 0.60 wt%)(Table 5).While Cu,Pb,Sn,and Mo contents are higher in the Spp, Fe, Au, and Cd are more in Sps. When compared with the theoretical concentrations of S and Zn in sphalerite(S = 32.90 wt%and Zn = 67.10 wt%)(Li et al.2008),S is greater, whereas Zn is lower in Sppand Spsalike, indicating enrichment of the former and a deficit of the latter.When the Fe content in sphalerite is more than 6%,then it is called marmatite(Li et al.2017).Hence,the sphalerite of the Yangla Spscan be characterized as marmatite. The sphalerite molecular formulae varied as Zn0.625Cu0.177-Fe0.178S to Zn0.927Cu0.036Fe0.045S in Spp, and as Zn0.79-Fe0.14S to Zn0.80Fe0.13S in Sps.
5 Discussion
5.1 Occurrence of trace elements in the sulfides
Previous studies have shown that a positive correlation between the major and trace elements in a mineralindicates an occurrence state of mineral inclusion,negative correlation implies isomorphism, while the absence of any correlation may indicate various forms(Bajwah et al.1987;Li et al. 2014b; 2019a). Therefore, for a better understanding of the occurrences of each trace element in the sulfides of the YCD,we carried out correlation analyses of some major and trace elements in pyrite, chalcopyrite,molybdenite, sphalerite, and pyrrhotite.

Table 4 The results of electron probe micro-analyzer (EPMA) (wt %) of sulfides, skarn Cu orebodies, Yangla Cu deposit
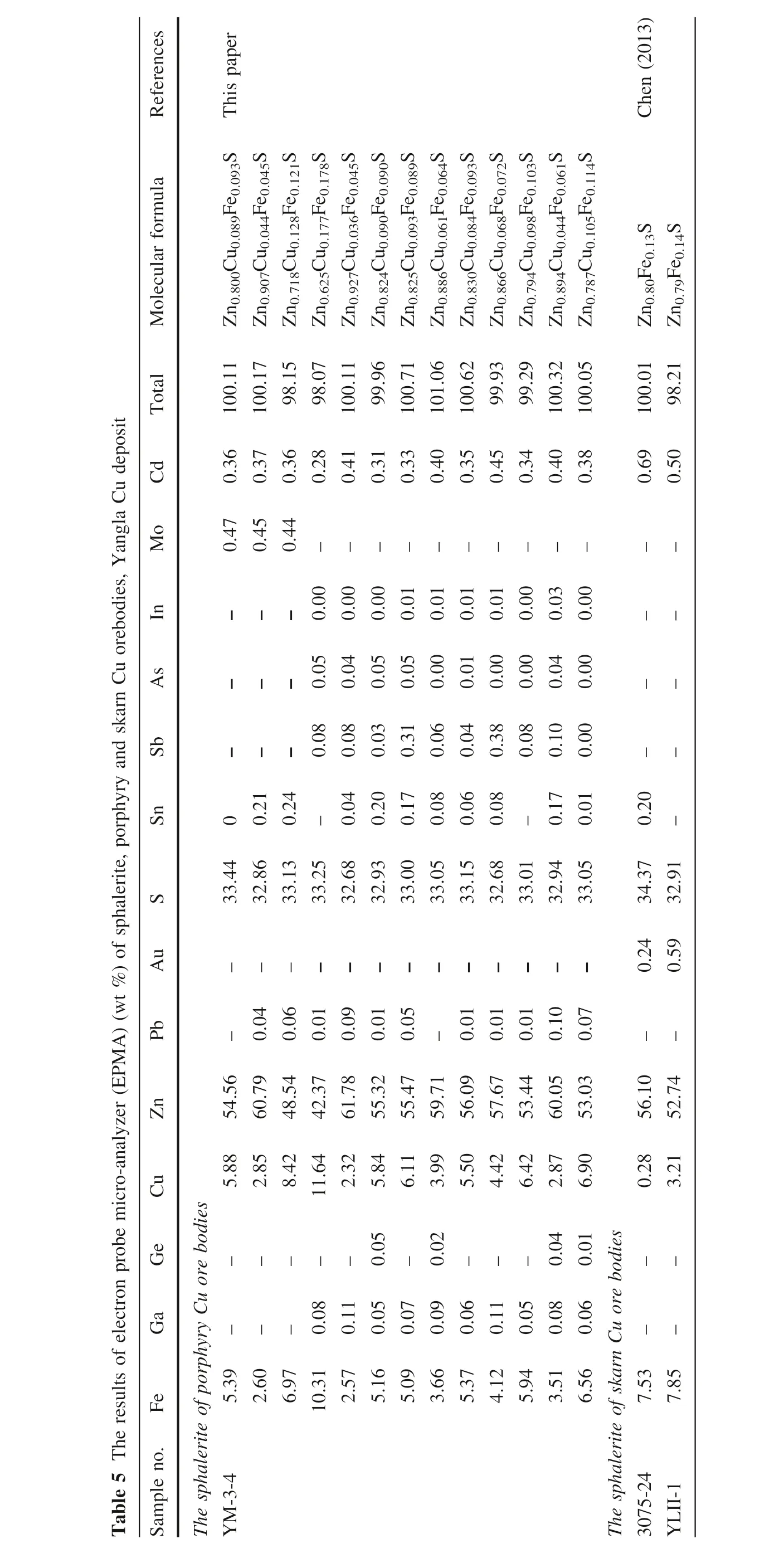

Table 6 The results of electron probe micro-analyzer (EPMA) (wt %) of pyrrhotine, skarn Cu orebodies, Yangla Cu deposit
In pyrite, Fe exhibits a negative correlation with the(Co + Ni + Cu + Zn + Pb + Sb + Mo) content in the porphyry Cu ore, against a positive relation in skarn as displayed in Fig. 5A.Figure 5B,D demonstrates that in the porphyry,Fe is positively correlated to both Zn and Co,as opposed to skarn, where it is positively related to Zn, but negatively to Co. The Pypalternately displays a negative trend of the relation between As and S,Ni and Fe,and Mo and Fe,while a relatively steady correlation with respect to As and S and positive relations between the others in the Pys(Fig. 5C,E,F).This indicates that trace elements such as Zn, As, Co, Ni, and Mo in the pyrite of the YCD may exist mostly in the form of isomorphism or as mineral inclusions, rather than a single occurrence state. Arsenic and Mo are particularly more likely to be enriched in the porphyry Cu ore.
In chalcopyrite,the major and trace elements exhibit the following relations:(a) the Zn and Fe,Zn and Cu,and Mo and Cu occurrences show negative correlations in the porphyry and skarn Cu ores alike (Fig. 6A, B, D); (b) As and S show a negative trend in the porphyry ore, but a relatively steady correlation in skarn(Fig. 6C);and(c) the Fe-(Co + Ni + Zn + Pb + Sb + Mo), and Cu- (Co +Ni + Zn + Pb + Sb + Mo) correlations are again negative in the porphyry,whereas positive in skarn(Fig. 6E,F).Hence, from these occurrence trends, it can be suggested that the trace elements such as Zn, As, and Mo may exist mostly in the form of isomorphism or mineral inclusions in the chalcopyrite of the YCD. Consequently, elements like As and Mo are possibly enriched in the porphyry Cu ore.
Likewise, the major and trace element correlations in molybdenite show negative trends between Pb and Mo,Zn and Mo, and Mo and (Fe + Co + Ni + Cu + Zn +Pb + Sb)(Fig. 7A–C)in the porphyry Cu ore.In the skarn Cu ore, there are negative relations between Zn and Mo,and Mo and (Fe + Co + Ni + Cu + Zn + Pb + Sb)contents. Thus, the trace elements like Pb and Zn mostly exist in isomorphism in the YCD molybdenite, with possible enrichment of Pb in the porphyry Cu ore.
Previous studies have shown that Fe2+,Cd2+,and Zn2+in sphalerite have similar ionic radii and thus can substitute each other as Zn2+↔(Fe2+, Cd2+), during the mineralization process(Cook et al.2009;Wu et al.2019;Ren et al.2019). Similarly, the correlation plots of Zn-Fe and Cd-Zn in Fig. 8A and B show that Zn has a positive correspondence with Fe and Cd in the sphalerite of the YCD,wherein the two elements isomorphically replace Zn. The Cu–Zn, Zn- (Fe + Co + Ni + Cu + Zn + Pb + Au +Sn + Mo + Cd), Ga-Fe, and Cd–Fe plots show negative correlation trends in both Spp(Fig. 8B–H) and Sps. The abundance of Cu (avg. 5.63 wt%, Fig. 8E) in sphalerite could be owed to its isomorphic replacement of Zn in the sphalerite crystal lattice (Li et al. 2014b). Likewise, Ge also replaces Zn in three ways, i) Zn2+↔Ge2+(Cook et al. 2009), ii) 2Zn2+↔Ge4++□(vacancy) (Cook et al.2015;Belissont et al.2016),and iii)3Zn2+↔Ge4++2Cu+(Wu et al. 2019). Given that no significant correlation could be found between Ge and Zn, and Ge and Cu in the sphalerite of the YCD, Ge mainly replaced Zn as per relation(i).Ga mainly replaced Zn by the way of 2Zn2+↔(Cu,Ag)++ (Ga,As,Sb)3+(Wu et al.2019).The Ga and Sb, Ga and Cu, and Sb and Cu display significant correlations in the sphalerite of the YCD, wherein Ga replaces Zn primarily as 2Zn2+↔(Cu, Ag)++ (Ga, As, Sb)3+.The obtained correlation patterns imply that trace elements such as Fe,Cu,Ga,Ge,Sb,and Cd in the Yangla sphalerite may mostly exist in the form of isomorphism.
In pyrrhotite, the Co and Fe, Mo and Fe, and As and S occurrences show positive correlations in the skarn Cu ores alike(Fig. 9A–C);Hence,these occurrence trends it can be suggested that the trace elements such as Co, Mo, and As may exist mostly in the form of mineral inclusions in the pyrrhotite of the YCD. Consequently, Mo is also possibly enriched in the skarn Cu ores.
5.2 Indication of metallogenic temperature
The concentration of S and Fe in all four types of sulfides were found to be higher in the skarn Cu ores of the YCD(Fig. 10A, B). The skarn ore also shows a higher abundance for Cu in chalcopyrite (Fig. 10C) and Mo in molybdenite, (Fig. 10D) as compared to their concentrations in porphyry ores. In terms of sphalerite, the Zn content is equivalent in porphyry and skarn (though much lower than the theoretical value of 67.10 wt%) (Fig. 10E);Cu is higher in porphyry,and Fe and Cd are higher in skarn(Fig. 10F). In addition, there is an overlap in the concentrations of each element or they vary uniformly (Fig. 10),indicating that the sulfides in the porphyry and skarn ores may have formed during different stages of the same mineralization process. The precipitation of metal sulfides from the ore-forming fluid consumes the ore-forming elements(S,Cu,Fe,Mo,Zn,Cd),and thus results in depletion of the concentrations of those elements in the hydrothermal system. Prior researches have suggested that the oreforming fluid in the YCD shows a decreasing trend of temperature,capture pressure,and salinity from the early to the late stage of the ore-forming process. The mechanism gradually changed from boiling of the ore-forming fluid in the early stage to cooling and unequal mixing in the late stage (Yang 2012; Chen et al. 2013a; Du 2017). While the ore-forming fluid composed dominantly of magmatic water in the early stage, mixing of meteoric water in the latestage resulted in dilution of the fluid and consequent reduction of the ore-forming temperature(Pan et al.2000a;Chen et al.2013a;Chen 2013;Yang et al.2014a).This has caused the metal sulfides formed in the late stage to have relatively lower trace elements contents as compared to those formed in the early stage, which reflects that the sulfides in the Yangla porphyry Cu ore were formed later and at lower temperatures than those of the skarn Cu ore.

Fig. 5 Diagrams showing relationships of the Fe–(Co + Ni + Cu + Zn + Pb + Sb + Mo), Zn–Fe, As–S, Co–Fe, Ni–Fe, and Mo–Fe of pyrite, Yangla Cu deposit, Yunnan, China

Fig. 6 Diagrams showing relationships of the Zn–Fe, Zn–Cu, As–S, Mo–Cu, Fe–(Co + Ni + Zn + Pb + Sb + Mo), and Cu–(Co + Ni +Zn + Pb + Sb + Mo) of chalcopyrite, Yangla Cu deposit, Yunnan, China
Cobalt and Ni can isomorphically replace Fe in pyrite and further form FeS2–CoS2and FeS2–NiS2solid solution series. High temperature is conducive to the progress of isomorphism, therefore, higher Co and Ni contents and Co to Ni ratio in pyrite reflects the higher temperature of mineral formation(Gong and Ma 2011;Yan et al.2012;Li et al. 2017; Fu et al. 2018). Accordingly, the Co and Ni concentrations, and Co/Ni ratios in pyrite in the Yangla porphyry ore range from 0.01–0.16 wt%, 0.043–0.05 wt%,and 0.5–4.0(avg.2.19),respectively;while in the skarn as,0.03–0.23 wt%, 0.02–0.09 wt%, and 1–22 (avg. 6.89),respectively. All of them may have formed in medium metallogenic environment temperatures.
When the chalcopyrite formation temperature is less than 200 °C, its composition is consistent with the theoretical chemical formula, n(Cu + Fe)/n(S) = 1. Whereas a higher formation temperature results in n(Cu + Fe)/n(S)>1(Li et al. 2008). In case of the YCD, the n(Cu +Fe)/n(S)of Ccppranges between 0.99–1.02(avg.1.01)and 0.96–1.04 (avg. 1.01) in the Ccps. Therefore, these higher average n(Cu + Fe)/n(S)ratios(i.e.,>theoretical unity)in the porphyry and skarn ores (Fig. 11) indicates that the formation temperatures of chalcopyrite in these ore bodies in the YCD are mostly >200 °C, possibly at medium temperature ranges of metallogenic environments.
The distribution coefficient of elements is constant at given temperature and pressure conditions, which means that the mineralization temperature can be estimated by using the distribution coefficient of trace elements in coexisting mineral pairs (Wen and Duo 2009). In the chalcopyrite (Ccp)-pyrite (Py) mineral pair, the contents of Co can be used as a geological thermometer to estimate the mineralization temperature, and the calculation formula is(Wen and Duo 2009):

where the distribution coefficientand (Co)Ccpand (Co)Pyare the concentrations of Co in chalcopyrite and pyrite,respectively. The Co content in chalcopyrite and pyrite in the Yangla porphyry ore ranges between 0.01–0.06 wt%(avg. 0.03wt. %) and 0.03–0.16 wt% (avg. 0.07 wt%),respectively, and the formation temperatures were calculated considering the respective avg. values of 0.03 and 0.07 wt. %. Accordingly, the equilibrium temperature of the formation of the chalcopyrite-pyrite pair in the porphyry ore was obtained as T = 185 °C, indicating that it was formed in a medium temperature mineralization environment. Similarly, the equilibrium temperature of chalcopyrite-pyrite formation in the skarn ore was calculated as T = 180 °C, based on avg. (Co)Ccpand (Co)Pycontents of 0.05 and 0.10 wt%,respectively(the respective ranges being 0.02–0.08 and 0.03–0.23 wt%). The equilibrium temperature thus obtained is comparable to the formation temperature in porphyry ores, i.e., the medium temperature range of the mineralization environment.
Studies have shown that molybdenite crystallizes from hydrothermal fluid, and is unlikely to form directly by magmatic differentiation, and/or metamorphic deposition(Wang et al.2012a,b),wherein temperature plays a pivotal role in its crystal and morphological characteristics.Therefore, the temperature conditions of molybdenite formation can be estimated using the mineral’s morphology and polytypic characteristics.Molybdenite has two types of polytypes: hexagonal two-layer (2H-type) and trigonal three-layer(3R-type),while 2H + 3R mixed type can also exist(Mao et al.1988;Wang et al.2012a,c;Wang and Liu 2013). Among them, the 2H and 3R-types are homogeneous polymorphic variants and are products of different thermodynamic conditions. Experimental results show that the 2H-type molybdenite is generally scaly and forms between 900 and 1300 °C; the 2H + 3R-type is generally scaly and fine-grained, forming between 700 and 800 °C;and the 3R-type is fine-grained, spherical, granular, acicular, or radial, and forms between 700 to 400 °C. At temperature slower than 400 °C, amorphous molybdenite forms as a dense or loose black powder(Shao 1984).As the temperature decreases, continuous material exchange occurs with the surrounding rocks, causing an increase in the concentration and types of impurities in the hydrothermal fluids. This corresponds to the polymorphic transformation of 2H-type molybdenite to 3R-type, where 2H contains lesser impurities than 3R (Huang et al. 1985).The molybdenite present in the quartz veins of the Yangla porphyry Cu ore is fine-grained, acicular, and radial, and contains minor concentrations of Fe (0.01–0.05 wt%), Zn(0.06–0.12 wt%), Pb (0.01–0.52 wt%), Cu (0.05–0.07 wt%),and Co(0.01–0.03 wt%)as impurities.However,the molybdenite in the Yangla skarn Cu ore shows fine granular, radial, and scattered occurrence in the mineral assemblages and quartz veins (Zhu 2012; Du 2017), with minor concentrations of Fe (0.02–0.05 wt%), Zn(0.06–0.07 wt%), Cu (0.01 wt%), and As (0.01 wt%) as impurities. Based on the crystal morphological characteristics of molybdenite in the Yangla Cu deposit and the concentrations of the impure elements, we argue that the molybdenite in the Yangla porphyry and skarn ore bodiesis most likely of 3R-type, which may have formed in a medium to high-temperature ore-forming hydrothermal environment (700–400 °C). It also indicates that the oreforming fluid itself was relatively enriched in Cu, Pb, Zn,and other ore-forming elements (Wang et al. 2012b, c).
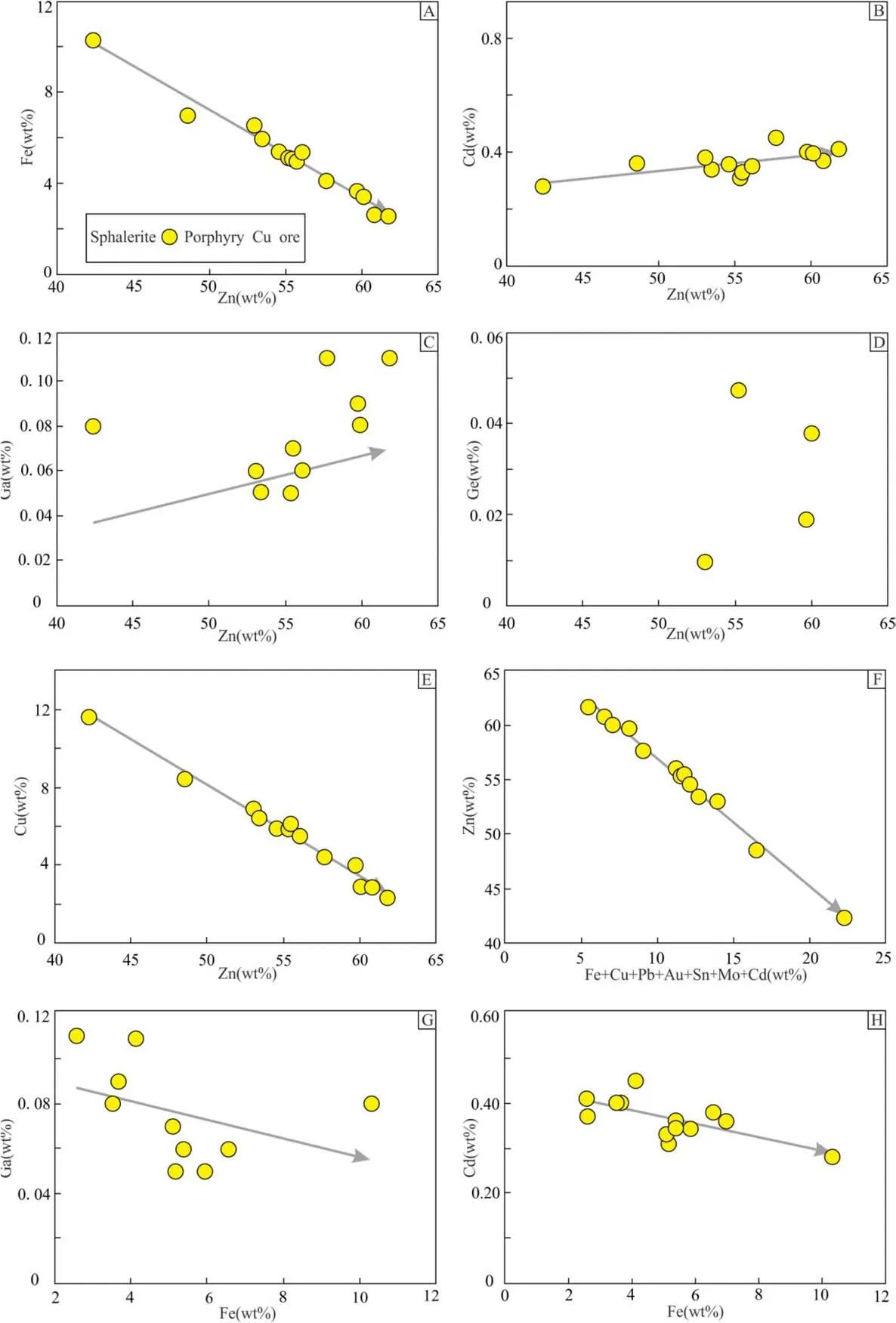
Fig. 8 Diagrams showing relationships of the Fe–Zn, Cd–Zn, Ga–Zn, Ga–Sb, Cu–Zn, Zn-(Fe + Cu + Pb + Au + Sn + Mo + Cd),Ga–Fe, and Cd–Fe of sphalerite, Yangla Cu deposit, Yunnan, China

Fig. 9 Diagrams showing relationships of the Co-Fe, Mo-Fe, and As-S of pyrrhotine, Yangla Cu deposit, Yunnan, China

Fig. 10 The comparisons values of major elements between porphyry and skarn sulfides in Yangla Cu deposit,Yunnan,China.Pyp = the pyrite of porphyry Cu ore; Pys = the pyrite of skarn Cu ore; Ccpp = the chalcopyrite of porphyry Cu ore; Ccps = the chalcopyrite of skarn Cu ore;Mtp = the molybdenite of porphyry Cu ore;Mts = the molybdenite of skarn Cu ore;Spp = the sphalerite of porphyry Cu ore;Sps = the sphalerite of skarn Cu ore

Fig. 11 The binary plots of the n(Cu + Fe)/n(S) chalcopyrite, Yangla Cu deposit, Yunnan, China
The composition of trace elements (such as Fe, Ga, Ge,In, and Cd) in sphalerite can effectively indicate the metallogenic temperature (Wu et al. 2019). In particular, a positive correlation exists between the Fe content and the temperature (Kullerud 1953; Yin and Hu 2004; Liu et al.2010; Li et al. 2017). The sphalerite formed under hightemperature conditions is dark and rich in elements like Fe,Mn, In, Se, and Tl, with small Ge/In and Ga/In ratios(<0.1) and Ge <5 ppm. On the other hand, Cd and Garich sphalerites with low In and Ge content (5 to 50 ppm)and a Ga/In ratio of 0.1 to 0.5 are normally formed at moderate temperatures (Wu et al. 2019). Low-temperature conditions form sphalerites that are light-colored, have high Ga,Ge(>50 ppm),and Ag contents,and Ga/In ratios between 1 to 100 (Liu et al. 1984; Cook et al. 2009; Ye et al.2011,2012;Gao et al.2016).The Ga and Cd contents in the Sppand Spsin the YCD occur in the ranges of 0.05–0.11 wt%and 0.28–0.69 wt%,respectively,and show Ga/In and Ge/In ratios varying as 2.7 to 11 and 1 to 2,respectively. This suggests that the sphalerite in the YCD formed in a medium–low temperature of the metallogenic environment.In addition,the Zn/Fe and Zn/Cd ratios in the sphalerite can be used as a geological thermometer to indicate the metallogenic temperature. A Zn/Fe ratio greater than 100, between 10 to 100, and less than 10 indicates a low temperature (<150 °C), intermediate temperature (150–250 °C), and intermediate-high temperature(250–300 °C),respectively(Dai 2016).Similarly,Zn/Cd >500 indicates a high temperature, while a ratio between 100 and 500 indicates medium–low temperature(Li et al. 2014b; 2017; 2019). In the YCD, the Zn/Fe and Zn/Cd ratios in the Spprange from 6.96 to 23.38 (avg.13.49) and134.83 to 164.30 (avg. 150.23), respectively;while in the Sps,from 6.72 to 7.45(avg.7.08)and 81.30 to 105.48 (avg. 93.39), respectively. Therefore, based on the Ga,Ge,and Cd contents,and Ga/In,Ge/In,Zn/Fe,and Zn/Cd ratios in sphalerite,we argue that the Sppand Spsin the Yangla copper deposit were formed in medium–low temperature,which is consistent with the temperature estimates of fluid inclusion studies (Yang 2012; Chen et al. 2013a;Yang et al. 2014b; Du 2017).
Previous studies have shown that clino-pyrrhotite is often formed in medium–low temperature (<300 °C),while hex-pyrrhotite is often formed in a medium–high temperature environment (>300 °C) (Yang et al. 2014a).
The distribution range of Fe atom percentage composition diagram of pyrrhotite (Fig. 12A), shown that the pyrrhotite in the Yangla skarn Cu ores is mainly plotted within the field of monoclinic pyrrhotite region, which indicated that the pyrrhotite was formed in medium–low temperature environment (<300 °C) (Yang et al. 2014a).The equilibrium diagram (Fig. 12B) of the Fe-S mineral phase system further shown that the Yangla pyrrhotite is mainly plotted with the field of monoclinic-pyrrhotite +pyrite region, which suggested that the pyrrhotite were formed the medium temperature environment (~250 °C)(Yang et al. 2014a). Therefore, we suggest that the pyrrhotite in the Yangla skarn Cu ores may be formed in a medium ore-forming temperature environment(~250 °C).

Fig. 12 The distribution range of Fe atom percentage composition (A) and the equilibrium diagram of the Fe-S mineral phasesystem (B) of pyrrhotite, Yangla Cu deposit, Yunnan, China (after Gu et al. 1995; Yang et al. 2014a)
5.3 Indication of Genetic Type of the Yangla Cu Deposit
The Fe/S ratio of pyrite can reflect its genesis and S deficiency,wherein a high Fe content(Fe/S >0.875)indicates hydrothermal origin,while S enrichment and Fe deficiency(Fe/S <0.875) indicates sedimentary origin (Xu and Shao 1980).The avg.Fe/S values of Pypand Pysin the YCD are 0.889 and 0.874, respectively. The higher Fe/S in the porphyry (>0.875) and lower in the skarn ore (<0.875)indicates that the pyrites in the YCD are not of a single genetic type. The Pypis mainly hydrothermal, while the Pyshas both hydrothermal and sedimentary origin,which is mainly manifested by sedimentary genesis (Fig. 13A).Previous studies have also confirmed the existence of typical sedimentary oolitic pyrite(with concentric or radial texture)in the skarn type ores of the YCD(Zhu 2011;Chen 2013).Additionally,the Co and Ni contents and the Co/Ni ratio of pyrite are also indicative of its genetic type(Xu and Shao 1980).The Co and Ni contents in sedimentary pyrite are relatively low, with Co/Ni <1 and a corresponding avg. of 0.63, whereas the Co and Ni contents of hydrothermal pyrite vary widely, with Co/Ni ratio ranging between 1.17 to 5.5 (Xu and Shao 1980). The volcanic massive sulfide deposits show Co/Ni between 5 and 50,with an avg. of 8.70 (Bajwah et al. 1987; Gong and Ma 2011; Li et al. 2017). With regards to the YCD, the Co(0.01–0.16 wt%), Ni (0.04–0.05 wt%), and Co/Ni ratios(0.5 to 4.0,avg.2.19)of Pypare lower than those Pys(Co,0.03–0.23 wt%; Ni, 0.02–0.09 wt%; and Co/Ni = 1 to 22,avg. 6.89). In addition, Pypand Pysare mainly plotted within the field of magmatic-hydrothermal genesis region in the Co versus Ni diagram (Fig. 13B), which is different from the sedimentary and sedimentary-reworked pyrite,and further indicated that the pyrite (Pypand Pys) in the YCD may belong to the magmatic-hydrothermal pyrite.Therefore, combined with the general lower Co and Ni contents, and variable Co/Ni ratiosin the pyrites of the YCD suggests its hydrothermal origin.
The formation of pyrite in the YCD continues through the whole mineralization process(Du 2017).The pyrite Co/Ni ratio in the skarn ore varies greatly (1 to 22), with an avg. Fe/S ratio lower than 0.875 (i.e., 0.874), thereby showing characteristics of sedimentary genesis. Therefore,we argue that the Pysmay have inherited some characteristics of the early sedimentary origin, causing it to have both sedimentary and hydrothermal genesis. Earlier researches have proven the existence of exhalative hydrothermal sedimentary siliceous rocks (Rb–Sr isochronal age 272 ± 6 Ma) in the Yangla Cu deposit (Pan et al. 2001). The Pb isotopic composition and rare earth elements characteristics of the massive sulfide ores formed during this period are consistent with that of the exhalative hydrothermal sedimentationof the siliceous rocks.The oreforming materials are mainly from the upper crust and the products of exhalative sedimentation, which are significantly different from the sulfide ores formed by skarn mineralization (Pan et al. 2000a; 2001; Yang et al. 2012c;2012d;Chen et al.2013b;Chen 2013;Xie 2018).The Re–Os age of the molybdenite from the skarn copper ore body is 229.7 to 234.8 Ma (Li et al. 2010; Yang et al. 2011;Yang 2012; Zhu 2012), with an avg. of 231 Ma, which is consistent with the granodiorite emplacement age of 202 to 239 Ma(avg.230 Ma)(Wang et al.2010;Gao et al.2010;Yang et al. 2011; Zhu 2012; Meng 2016). Moreover, isotope geochemistry also confirms that the ore-forming materials of the YCD were mainly derived from a combination of the lower crust and the mantle (with magmatic inputs) and as products of hydrothermal mineralization(Zhu et al.2011b;Yang 2012;Chen et al.2013a;Du 2017).All of these indicate that there are both exhalative-sedimentation and skarn mineralization in the YCD, and the skarn mineralization superimposed and transformed the early exhalative-sedimentation ore body to further enrich the metallogenic elements(Pan et al.2000a,b,2001;Yang et al.2012c;2012d;Chen et al.2013b;Xie 2018).In recent years, Cu-mineralized porphyry intrusion (U–Pb age of zircon is 195 to 213 Ma) has been discovered in the depth of the Yangla Cu deposit (Wang 2019; Li et al. 2020),indicating the presence of porphyry mineralization.

Fig. 13 The diagrams showing relationships of the Fe-S and Co–Ni of pyrite, Yangla Cu deposit, Yunnan, China (after Zhu 2011; Yang et al.2012e, 2014a; Li et al. 2019b)

Fig. 14 The binary plots (A) Cd versus Fe and (B) Sn versus Cu for sphalerites (The data of area from Ye et al. 2011) in Yangla Cu deposit,Yunnan, China
The Mtpis relatively rich in Cu, Pb, and Zn, while the one in the skarn ore is predominant in Bi,Cu,and W,with trace element contents (total amount of impurities) in both being lower than 1 wt% (Huang et al. 2014). The molybdenite As + Fe + Co + Pb + Ni + Sb + Cu + Zn ranges between 0 to 0.65 wt%in the Yangla porphyry ore,and between 0.10 and 0.16 wt% in the skarn ore (Yang 2012).A certain amount of Cu, Pb, Zn, and other elements are also enriched in the molybdenite of the YCD,which further confirms the existence of skarn-porphyry mineralization.This indicates that the YCD may be a composite superimposed genetic deposit, which has undergone exhalativesedimentation to skarn-porphyry mineralization.
Song (1984) summarized the relationship between Zn/Cd and the genetic type of the deposit, and obtained that the Zn/Cd ratio of sphalerite is 417 to 531 in volcanic sedimentary deposits; 252 to 330 in sedimentary and carbonate rocks;and 102 to 214 in hydrothermal deposits.The Zn/Cd ratios of 134.83–164.30 and 81.30–105.48, respectively, in the Yangla Sppand Spswere found to be consistent with the Zn/Cd ratio of the hydrothermal deposits.Additionally, the Cd content of Sppand Spsare 0.28–0.45 wt%(avg.0.36 wt%)and 0.50–0.69 wt%(avg.0.60 wt%),respectively, which is consistent with the characteristic Cd enrichment in magmatic-hydrothermal deposits (Zhang 1987). Thus, it can be suggested that the YCD may arise from magmatic-hydrothermal deposits.
As substantiated by earlier research, sphalerite in skarn ore bodies and massive sulfides deposits usually have low Cd/Fe ratios (<0.30) (Cook et al. 2009; Leng and Qi 2017).Accordingly,the Cd/Fe ratios of Yangla Sppand Spsvary between 0.05–0.14 and 0.06–0.09, respectively, indicating that the YCD may be skarn-type or a massive sulfide deposit. These deposits can be further distinguished from the genesis based on the sphalerite Cd–Fe (Fig. 14A) and Sn–Cu (Fig. 14B) correlation plots (Ye et al. 2011). Sppand Spsin the Yangla Cu deposit were mainly collected close to the skarn deposit, indicating that the YCD may have a similar genetic mechanism as skarn deposits. Furthermore,the skarn and porphyry Cu ores should be formed the same magmatic-hydrothermal metallogenic system in the Yangla Cu deposit (Chen et al. 1999; Hu 2015; Wang et al. 2020). Therefore, the sphalerite of the skarn and porphyry Cu ores will exhibit similar geochemical features and may plot within the same range of variation in Fig. 14.
Studies have shown that the Co versus Ni diagram of pyrrhotite is also indicative of the genesis of the ore deposit(Yang et al. 2014a). The pyrrhotite of Yangla Cu ores mainly plotted within the field of the skarn Cu deposit,which is significantly different from Cu-Ni sulfide deposits and consistent with the Yangla geological facts, and suggested that Yangla deposit is a skarn Cu deposit (Fig. 15).
In summary, the YCD demonstrates features of exhalative-sedimentation-skarn-porphyry deposits and may be a composite superimposed genetic deposit.

Fig. 15 The relationship between Co and Ni of the pyrrhotite in Yangla Cu deposit, Yunnan, China (after Yang et al. 2014a)
6 Conclusions
1. The correlation analyses of the major and trace elements of sulfides in the YCD show that the Fe,Co,Ni,Cu, Pb, Zn, Ga, Ge, Sb, As, and other trace elements primarily occur in the form of isomorphism and/or as mineral inclusions.
2. The trace element contents in sulfides geo-thermometers indicate that the YCD were formed in a medium–high temperature metallogenic environment.
3. The chemical compositions of each sulfide in the deposit show that its formation may have undergone the process of exhalative-sedimentary to skarn-porphyry mineralization, implying that the YCD is a composite superimposed genetic ore deposit.
AcknowledgementsThis study was supported jointly by the National Natural Science Foundation of China (41862007 and 41402072), Yunnan Ten Thousand Talents Plan Young & Elite Talents Project (No. YNWR-QNBJ-2018-093), the Key Disciplines Construction of Kunming University of Science and Technology(No.14078384), and the Analysis and Testing Foundation of Kunming University of Science and Technology (2017T20160006). We are grateful to Dr.Jianping Liu,Dr.Weikang Chen,and Dr.Shaoqing Liu(School of Geosciences and Info-physics, Central South University)for sulfides EPMA analyses; and Dr. Yuedong Liu, Dr. Cheng Luo,Dr. Xiaoqing Liu and Dr. Zaizao Li (Yunnan Diqin Mining Industry Group) for their field work. The authors would also like to thank anonymous reviewers for their useful comments and constructive reviews, which significantly improved the manuscript.
杂志排行
Acta Geochimica的其它文章
- Germanium/silica ratio and trace element composition of Early Cambrian siliceous rocks in Keping:implications for the siliceous rocks’ formation and paleoenvironment interpretations
- In situ LA-ICP-MS analyses of mica and wolframite from the Maoping tungsten deposit, southern Jiangxi, China
- Mineralogy and geochemistry of pozzolans from the Tombel Plain, Bamileke Plateau, and Noun Plain monogenetic volcanoes in the central part of the Cameroon Volcanic Line
- Zircon U-Pb geochronology, whole-rock geochemical,and Sr-Nd-Pb isotopic constraints on the timing and origin of Permian and Triassic mafic dykes from eastern North China Craton
- Geotectonic significance of the Neoproterozoic ophiolitic metagabbros of Muiswirab area, South Eastern Desert, Egypt:constraints from their mineralogical and geochemical characteristics
- Contribution of Asian dust to soils in Southeast China estimated with Nd and Pb isotopic compositions
