Statistical Analysis of Defective Items of Pharmaceutical Wholesale Enterprises
2020-12-24CaoLinlinWuZhiang
Cao Linlin,Wu Zhi’ang
(1.School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China;2.Center for food and Drug Evaluation and Inspection of HMPA,Henan 450002,China)
Abstract Objective To provide some references and suggestions for promoting the healthy development of pharmaceutical wholesale enterprises,improving the scientific supervision level of the drug regulatory department and ensuring the quality of drugs in the circulation.Methods Retrieving the items on the official websites of the national and provincial drug regulatory departments from 2017 to 2019 that didn’t meet the requirements of the 2016 edition of the Good Supplying Practice in pharmaceutical wholesale enterprises under various inspections,the defective items were summarized and analyzed,and further study of the changes of defective items was conducted.Results and Conclusion 908 pharmaceutical wholesale enterprises had definite defective items,and 218 items violated the Guidelines for On-site Inspection of the Good Supplying Practice,with a cumulative frequency of 3 874 defects.Defective items with high-frequency mainly occurred in storage and maintenance,facilities and equipment,personnel and training,general rules and sales.The average defect frequency in each pharmaceutical wholesale enterprises increased year by year,but the proportion of serious defective items decreased significantly.It is recommended that based on improving drug quality,pharmaceutical wholesale enterprises should strengthen personnel training to enhance their awareness of quality responsibility.Besides,the drug regulatory department should increase inspection and crackdown on illegal business operations and the deception of pharmaceutical wholesale enterprises.
Keywords:pharmaceutical wholesale enterprise; good supplying practice; defective item
The pharmaceutical distribution industry is an important part of national medical and health industry,because it is closely related to people’s health and life safety[1].Pharmaceutical wholesale enterprises are the important subjects in the circulation field.Good Supplying Practice (GSP) is the basic criterion for regulating drug business management and quality control[2],which plays an important role in regulating the conditions and behaviors of pharmaceutical enterprises and ensuring the quality of pharmaceutical business.In June 2013,the GSP was issued by the former Health Ministry (revised in 2012),clearly defined the goal of comprehensively promoting the management,strengthening two key links and solving three difficult problems[3].In July 2016,the GSP issued by the former China Food and Drug Administration (CFDA) clarified the relevant content of drug traceability.In December of the same year,it issued the Guidelines for On-site Inspection of the Good Supplying Practice (hereinafter referred to as “Guiding Principles”),which,as a supporting document of GSP,was the evaluation standard in GSP inspection[4].Since the implementation of GSP,the software,hardware and quality management level of domestic pharmaceutical wholesale enterprises have been significantly improved.Therefore,drug management is more standardized,and its quality management has reached a new level.However,violations of GSP by pharmaceutical wholesale enterprises still occur from time to time,which seriously affects the quality and safety of drugs.In this study,we summarized the items that didn’t meet the GSP requirements in pharmaceutical wholesale enterprises under various inspections from 2017 to 2019 on the official websites of national and provincial drug regulatory departments.Then they were analyzed to provide some reference and suggestions for promoting the healthy development of pharmaceutical wholesale enterprises,improving the scientific supervision level of drug regulatory departments and ensuring the quality of drugs in circulation.
1 Data and methods
1.1 Source of data
By searching the official websites of the National Medical Products Administration (NMPA),the former CFDA, Drug and Food Administration of 31 provinces (municipal,autonomous region),the former Food and Drug Administration and the Administration for Market Regulation,we obtained the inspection results that didn’t meet GSP requirements from 2017 to 2019.
1.2 Methods
First,we entered the information such as the names of pharmaceutical wholesale enterprises,penalty measures and problems found in the inspection into Excel.Then we matched the terms of defective items according to GSP and Guiding Principles,referring to relevant documents of pharmaceutical wholesale enterprises and related content from WeChat,Sougou and Baidu for an auxiliary matching.Lastly,Excel was used to count the frequency of various clauses,and relevant charts were drawn for visualization.
Each clause in the Guiding Principles is composed of a 5-digit code,which corresponds to specific clauses in GSP.For example,item 02601 in the Guiding Principles corresponds to item 26 in GSP (this article is about the training content,which includes relevant laws and regulations,pharmaceutical professional knowledge and skills,quality management systems,responsibilities and post-operation procedures.)
2 Results
2.1 Distribution of pharmaceutical wholesale enterprises that didn’t meet GSP requirements
In this paper,a total of 1 545 pharmaceutical wholesale enterprises were counted that didn’t meet the GSP requirements in their operation published on the official websites of government departments.Among them,908 pharmaceutical wholesale enterprises had definite defects.See Table 1 for details of the top ten provinces with enterprises that didn’t meet GSP requirements from 2017 to 2019 in China.Guangdong,Anhui and Hebei ranked top three in terms of inspection information disclosure of pharmaceutical wholesale enterprises.Some provinces,such as Jiangsu,announced fewer inspection defects in wholesale enterprises.The reason may be due to their institutional reforms in 2018,the websites of the former Provincial Food and Drug Administration were cancelled or their official websites were moved to the Provincial Drug Administration with incomplete content.Therefore,they had less relevant information about pharmaceutical wholesale enterprises.
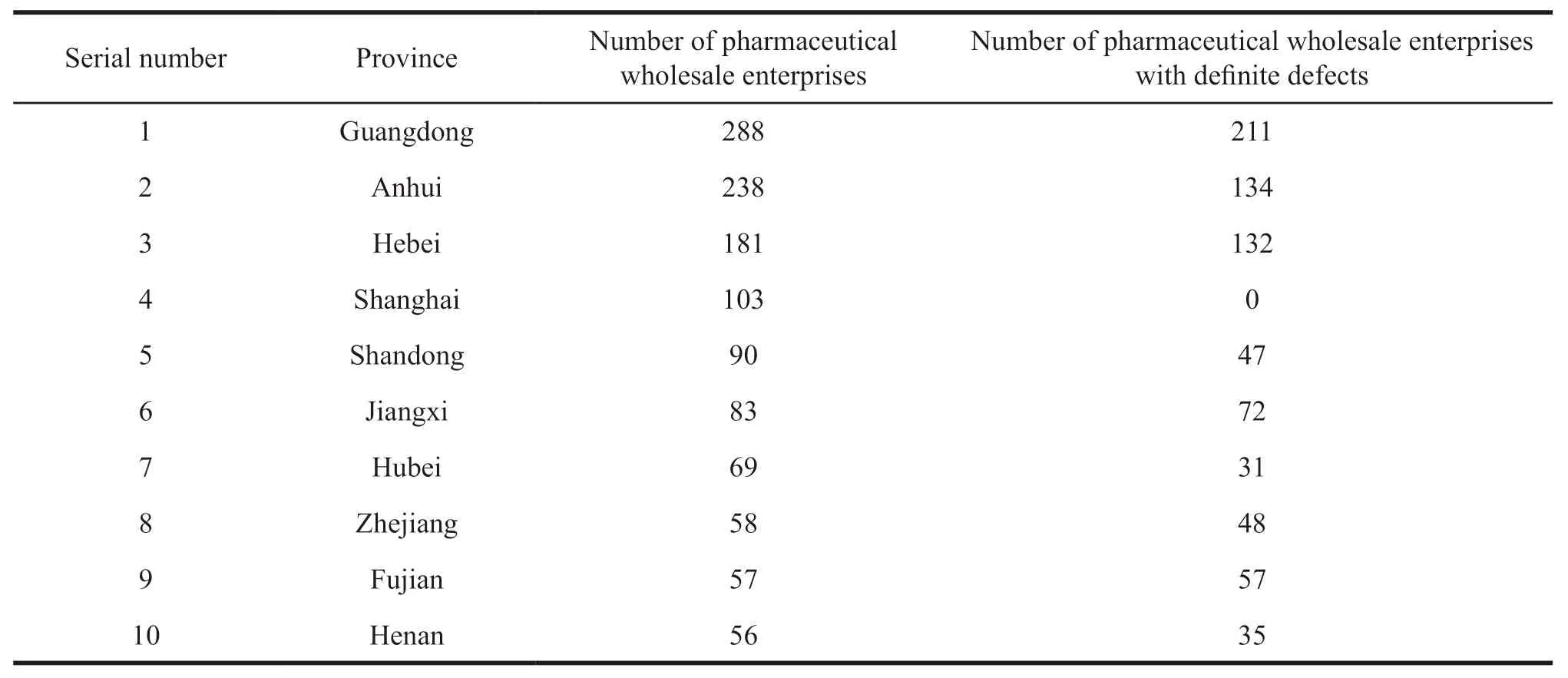
Table 1 The top ten provinces with pharmaceutical wholesale enterprises that didn’t meet the requirements of GSP
2.2 Result analysis of defective items in pharmaceutical wholesale enterprises
A total of 908 pharmaceutical wholesale enterprises involved 218 defects in the Guiding Principles,with a cumulative defect frequency of 3 874 times.See Table 2 for details.
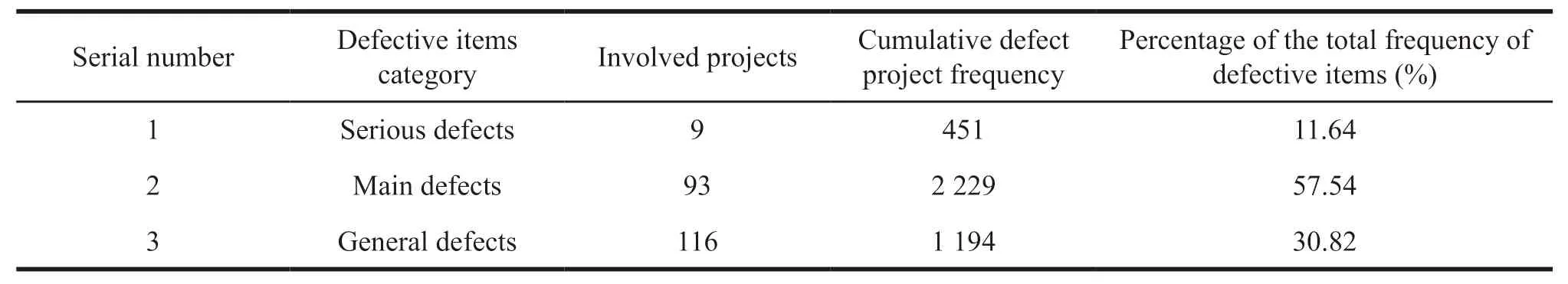
Table 2 General situation of defective items in pharmaceutical wholesale enterprises
2.2.1 Analysis of defective items
As shown in Fig.1,the most frequent defective items are:storage and maintenance,facilities and equipment,personnel and training,responsibilities and general rules of quality management departments,accounting for 17.35%,13.50%,10.45%,6.87% and 6.63% of the total defect frequency respectively.After analyzing the results of GSP certification on-site inspection of 215 drug wholesale enterprises in Liaoning Province in the literature published in 2016,Yang Mu et al.[5]pointed out that the highest defect ratios were facilities and equipment,personnel and training,storage and maintenance,and quality management system documents.Liu Hua et al.[6]summarized the defect clauses of 49 pharmaceutical enterprises in Hebei Province in 2018 and pointed out that the most defective clauses were:storage and maintenance,facilities and equipment,and personnel and training.Wang Weijia[7]summarized the defective items of 480 pharmaceutical wholesale enterprises in Heilongjiang Province from June 2014 to September 2015 and pointed out that the top three clauses in terms of defects were storage and maintenance,facilities and equipment,and personnel and training.It can be seen that storage and maintenance,facilities and equipment,personnel and training are the links that pharmaceutical wholesale enterprises are most likely to violate GSP regulations.
2.2.2 Analysis of various types of defective items
(1) Analysis of serious defective items.It can be seen from Table 2 and Table 3 that the serious defective items cover all the important articles in the Guiding Principles.In the daily pharmaceutical business,the top three items with serious defects in pharmaceutical wholesale enterprises are **00401 (pharmaceutical trading enterprises should operate according to the law),**09101 (drug sales of enterprises should issue invoices truthfully to ensure the tickets,accounts,goods and payment are consistent) and **00402(pharmaceutical trading enterprises should adhere to honesty and trustworthiness,and prohibit any false or deceptive behavior).The risks of the above three are relatively high.Pharmaceutical wholesale companies also have invoice problems in the purchase and sales of drugs,with 115 times in total including**09101,**06601 and **06701.The main reasons are the purchase does not require invoice,the name and amount of the purchase invoice unit,the name of the product and the payment flow are inconsistent.Besides,there is the inconsistency of financial accounts with drug sales and payments.
(to be continued)
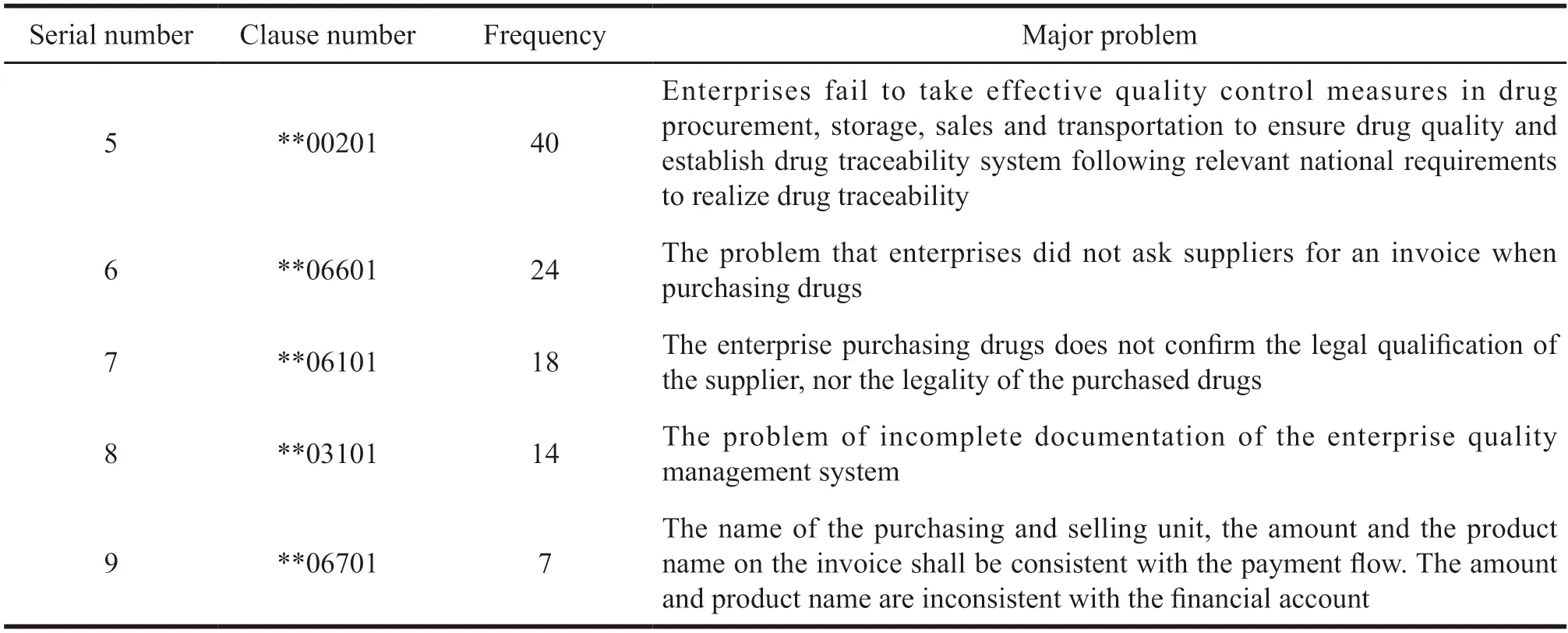
Continued Table 1
(2) Analysis of major defective items.As shown in Fig.2,in the frequency distribution of major defective items,the first one is storage and maintenance,reaching 19.29%,followed by facilities and equipment at 11.22%,personnel and training at 10.99% and responsibilities of quality management departments at 10.45%.
It can be seen from Table 2 and Table 4 that there are 93 major defective items,covering 90.29%of the 103 main defective items in the Guiding Principles.The major defective items with a frequency higher than 100 mainly involve two items,one is*04704 (equipped with temperature and humidity monitoring system) and the other is *08404 (failed to take effective control in time when the temperature and humidity exceed the standard) in facilities and equipment.Generally speaking,the problems related to temperature and humidity mainly include *04704 in facilities and equipment section,*08404 and *08302 in storage and maintenance section (the temperature of drug storage does not meet the requirements),with a total of 297 times.In the ranking of major defective items with a frequency greater than or equals to 40,there are no clauses in procurement,receipt and acceptance,delivery or transportation and distribution.
After-sales management (100%),sales (98.85%),storage and maintenance (86.74%) and personnel and training (76.74%) account for more than 60% of major defective items with high-frequency.Among them,the major defects in the sales are mainly *08901(data verification of illegal drug sales units,purchase unit,purchasers and delivery personnel) and *09301(sales management of special drugs),accounting for 50.57% and 48.28%,respectively.The main defect in computer is *05901 (the operation of computer system data modification,input and storage does not meet the file requirements),accounting for 53.02%.
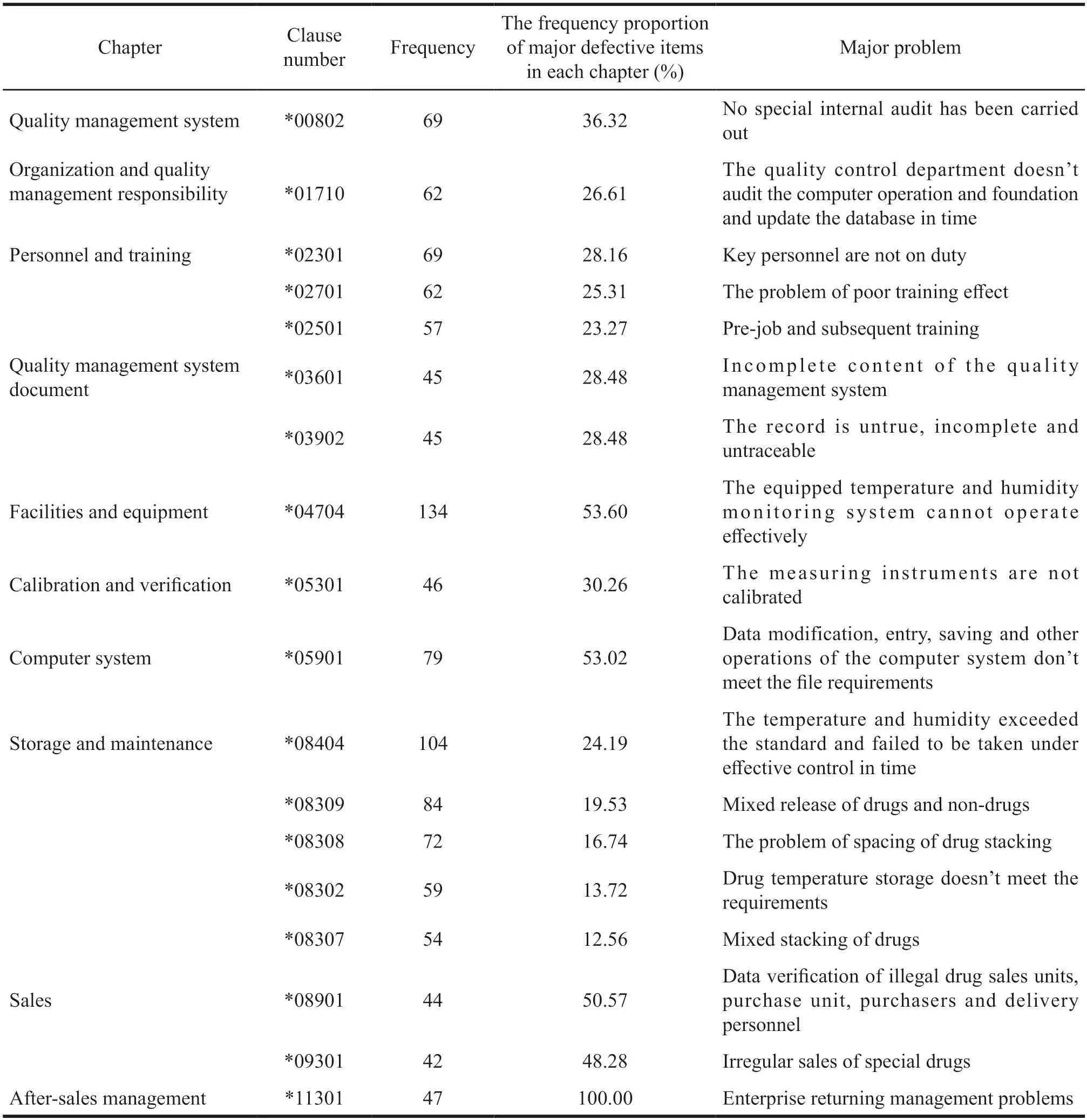
Table 4 Distribution of main defective items (frequency ≥ 40)
(3) Analysis of general defective items.As shown in Fig.3,in the frequency distribution of general defective items,facilities and equipment ranks first,accounting for 22.86%,followed by storage and maintenance at 20.27%,personnel and training at 13.40%,and receiving and acceptance at 10.30%.The computer,sales and delivery of cargo from storage are less than 1%.After analyzing the results of GSP certification on-site inspection of 215 pharmaceutical wholesale enterprises in Liaoning Province,Yang Mu et al.[5]pointed out that facilities and equipment (19.22%),storage and maintenance (14.41%),and personnel and training(12.44%) accounted for a higher percentage of general defects in an article published in 2016.There is little difference between the data in this paper and Yang Mu’s paper.The difference between storage and maintenance is about 6%,which may be caused by the statistical data in this paper which contains defect information of different inspection methods.Therefore,the inspection standards are inconsistent.
It can be seen from Tables 2 and Tables 5 that there are 116 general defective items,covering 81.12%of the 143 general defective items in the Guiding Principles.16 general defective items occur 20 times or more,which are distributed into six sections:personnel and training,quality management system document,facilities and equipment,procurement,receipt and acceptance,storage and maintenance,with a total of 533 times,accounting for 44.64% of the frequency of general defective items in pharmaceutical wholesale enterprises.Among them,the top three are 04603 for facilities and equipment (inner wall and roof of the warehouse are not smooth,the ground is uneven)and 05201 (storage and transportation facilities and equipment are not regularly inspected and maintained,and their training records and files are imperfect),and 02601 for personnel and training (incomplete training content).As to the frequency composition of general defective items,06501 for the procurement (the quality assurance agreement signed by the enterprise and the supplier is incomplete),which accounts for more than 30% of the total frequency of general defective items.40.45% of the defective items are caused by subjective factors of personnel.
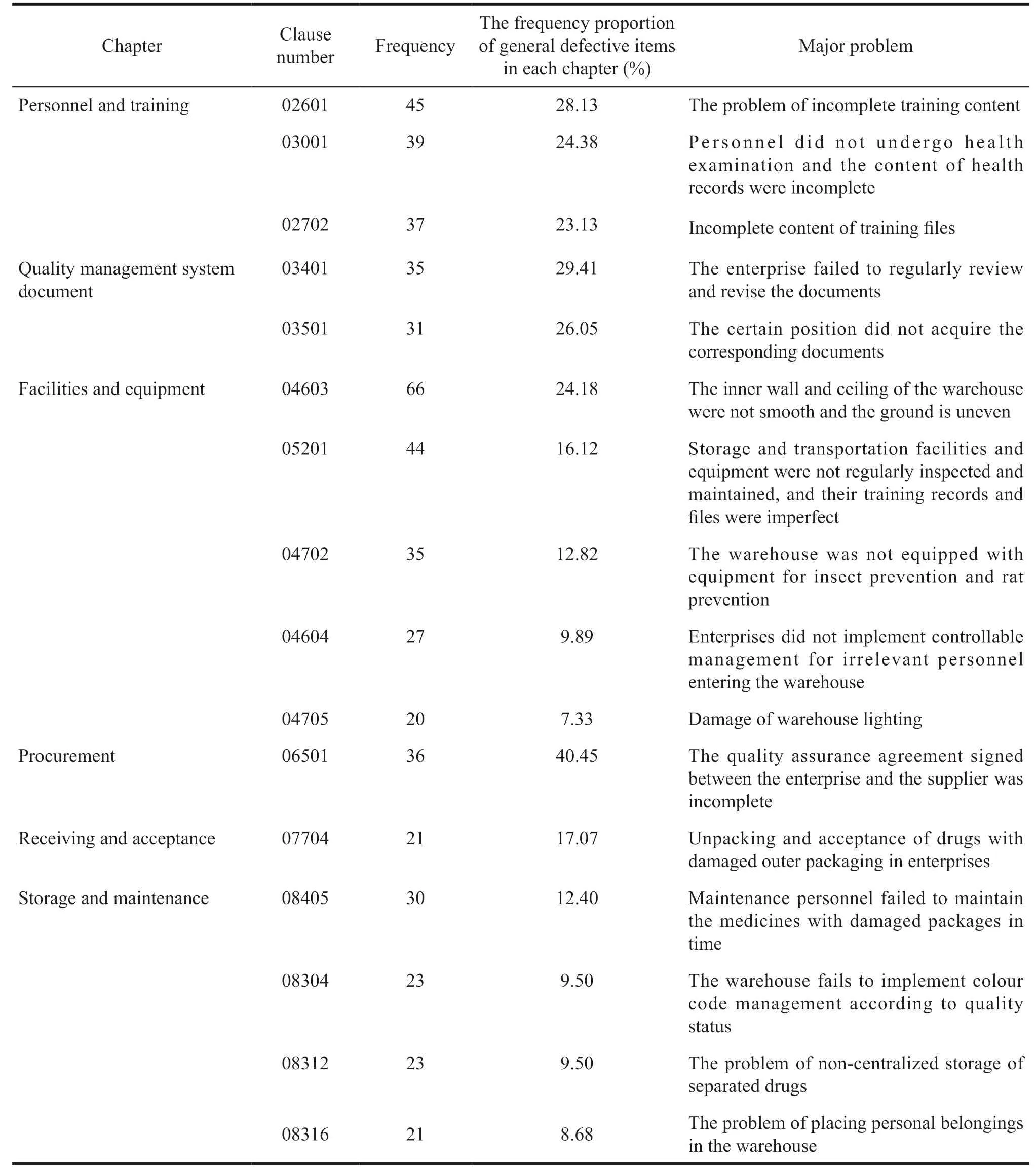
Table 5 Distribution of general defective items (frequency ≥ 20)
2.3 Yearly analysis of the changes of defective items in pharmaceutical wholesale enterprises
From 2017 to 2019,there were 465 279 and 164 pharmaceutical wholesale enterprises that did not meet the GSP requirements each year,and the average frequency of defective items for each pharmaceutical wholesale enterprises each year was 4.04,4.46 and 4.58 respectively.As shown in Fig.4,the frequency proportion of serious defective items and major defective items decreases year by year.In particular,the frequency proportion of serious defective items decreases obviously.In 2017,it is about twice the frequency proportion of defective items as in 2019.
Fig.5 shows the frequency distribution of defective items from 2017 to 2019.On the whole,from 2017 to 2019,the frequency proportion of defective items in general rules,responsibilities of quality management departments,procurement,calibration and verification,sales,delivery and others shows a decreasing trend every year.However,the frequency proportion of defective items in facilities and equipment,storage and maintenance chapters is increasing,and the frequency proportion of defective items in facilities and equipment,storage and maintenance reaches 10% and above.On the one hand,pharmaceutical wholesale enterprises may have less awareness and ability to implement GSP in those two aspects.On the other hand,the drug regulatory authorities might have stronger supervision over these two aspects.
As shown in Fig.6,the frequency proportion of the most serious defective items in each year is declining.In 2017,the frequency proportion of**00401,**00402 (pharmaceutical trading enterprises should adhere to honesty and trustworthiness,and prohibit any false and deceptive behavior) and**09101 (enterprises should issue invoices truthfully to ensure the consistency of bills,accounts,goods and funds) is higher than that of other years.Besides,the frequency proportion of these three categories in 2017 is more than twice as that of 2019[8]because the CFDA carried out a centralized rectification of illegal business practices in the field of drug circulation and the effect was better.In December 2016,the CFDA issued the Notice on Implementing the “Two-Vote System” in Drug Procurement of Public Medical Institutions (Trial)[9],and gradually conducted the “two-vote system” in drug procurement of public medical institutions,encouraging other medical institutions to use the same system in drug procurement.Every public hospital has strictly implemented the regulations on drug purchase and sale bills.Therefore,the implementation effect is good.In 2018,the NMPA issued the Notice on Further Strengthening the Supervision of Drugs,Medical Devices and Cosmetics during the Institutional Reform[10],requiring key inspections on unlicensed operation of drugs,medical devices,illegal trading of unqualified Chinese herbal medicines,failure to store and transport drugs in accordance with regulations,inconsistent goods and account,and false records.The awareness of strictly implementing the quality management standards in pharmaceutical wholesale enterprises has gradually strengthened.As a result,the frequency proportion of most serious defective items has decreased year by year since 2017.
As shown in Fig.7,in general,among the frequency proportion the major defective items each year,*04704 (the warehouse should possess equipment for automatically monitoring and recording the temperature and humidity of the warehouse) had the highest frequency proportion in 2017,reaching 3.67%.Besides,the proportion of major defective items each year was less than .3.5%.In the storage and maintenance section,the frequency proportion of *08309 (drugs and nondrugs,drugs for external use should be stored separately) and *08307 (drugs should be stacked according to the batch number,and drugs with different batch numbers shall not be mixed) was the highest in 2019,showing that pharmaceutical wholesale enterprises did not pay attention to drug placement.The frequency proportion of *04704 and*08404 (maintenance personnel should effectively monitor and control the temperature and humidity of the warehouse) decreased year by year,which could be attributed to the increasingly strict management of temperature and humidity by enterprises.
As shown in Fig.8,on the whole,the frequency proportion of general defective items (frequency≥20) is below 2.5%.In addition to the frequency of three defective items of 03401,08316 and 04705,the frequency proportion of other defective items was the highest in 2019.It can be seen that pharmaceutical wholesale enterprises need to strengthen the management of details in operation
3 Discussion and analysis
Through the above analysis,the following suggestions are made for the common problems of pharmaceutical wholesale companies in five aspects:general rules,personnel and training,facilities and equipment,storage and maintenance,and sales.
3.1 General rules
The defects in general rules are caused by subjective factors of personnel[11].In the process of pharmaceutical companies’ operation,they must run their business according to law and being honest to customers.In daily operations,some pharmaceutical wholesale enterprises still violate the regulations.For example,the enterprise changed its registered address without authorization,it was found that the registered address of the enterprise was XX,but the enterprise’s quality control department,purchasing department,storage and transportation department,invoice printing and other related facilities and equipment were all at other places,that is,the address of current warehouse XXX.Therefore,the enterprise did not perform legal exchange procedures.After reviewing the qualification of the supplier A,it was found that this company had no production approval for toxic Chinese herbal pieces,germination and frost making.In addition,it could not provide the Record Form for Dispensing Toxic Chinese Herbal Pieces by Pharmaceutical Manufacturers.The worst thing is this company offered fake certificates of qualifications,bills,etc.to other people in drug business.Some salesmen in this company rented their licenses.Drug sales records of the company were inconsistent with the real flow.The main reasons leading to the above defects are that the managers of pharmaceutical wholesale enterprises did not have quality consciousness,and just paid attention to profits.Therefore,it is necessary to implement management responsibility in pharmaceutical wholesale enterprises and strengthen the managers’awareness that enterprises must be responsible for drug quality.Managers of pharmaceutical wholesale enterprises should be aware of the risks of violating laws.The drug supervision department should strictly implement the qualification penalty so that the industry access of the pharmaceutical wholesale enterprises is linked to the implementation of GSP.Lastly,the government must intensify the crackdown on illegal business operations,dishonest behaviors of pharmaceutical wholesale enterprises.
3.2 Personnel and training
The defects in personnel and training are mainly caused by the subjective factors of personnel.Personnel defects will lead to the lack of quality system activities and bring greater risks to the quality and safety of drugs.Training is the most critical issue in this link.For example,the company did not conduct pre-job training for Mr.He,the inspector who joined the company in April 2017.Mr.Liu,the quality controller,was unfamiliar with the job responsibilities due to inadequate training.The prejob training for the newly recruited employee Mr.Zhu in retail and terminal sales department on May 9th,2018 did not include computer system operation.The problem that the person in charge of quality,the person in charge of the organization and the person in charge of acceptance work are not on duty is also serious.The main reasons for the above defects include the followings.First,enterprises do not pay attention to training,and the relevant training cannot be implemented according to documents.Second,quality management personnel is not enthusiastic about their job.It is suggested that enterprises should strengthen the importance of the training system and improve the training content,such as covering relevant laws and regulations,pharmaceutical professional knowledge and skills and post-operation procedures.Then,the sense of responsibility of quality management personnel must be enhanced,and some necessary reward and punishment measures should be taken to ensure that they can effectively perform their duties.During the inspection,the drug administration department shall judge whether the enterprise training plan is implemented based on the on-site operation effect of enterprise personnel.
3.3 Facilities and equipment
The defects in facilities and equipment may be caused by insufficient hardware of pharmaceutical wholesale enterprises or subjective factors of personnel.Facilities and equipment are the foundation and guarantee for enterprises to carry out drug business.The management of temperature and humidity automatic monitoring system is a big problem in this link.For example,if the temperature and humidity monitoring system has abnormal data,the data in the computer system will be zero.Another example is the temperature test.When the temperature exceeded the standard at 9:06,the mobile phone received an alarm message at 9:10,but the computer had no alarm record because the alarm list was not displayed.The main causes of the above defects could be the settings of temperature and humidity monitoring system,computer problems and personnel operation problems.The structural and external environment problems of the warehouse are also serious,such as peeling walls of the warehouse,spider webs in the corner,ground damage and accumulated water and dust,etc.The main reasons are the followings.First,enterprises want to save costs by ignoring the bad warehouse conditions.The quality of the staff is not high,and they are not aware of the risk caused by the environment to drug quality.It is recommended that pharmaceutical wholesale enterprises should organize staff to systematically study GSP content,clarify the requirements of individual post.Besides,they must report the facilities and equipment problems involved in their posts in time.Secondly,the training and assessment of the technical personnel of the temperature and humidity monitoring system should be strengthened.Lastly,drug administration departments should do more onsite testing of equipment instead of simply checking the temperature and humidity monitoring records.
3.4 Storage and maintenance
The defects in storage and maintenance are mainly caused by subjective factors of employees.Temperature and humidity are the worst problems in this link.For example,the historical data of the temperature and humidity monitoring of the enterprise during the on-site inspection showed that the temperature of the cool warehouse of Chinese and Western medicines in the enterprise (instrument number:01-25 right) from 20:00 on September 1st to 23:30 on September 3rd,2018 was between 21.1 ℃and 22.4 ℃,and no effective control measures were taken.Drug placement is another problem.For instance,some external drugs are mixed with other drugs.The following case shows the truth.1 piece of Methylrosanilinium Chloride Solution(batch number 170207) and 4 pieces of Ma Yinglong Musk Hemorrhoids Cream (batch number 160808)are mixed with other drugs in the western medicine room temperature warehouse on the second floor.The stacking distance of some drugs in the cool warehouse is less than 5 cm,and the distance of the temperature control pipeline facilities is less than 30 cm.The above defects may be caused by the low professional ability of personnel in the storage and maintenance and their slack thoughts.Besides,the training contents of pharmaceutical wholesale enterprises are incomplete.It is believed that pharmaceutical wholesale enterprises should focus on improving the professional competence of storage and maintenance personnel,and organize them to visit and study in other pharmaceutical wholesale enterprises.In addition,their sense of responsibility should be strengthened so that they can finish their job successfully in drug storage and maintenance.
3.5 Sales
The defects in sales are mainly caused by subjective factors of employees.For instance,in the process of sales,pharmaceutical wholesale enterprises do not issue invoices truthfully.The most common defect in sales is that the invoice,accounts,goods and payment are inconsistent.For example,enterprises sell drugs to some individual clinics and retail pharmacies without issuing invoices.There are 237 customers of Chinese herbal medicines in the downstream of the enterprise,only 4 enterprises ask for invoices.The company sells quetiapine fumarate tablets without issuing invoices,and the invoices,account,goods and payment are inconsistent.The problem that pharmaceutical wholesale enterprises do not strictly examine the qualifications of purchasing units is also obvious.Pharmaceutical wholesale enterprises should have be in accordance with the law in invoice management and strictly operate according to GSP.
4 Conclusions
The management level of domestic pharmaceutical wholesale enterprises is uneven,and the gap between enterprises in different regions is large.Therefore,their problems that can easily occur in operation are different.However,the problems in invoice management,drug temperature and humidity management,bad conditions of warehouse are the most common ones.On the one hand,pharmaceutical wholesale enterprises should strengthen personnel training based on improving facilities.On the other hand,pharmaceutical wholesale enterprises should strengthen the awareness of quality risk management to lay a good foundation for healthy development.The drug administration department can increase number of inspections on the serious problems of drug wholesale enterprises.Only through scientific supervision by drug regulatory authorities and the establishment of the quality management consciousness in pharmaceutical wholesale enterprises can the drug safety of the public be better guaranteed.
杂志排行
亚洲社会药学杂志的其它文章
- Empirical Study on Improving Customer Satisfaction of Retail Pharmacies by Using Quality Control Circle
- Research on Improving Customer Value by Using QCC in Drugstores Based on Customer Life Cycle
- Policy Suggestions on Procurement of Basic Infusion Against the Background of Volume Procurement
- Current Effect of “4+7” Drug Centralized Volume Procurement and Suggestions for Future Improvement
- Research on Development Strategy of Online Pharmacies Based on Blockchain Technology
- Research on Risk ldentification of Supply Chain Management in Pharmaceutical Wholesale Enterprises
——Taking Enterprise A as an Example
