吲哚美辛和胰管支架联合应用预防ERCP术后胰腺炎和高淀粉酶血症的临床研究
2021-01-05方剑锋陈志良沈志宏鲁葆春
方剑锋 陈志良 沈志宏 鲁葆春

[摘要] 目的 探討吲哚美辛和胰管支架在预防经内镜逆行胰胆管造影术(ERCP)术后胰腺炎和高淀粉酶血症中的临床应用价值。 方法 纳入2018年1月至2020年6月绍兴市人民医院肝胆胰外科收治的1063例ERCP患者,分成高危、中危、低危3组,再根据随机双盲原则将高危组分成高危A、高危B、高危C 3组,中危、低危组分成中危A、B和低危A、B各2组。在术后常规处理外,对高危、中危、低危A组予吲哚美辛栓肛塞,B组仅常规处理,高危C组予胰管支架置入联合吲哚美辛栓肛塞。观察术后3、24 h的血淀粉酶及是否有胰腺炎症状体征、CT表现。结果 高危C组高淀粉酶血症发生率为56.6%,胰腺炎发生率为27.7%,较A组、B组明显下降,差异有统计学意义(P<0.001),中危A组高淀粉酶血症发生率为35.0%,胰腺炎发生率为6.5%,较B组明显下降,差异有统计学意义(P<0.001),低危A组高淀粉酶血症发生率为4.1%,胰腺炎发生率为0.6%,较B组明显下降,差异有统计学意义(P<0.05)。 结论 对于ERCP术后胰腺炎高危患者,术中胰管支架置入联合术后吲哚美辛栓肛塞能显著降低术后胰腺炎和高淀粉酶血症的发生率。对于ERCP术后胰腺炎中低危患者,术后吲哚美辛栓肛塞能显著降低术后胰腺炎和高淀粉酶血症的发生率。
[关键词] 吲哚美辛;胰管支架;PEP;高淀粉酶血症
[中图分类号] R735.37 [文献标识码] B [文章编号] 1673-9701(2021)30-0106-05
[Abstract] Objective To investigate the clinical application value of pancreatic duct stent combined with indomethacin in preventing pancreatitis and hyperamylasemia after endoscopic retrograde cholangiopancreatography(ERCP). Methods A total of 1063 Patients received ERCP in the Department of Hepatobiliary and Pancreatic Surgery of Shaoxing People’s Hospital from January 2018 to June 2020 were divided into three groups as the high-, medium- and low-risk group. The high-risk group were further divided into three groups as the high-risk group A, B and C, according to the randomized double-blind principle. The medium-risk group were further divided into two groups as the medium-risk group A and B. The low-risk group were further divided into two groups as the low-risk group A and B. The high-, medium- and low-risk group A were given indomethacin anal embolization, in addition to conventional postoperative treatment. The high-, medium-and low-risk group B were only given conventional treatment. The high-risk group C were given pancreatic duct stent implantation combined with indomethacin anal embolization. The levels of blood amylase at 3 and 24 h after operation, whether there were symptoms and signs of pancreatitis, and CT manifestations were observed. Results The incidences of hyperamylasemia and pancreatitis in the high-risk group C were 56.6% and 27.7%, respectively, which were significantly lower than those in the high-risk group A and B,the difference was statistically significant(P<0.001). The incidences of hyperamylasemia and inflammation in the medium-risk group A were 35.0% and 6.5%, respectively, which were significantly lower than those in the medium-risk group B,the difference was statistically significant (P<0.001). The incidences of hyperamylasemia and pancreatitis in the low-risk group A were 4.1% and 0.6%, respectively, which were significantly lower than those in the low-risk group B,the difference was statistically significant (P<0.05). Conclusion For patients with high risk of pancreatitis after ERCP, intraoperative pancreatic duct stent placement combined with postoperative indomethacin anal embolization can significantly reduce the incidences of postoperative pancreatitis and hyperamylasemia. For patients with low and medium risk of pancreatitis after ERCP, postoperative indomethacin anal embolization can significantly reduce the incidences of postoperative pancreatitis and hyperamylasemia.
[Key words] Indomethacin; Pancreatic duct stent; PEP; Hyperamylasemia
经内镜逆行胰胆管造影术(Endoscopic retrograde cholangiopancreatography,ERCP)引进中国已有40多年历史,通过不断普及和发展,已成为临床诊断和治疗胆胰疾病不可或缺的重要手段,其具有诊断确切、疗效好、创伤小、副作用少等优点。但ERCP属于有创操作,术后有一定的并发症,包括疼痛、出血、高淀粉酶血症、胰腺炎等。以术后高淀粉酶血症最常见,多数无特殊的临床表现,可发展为ERCP术后胰腺炎(Post-ERCP pancreatitis,PEP),总发生率高达9.7%,病死率有0.7%[1],成为阻碍ERCP 技术发展与应用的因素之一。因此,有效地预防ERCP术后胰腺炎和高淀粉酶血症,降低ERCP术后并发症,减少病死率,对促进ERCP技术的发展具有极其重要的意义。
目前认为,PEP发生的高危因素主要可分为患者相关因素、手术相关因素及操作者相关因素三类[2-4]。其引起的炎症反应、胰管堵塞胰管内高压是PEP发生的主要原因。吲哚美辛在炎症反应初期能阻断炎症因子的瀑布效应,胰管支架能解除胰管梗阻,降低胰管内高压,从而预防PEP的发生。但它们也有副作用,如消化道出血、支架移位、堵塞等。本研究通过在各危险组患者中选择性使用吲哚美辛栓和胰管支架来明确吲哚美辛和胰管支架在预防ERCP术后胰腺炎和高淀粉酶血症中的临床应用价值,现报道如下。
1 资料与方法
1.1一般资料
绍兴市人民医院肝胆胰外科2018年1月至2020年6月符合纳入标准的ERCP患者共1063例,按ERCP术后胰腺炎风险评估表分成高危、中危、低危3组,再根据随机双盲原则将高危组分成高危A、高危B、高危C 3组,中危、低危组分成中危A、B和低危A、B各2组。各组性别、年龄比较,差异无统计学意义(P>0.05),具有可比性。见表1。
1.2 纳入与排除标准
1.2.1 纳入标准 ①本院肝胆胰外科因各种胆胰疾病有ERCP手术适应证者;②无禁忌证[5]者;③所有患者签署知情同意书。
1.2.2排除标准 ①胃肠道改道术后者(如BⅡ式胃术后);②单纯十二指肠镜检查者;③单纯胆道或胰管支架拔除者;④有吲哚美辛栓使用禁忌者;⑤对吲哚美辛过敏者;⑥急性上消化道出血者;⑦发病前2周应用抗凝药者;⑧肾功能不全者;⑨机械性肠梗阻者。
1.3 方法
在术后常规处理(禁食、输液抗炎解痉抑酸护胃补液治疗,不使用生长抑素、奥曲肽等胰酶抑制剂)外,对高危、中危、低危A组予吲哚美辛栓(湖北东信药业有限公司,国药准字 H42021462)100 mg肛塞,B组仅常规处理,高危C组予胰管支架置入联合吲哚美辛栓100 mg肛塞。观察术后3、24 h的血淀粉酶及是否有胰腺炎症状体征、CT表现。
1.4 观察指标
①高淀粉酶血症发病率。ERCP术后复查术后3 h和术后24 h血淀粉酶,如高于正常上限(碘比色法≥180 U/L),且无腹痛及胰腺炎相关影像学表现,则提示高淀粉酶血症。②ERCP术后胰腺炎发病率。ERCP术后新发生腹痛或原有腹痛加重,伴有术后24 h血清淀粉酶超过正常高值的3倍(碘比色法≥540 U/L),则提示ERCP术后胰腺炎。同时完善患者影像学检查,若患者无胰腺炎样腹痛或血淀粉酶升高超过正常上限3倍者,以影像学检查结果综合判断。
1.5 统计学方法
采用SPSS 25.0统计学软件进行数据处理,计量资料以均数±标准差(x±s)表示,组间比较采用t检验;计数资料以[n(%)]表示,组间比较采用χ2检验,P<0.05为差异有统计学意义。
2结果
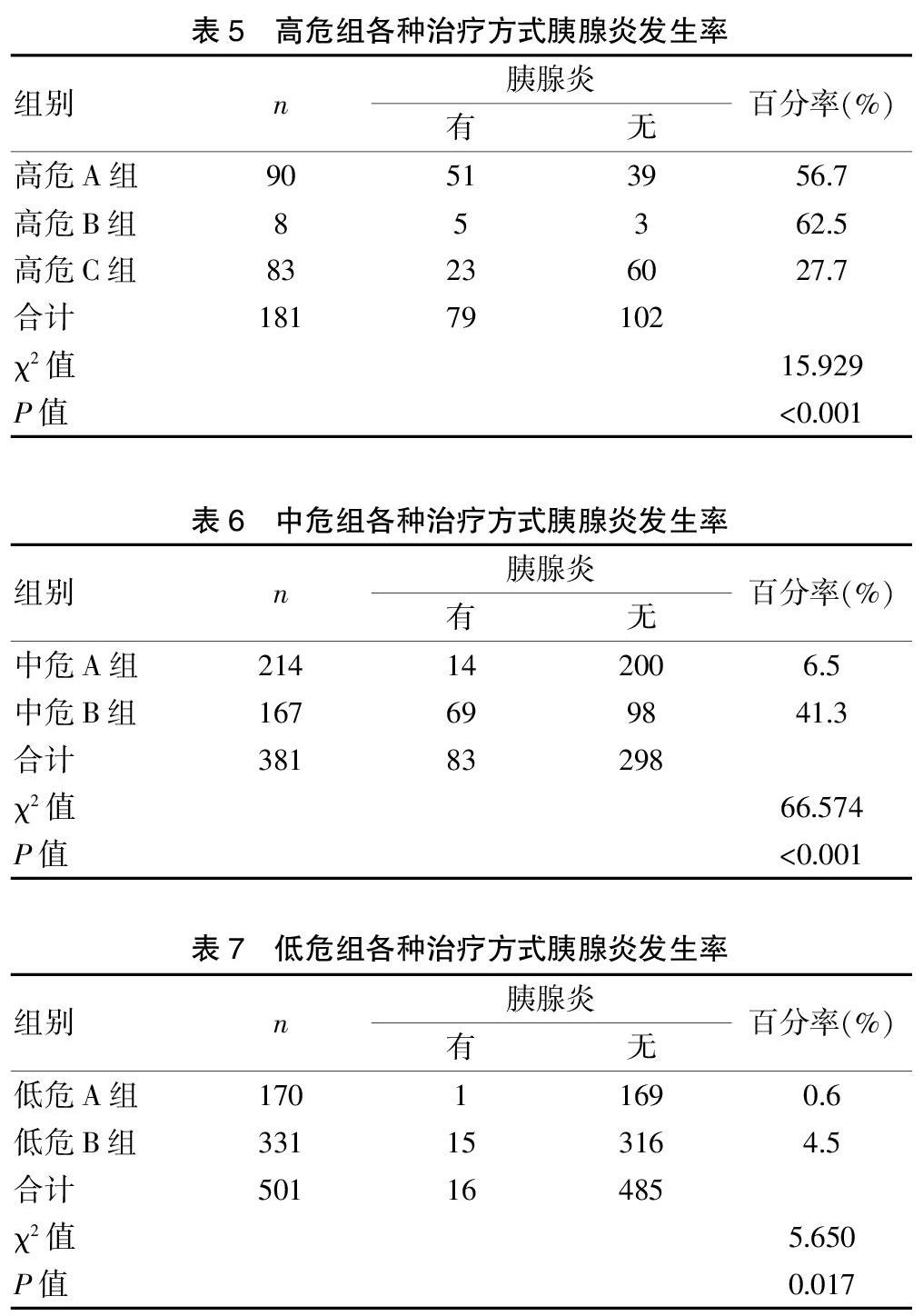
共统计ERCP患者1063例,其中高危组181例,中危组381例,低危组501例。结果发现高危A组90例均淀粉酶增高,高淀粉酶血症发生率为100.0%(90/90),高危B组高淀粉酶血症发生率为87.5%(7/8),高危C组高淀粉酶血症发生率为56.6%(47/83),三组比较差异有统计学意义(P<0.001);统计中危A组214例,高淀粉酶血症发生率为35.0%(75/214),低于中危B组的88.0%(147/167),差异有统计学意义(P<0.001);低危A组高淀粉酶血症发生率为4.1%(7/170),低于低危B组的13.3%(44/331),差异有统计学意义(P<0.05)。高危A组胰腺炎有51例,发生率为56.7%(51/90),高危B组胰腺炎发生率为62.5%(5/8),高危C组胰腺炎发生率为27.7%(23/83),三组比较差异有统计学意义(P<0.001);中危A组胰腺炎发生率为6.5%(14/214),低于中危B组的41.3%(69/167),差異有统计学意义(P<0.001);低危A组胰腺炎发生率0.6%(1/170),低于低危B组的4.5%(15/331),差异有统计学意义(P<0.05)。见表2~7。
3 讨论
ERCP由于其具有诊断明确、创伤小、恢复快、住院时间短等特点,已经成为诊断和治疗胆胰疾病不可或缺的手段。但ERCP也有其并发症,最常见的就是ERCP术后胰腺炎和高淀粉酶血症。PEP发生的主要原因是手术引起的炎症反应、胰管堵塞胰管内高压。
吲哚美辛是一种非甾体抗炎药物(NSAIDs),具有环氧合酶(COX)抑制活性,是磷脂酶A2(PLA2)的拮抗剂。PLA2在胰腺炎的发病机制中起重要作用,能调节前列腺素、白细胞介素和血小板活性因子等炎症前递质。故吲哚美辛在炎症反应初期能阻断炎症因子的瀑布效应[6-9]。但是,吲哚美辛的不良反应很多,最常见的是胃肠道损伤,包括炎症、溃疡、穿孔及消化道憩室等。所以ERCP术后预防性使用吲哚美辛栓应有明确的适应证,其应用范围需严格控制。
胰管支架置入可降低因困难的插管、Oddi括约肌狭窄高压、括约肌的预切开损伤胰管开口等引起的胰管高压,引流胰液,一直被认为是预防PEP发生、降低PEP严重程度的有效手段,且应用于PEP高危患者也能获得良好效果[10-15]。Troendle等[10]进行的一项单盲、随机对照试验也表明,对于高危患者,胰腺支架置入是一种防治ERCP 术后胰腺炎安全、有效的技术。Mazaki等[11]通过一篇涉及680例患者的Meta分析指出,PEP的发生率从19%明显下降至7%。Freeman[16]认为对于那些反复发生胰腺炎、Oddi括约肌功能异常等患者,胰管支架对于预防PEP是必须的,是任何药物治疗不可替代的。但应用塑料支架也有其并发症,最主要的是支架堵塞[10]、移位[17-18]、胰管狭窄[19]、胰管损伤[20]及出血、穿孔等。同时,胰管支架的置入增加了患者的费用,其经济性不佳。
但目前国内外对预防ERCP术后胰腺炎及高淀粉酶血症的研究多为单独、随意使用吲哚美辛或胰管支架,没有明确的使用适应证和风险度分级。与常规处理相比,應用组胰腺炎及高淀粉酶血症几率有降低,但仍有一定量的发生率,且出现副作用、并发症较多。为避免这个问题,所以本研究对ERCP术后患者行胰腺炎危险因素评定、分级,区别化处理,统计各危险组使用吲哚美辛和胰管支架的效果,以个体化使用吲哚美辛、胰管支架,在达到效果的同时,减少其并发症。
本研究发现,对PEP高危患者,术后吲哚美辛栓肛塞联合术中胰管支架置入与单独使用吲哚美辛栓和术后常规处理组比较,联合使用组术后胰腺炎和高淀粉酶血症的几率明显降低(高淀粉酶血症发生率56.6%,胰腺炎发生率27.7%),差异有统计学意义(P<0.001)。这可能跟胰管支架置入能解除胰管梗阻,降低胰管内高压,引流胰液有关。同时,吲哚美辛在炎症反应初期能阻断炎症因子的瀑布效应,另有研究显示,非甾体抗炎药物(NSAIDs)可抑制环磷酸腺苷的合成、过氧化物的产生、溶酶体酶的释放等伴随中性粒细胞活化的一系列现象,从而减轻炎症反应[21]。所以,吲哚美辛和胰管支架联合应用更能降低术后胰腺炎和高淀粉酶血症的发生。
对于中低危患者,术后使用吲哚美辛的中危A组高淀粉酶血症发生率为35.0%(75/214),胰腺炎发生率为6.5%(14/214),较中危B组明显下降[高淀粉酶血症发生率为88.0%(147/167),胰腺炎发生率为41.3%(69/167)],低危A组高淀粉酶血症发生率为4.1%(7/170),胰腺炎发生率为0.6%(1/170),也较低危B组明显下降[高淀粉酶血症发生率为13.3%(44/331),胰腺炎发生率4.5%(15/331)],差异有统计学意义(P<0.05)。由此可见,吲哚美辛对于PEP中危、低危的患者,均有很好的预防ERCP术后胰腺炎和高淀粉酶血症的效果。
对于ERCP术后胰腺炎高危患者,术中胰管支架置入联合术后吲哚美辛栓肛塞能显著降低术后胰腺炎和高淀粉酶血症的发生率。对于ERCP术后胰腺炎中危患者,术后吲哚美辛栓肛塞能显著降低术后胰腺炎和高淀粉酶血症的发生率。对于ERCP术后胰腺炎低危患者,术后吲哚美辛栓肛塞也能降低术后胰腺炎和高淀粉酶血症的发生率,加快患者恢复,缩短住院时间。
[参考文献]
[1] Kochar B,Akshintala VS,Afghani E,et al. Incidence,severity,and mortality of post-ERCP pancreatitis:A systematic review by using randomized,controlled trials[J]. Gastrointest Endosc,2015,81:143-149.
[2] Lin Y,Liu X,Cao DQ,et al.Analysis of risk factors and prevention strategies of post-ERCP pancreatitis[J].European Review for Medical & Pharmacological Sciences,2017,21(22):5185.
[3] Zheng L,Wang XP,Tao Q,et al.Different pattern of risk factors for post-ERCP pancreatitis in patients with biliary stricture[J].Scand J Gastroenterol,2018,53(5):604-610.
[4] Miyatani H,Matsumoto S,Mashima H.Risk factors of post-endoscopic retrograde cholangiopancreatography pancreatitis in biliary type sphincter of oddi dysfunction in Japanese patients[J].J Dig Dis,2017,18(10):591-597.
[5] 王书智,胡冰.ERCP护理培训教程[M].上海:上海科学技术出版社,2016:114.
[6] Akbar A,Abu Dayyeh BK,Baron TH,et a1.Rectal nonsteroidal anti-inflammatory drugs are superior to pancreatic duct stents in preventing pancreatitis after endoscopic retrograde cholangiopan-creatography:A network meta-analysis[J].Clin Gastroenterol Hepatol,2013,11(7):778-783.
[7] 周世文,刘斌,石向阳,等.吲哚美辛对ERCP术后高淀粉酶血症及胰腺炎的预防作用[J].肝胆外科杂志,2017, 25(2):129-133.
[8] 李国栋,董海燕,庞秋萍,等. 选择性胰管支架和非甾体类抗炎药预防ERCP术后胰腺炎的倾向性评分匹配分析[J].中华消化内镜杂志,2016,33(4):219-222.
[9] 刘振,郝建宇. 环氧酶-2选择性抑制剂在预防内镜逆行胰胆管造影术后胰腺炎、高淀粉酶血症中的作用研究[J].中华消化内镜杂志,2016,33(7):458-462.
[10] Troendle DM,Abraham O,Huang R,et al Factors associated with post-ERCP pancreatitis and the effect of pancreatic duct stenting in a pediatric population[J]. Gastrointest Endosc,2015,81:1408-1416.
[11] Mazaki T,Masuda H,Takayama T.Prophylactic pancreatic stent placement and post-ERCP pancreatitis:A systematic review and meta-analysis[J].Endoscopy,2010,42(10):842-853.
[12] 孫备,苏维宏,2013年国际胰腺病学会与美国胰腺病学会《急性胰腺炎治疗的循证性指南》解读[J].中华消化外科杂志,2013,12(12):937-943.
[13] Kawaguchi Y,Ogawa M,Omata F,et al.Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography[J].World J Gastroenterol,2012,18(14) :1635-1641.
[14] Conigliaro R,Manta R,Bertani H,et a1.Pancreatic duct stenting for tim duration of ERCP only does not prevent pancreatitis'after accidental pancreatic duct cannulation:A prospective randomized trial[J].Surg Endosc,2013,27(2):569-574.
[15] 李运红,姚玉玲,贺奇彬,等. 胰管支架预防困难胆管插管ERCP术后急性胰腺炎的前瞻性研究[J].中华消化内镜杂志,2014,31(7):403-406.
[16] Freeman ML. Pancreatic stents for prevention of post-ERCP pancreatitis:The evidence is irrefutable[J]. J Gastroenterol,2014,49: 369-370.
[17] Lee TH,Moon JH,Choi HJ,et a1.Prophylactic temporary 3F pancreatic duct stent to prevent post-ERCP pancreatitis in patients with a difficuh biliary cannulation:A multicenter,prospective,randomized study[J].Gastrointest Endosc,2012,76(3):578-585.
[18] Wang ZK,Yang YS,Cat FC,et a1.Is prophylactic somatostatin effe(ttive to prevent post—endoscopic retrograde cholangiopancre-atography pancreatitis or hyperamylasemia?A randomized,place-bo-controlled pilot trial[J].Chin Med J(En91),2013,126(13):2403-2408.
[19] Eleftherladis N,Dinu F,Delhaye M,et al.Long-term outcome after pancreatic stenting in severe chronic pancreatitis[J].Endoscopy,2005,37(3):223-230.
[20] Morgan DE,Smith JK,Hawkins K,etal.Endoscopic stent therapy in advanced chronic pancreatitis:Relationships between ductal changes,clinical response,and stent patency[J].Am J Gastroenterol,2003,98(4):821-826.
[21] Slater D,Kunnathil S,McBride J,et al. Pharmacology of nonsteroidal antiinflammatory drugs and opioids[J]. SeminIntervent Radiol,2010,27:400-411.
(收稿日期:2020-11-09)
