Policy Suggestions on Procurement of Basic Infusion Against the Background of Volume Procurement
2020-12-24CuiYuepengWangZuoyuYuanHongmei
Cui Yuepeng,Wang Zuoyu,Yuan Hongmei
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To evaluate the clinical value of basic infusion packaging materials for providing suggestions on formulating basic infusion volume procurement policies.Methods Combined with market data,questionnaire surveys were used to rank different basic infusion packaging materials.Pharmacists,nurses,and experts in the field of packaging respectively assess the safety,compatibility,effectiveness,convenience,and environmental friendliness of the materials.Results and Conclusion Products with the best clinical value have a lower market share than other products with average clinical value.The best three packaging materials only account for 15%of the market share.The order of clinical value from good to bad is inner-sealed polypropylene infusion bag(BFS) > soft aseptic bag with double valves and layers > upright polypropylene infusion bag > non-PVC soft bag > plastic bottle > glass bottle.The order of market share is non-PVC soft bag > soft aseptic bag with double valves and layers > plastic bottle > vertical polypropylene infusion bag > inner-sealed polypropylene infusion bag(BFS) > glass bottle.Therefore,the clinical values of various basic infusion packaging materials are different.The following countermeasures are put forward such as separating bidding for basic infusions with different packaging materials,combining volume and price with procurement,and priority procurement of packaging materials with better clinical values.
Keyword:volume procurement; basic infusion; packaging material; clinical value assessment
In 2018,the National Healthcare Security Administration issued a document in 11 pilot areas,represented by Shanghai,which stipulated the varieties (specified specifications) and agreed purchase quantities of relevant drugs.This regulation is called volume procurement.Drug procurement with volume refers to the drug tender procurement method that takes price and procurement volume into account in the process of drug centralized bidding and purchases,which aims to reduce drug prices through pricefor-quantity[1].Most of the varieties in this volume procurement catalogue were oral dosage forms.In the procurement plan,the quality level of the bid drugs did not matter,but only drug could win the bid.In the same year,the government successively issued several policies,from clarifying the general idea to piloting,and then further standardizing and optimizing the plan of centralized drug procurement to promote its implementation[2].Besides,various pilot regions also introduced some supporting documents to guarantee the smooth progress of the drug purchase process[3,4].
As a product with a huge clinical use,the procurement of basic infusion can effectively promote the centralized procurement of drugs in China.
Basic infusions mainly include sodium chloride injection,glucose injection and glucose sodium chloride injection.The usual volumes are 50 ml,100 ml,250 ml,and 500 ml.The function of the basic infusion is to regulate the patient’s metabolism,maintain the osmotic pressure and correct the acidbase balance of the body fluid.Many therapeutic drugs need to be integrated into the basic infusion for the treatment.It is both a solvent and a carrier.It plays an irreplaceable role in clinical medicine,especially in the rescue of critically ill patients.Since the quality of basic infusions cannot be determined only by whether they pass consistency evaluation,and the volume procurement makes different basic infusions being bought at a uniform price,it may result in quality problems of the selected basic infusion.Therefore,from the perspective of basic infusion packaging materials,this study used the method of questionnaire survey to evaluate the clinical values of basic infusions with different packaging materials so as to provide reference for the government to formulate basic infusion volume procurement policies.
1 Classification of basic infusion packaging materials and market data of Liaoning Province
1.1 Classification of basic infusion packaging materials
At present,there is no authoritative document to clarify the specific division of packaging materials for basic infusions.Based on the literature review of basic infusions,we found that the classification of packaging materials is more general,which cannot be further subdivided.In terms of packaging form,researchers divided the basic infusions into several types[5,6].As to clinical value of basic infusion materials,some researchers analyzed the characteristics of these materials from different aspects[7-11].Combined with the research purpose and the iterative update of basic infusion packaging materials in the market,the packaging materials are determined as follows:glass bottle,plastic bottle,non-PVC soft bag,vertical polypropylene infusion bag,inner-sealed polypropylene infusion bag (BFS),soft aseptic bag with double valves and layers.
1.2 Market data of basic infusion in Liaoning Province
The data in this article comes from the procurement data of the centralized procurement platform for drugs and medical consumables in Liaoning Province.In 2019,hospitals in Liaoning Province spent a total of 525 million yuan on basic infusions.Hospitals in the pilot cities such as Dalian and Shenyang spent 229 million yuan,accounting for 43.6% of the total.According to 60% of the bidding,its market value was 137 million yuan.
From the market data of different packaging materials in Liaoning Province in 2019 (see Table 1),non-PVC soft bags had the largest market share among the above six packaging materials,followed by soft aseptic bags with double valves and layers and plastic bottles the third.The sum of these three types of infusion purchases accounted for about 57.1% of the total.It can be seen that the price range of packaging materials was small,and the average price was close to the median price.

Table 1 Market data of basic infusion with different packaging materials in Liaoning Province in 2019
(to be continued)

Continued Table 1
Since it is impossible to determine the quality of basic infusions based on consistency evaluation,and centralized drug procurement cares about the price and only one can win the bid.This may result in unsatisfactory quality of the selected basic infusion.Therefore,it is necessary to further study the quality of different basic infusions to determine the appropriate bidding standards.
2 Research methods
In order to make the survey results more authentic to reflect the real situation,this study uses the literature research method to determine the types of packaging materials for evaluating the clinical values of basic infusions.Then,the questionnaire is formed.After that,the data of the returned questionnaire is analyzed through the questionnaire survey method,Delphi method as well as the analytic hierarchy process (AHP)[12].The combination of subjective and objective can comprehensively reflect the clinical values of basic infusions based on different packaging materials.
3 Research on basic infusion rating based on packaging materials
3.1 Clinical indicators of basic infusion
According to the guidelines for evaluation of clinical use of basic transfusion compiled by Chinese Medical Association and Chinese Pharmaceutical Packaging Association commissioned by the health commission of China in 2018,10 indicators of basic infusion were determined such as residual volume,transparency and dosing space[13-17].Some indicators of the subject such as compatibility,environmental friendliness were determined through literature review[6,17].Eight indicators,including quality stability and air pollution isolation,were determined through expert demonstration,enterprise research and clinical user survey[18,19].The specific indicators are as follows.
3.1.1 Safety of infusion products
Firstly,the safety of infusion products refers to their safety in clinical use,mainly including bacterial endotoxin,insoluble particles,liquid leakage rate and quality of rubber plug.Then,the quality system of infusion production enterprises has been established,which is conducive to control the quality of drugs from the source.Therefore,it can ensure the safety of public medication.The indicators in this questionnaire include isolation of air pollution,quality stability,insoluble particles,bacterial endotoxin and air embolism.
The quality standard of basic infusion package materials should be based on the national stipulation,and the production enterprise should formulate internal control regulations based on the national standard.The internal control standard of enterprises should be higher or equal to the national (or industrial)one[20].Because the requirements of basic infusion production and storage determine the safety standard,the proportion of safety index in this questionnaire is low.Considering all the indicators and previous literature research[6,21],the above related indicators are added to this questionnaire.
3.1.2 Compatibility of drugs and packaging materials
The production of infusion is basically sterilized at high temperature.The packaging materials and the liquid have long-term direct contact,so the risk of interaction between the packaging materials and the preparation is high.The indicators in this questionnaire are both compatibility of drug solution and packaging materials.
3.1.3 The effectiveness of clinical use
The effectiveness of clinical use refers to administer a certain amount of preparations at a certain speed according to the requirements of treatment.The specific indicators in this questionnaire are rapid infusion under pressure,infusion residual fluid volume,patient and nurse compliance.
3.1.4 Convenience of clinical use
The convenience of clinical use means that the design of packaging materials should match the specific dosage form,delivery route and performance.The indicators in this questionnaire are transparency,drug adding space,convenient nursing and hanging,easy checking label information,storage and stacking,needle stabbing probability,convenience for light avoidance and heating.
3.1.5 Special use for emergency
Emergency refers to natural disasters,accidents,public health events and social safety events that suddenly occur and cause or may cause serious social hazards which need to be dealt with immediately.In recent years,emergencies such as floods,earthquakes,typhoons and epidemics occur more often.Therefore,it is important for the basic infusions to have such features as convenience,efficiency and practicality.This index is critical among the others.The indicators in this questionnaire are special cases.
3.1.6 Environmental friendliness
Environmental friendliness refers to whether the empty bottles and bags and other packing materials can be recovered after infusion are used.These materials must be convenient and easy to recycle.The indicators in this questionnaire are the convenience of recycling and environmental protection.

Table 2 Evaluation index system of clinical value of basic infusions
(to be continued)

Continued Table 2
In order to reflect the real situation of basic infusions with different packaging materials,three questionnaires were designed in this study and they were questionnaire for pharmacists,questionnaire for nurses and questionnaire for expert group.Considering their contact with different packaging materials for basic infusions,each questionnaire was designed with corresponding indicators for different groups,aiming to comprehensively and objectively reflect the clinical application value of basic infusions with different packaging materials.
According to the purpose of “Research Project on Clinical Application and Industrial Development of Basic Infusion”,in order to make the evaluation results of clinical value of basic infusion more universal and authentic,the research objects include hospital pharmacists,nursing staff and other experts.
On May 19,2020,1 173 and 1 988 questionnaires were collected from pharmacists and nurses.According to the pre-investigation,if pharmacists spend more than 150 seconds and the nurses spend more than 200 seconds to answer their questionnaire,it is regarded as a valid questionnaire.The returned questionnaires were screened,and some discrete data was removed.Then,the valid questionnaires were obtained,1 037 for pharmacists and 1 724 for the nurses.The recovery rates of valid questionnaires were 88.4% and 86.7%.Besides,a total of 56 questionnaires were returned from the expert group,including 36 questionnaires from hospital experts and 20 from enterprise experts.
3.2 The secondary index scores of the questionnaires for pharmacists and nurses
After removing the invalid questionnaires,the left questionnaires for pharmacists and the nurses were integrated.Lastly,the integrated scores of different indicators were averaged as the scores of secondary index.The results are shown in Table 3.
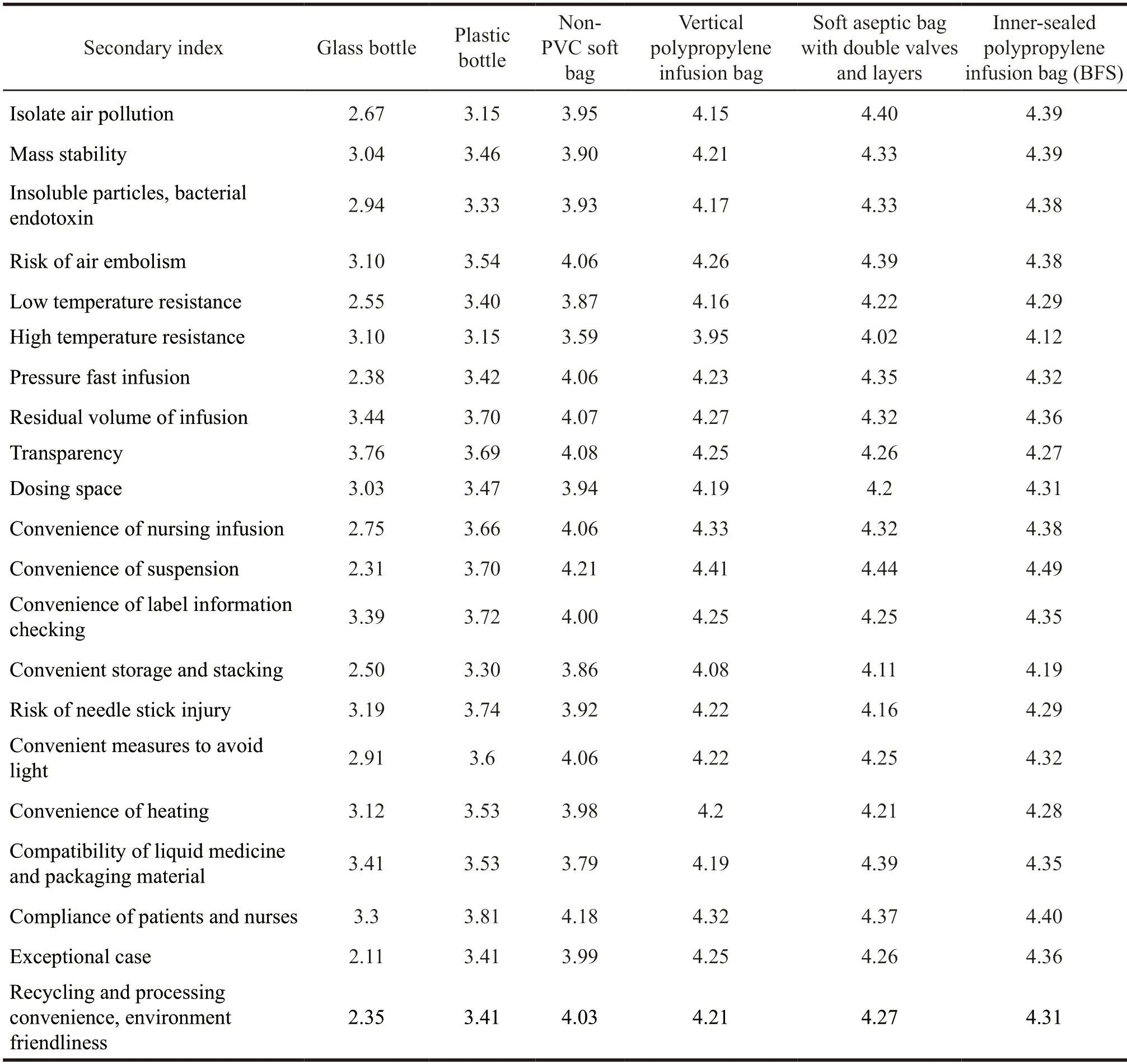
Table 3 The comprehensive scores of the six types of packaging materials in the questionnaires for pharmacist and nurse
Judging from the integrated scores of the pharmacists and nurses,the overall order is inner-sealed polypropylene infusion bag (BFS) > soft bag with double-valve aseptic packaging > vertical polypropylene infusion bag > non-PVC soft bag > plastic bottle >glass bottle.Judging from the scores of secondary index,the inner-sealed polypropylene infusion bag(BFS) has the following qualities such as stability,insoluble particles,bacterial endotoxin,low temperature resistance,high temperature resistance,low residual volume,transparency,dosing space,convenience of nursing and easy to check label information,probability of needle stick injury,convenience of avoiding light and heating,compliance of patients and nurses,convenience of recycling and disposal,environmental friendliness and safety,which are better than other packaging materials.The soft aseptic bag with double valves and layers has certain advantages in terms of isolation of air pollution,reducing air embolism,pressurized rapid infusion,and compatibility of liquid.The vertical polypropylene infusion bag also performs well in indicators of transparency,convenience of nursing and checking label information,risk of needle stick injuries,and convenience of heating.Although there are few differences in scores among the innersealed polypropylene infusion bag (BFS),the soft aseptic bag with double valves and layers and the vertical polypropylene infusion bag,they are within the same range.If we take the subjective preference factors of pharmacists and nurses and the limitations of survey sample selection into account,these scores should not be taken as determining factors.
3.3 The first-level index scores of the questionnaires for pharmacists and nurses
The scores of each secondary indicator of the questionnaires for pharmacists and nurses in Table 3 is multiplied with the weight of each secondary indicator,in Table 2,we can obtain the scores of each primary indicator of the questionnaires for pharmacists and nurses,see Table 4.

Table 4 Summary of the scores of each indicator of questionnaires for pharmacists and nurses
Judging from the scores of each basic infusion products integrated by the questionnaires for pharmacists and the nurses,it can be seen that the inner-sealed polypropylene infusion bag (BFS) has better scores than other basic infusion packaging materials in the four aspects of safety,convenience,particularity,and environmental friendliness.But as to compatibility and effectiveness,the soft aseptic bag with double valves and layers has certain advantages.
3.4 Final scores of the clinical value of basic infusions with different packaging materials
This study uses AHP to determine the weights of the first-level indicators.The specific weights are shown in Table 5.
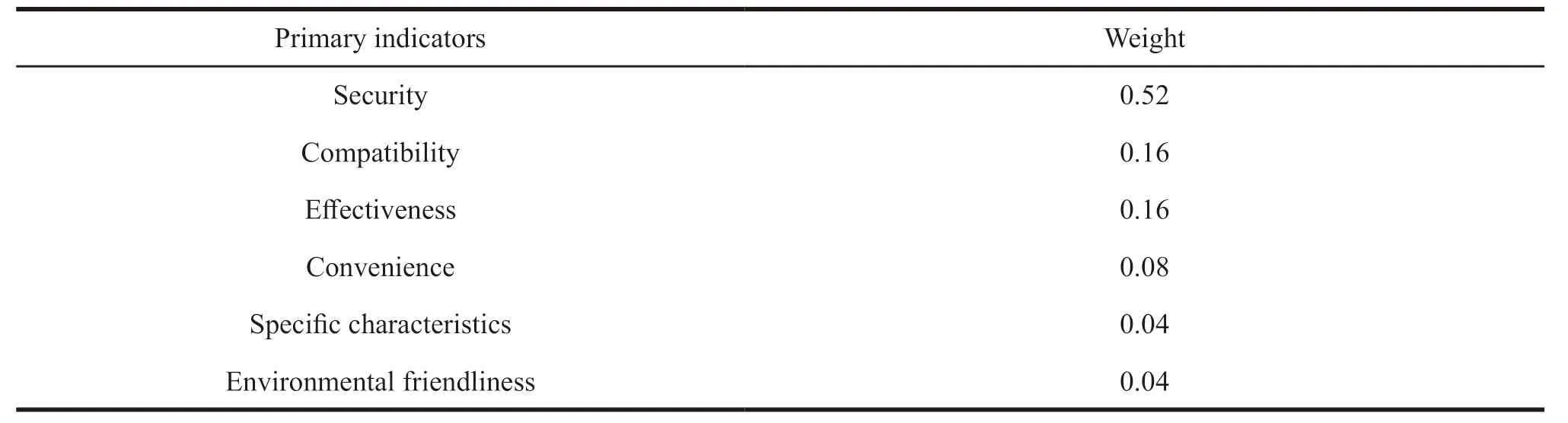
Table 5 10 experts from hospitals and 4 from enterprises determine the weights by AHP
This study adopts three forms to determine the final scores of the clinical value of the basic infusions with different packaging materials.One is the result of multiplying the scores of the first-level indicators determined above by the weights of the respective first-level indicators according to the constructed basic infusion clinical value evaluation system.Then,the sum should be done after multiplying the firstlevel indicators of different packaging materials by the weights,which will be used as the final scores.According to the last question in the questionnaire,the respondent’s scoring results on the clinical value of the basic infusions with different packaging materials,the average of the scores of different respondents can be taken as the final scores.Lastly,based on the scoring results of the experts on the clinical value of the basic infusions with different packaging materials,the average of the scores of the experts is used as the final score of each packaging material.See Table 6 for specific scores.

Table 6 Final scores of different packaging materials
According to the evaluation results of the clinical value of the basic infusions with 6 different packaging materials,the new packaging materials for basic infusions emerged in recent years such as vertical polypropylene infusion bags,soft aseptic bag with double valves and layers,and inner-sealed polypropylene infusion bags (BFS) have better clinical value,and glass bottles have the lowest clinical value.
4 Conclusions and recommendations
This article distinguishes the qualities by grading the clinical value of basic infusions with different packaging materials,and provides suggestions for formulating volume procurement policies.The results show that the clinical value in order from good to bad is inner-sealed polypropylene infusion bag(BFS) > soft aseptic bag with double valves and layers > vertical polypropylene infusion bag >non-PVC soft bag > plastic bottle > glass bottle.Combining the procurement data of basic infusions in Liaoning Province,the market share of these six products is in the order of non-PVC soft bag > soft aseptic bag with double valves and layers > plastic bottle > vertical polypropylene infusion bag > innersealed polypropylene infusion bag (BFS) > glass bottle.The top three packaging materials account for 15% of market share.It can also be seen from the price data that,except for the lower prices of plastic bottles and glass bottles,the price range and average price of other packaging materials are almost the same.
The suggestions made in this article are as follows.
(1) According to the national drug centralized procurement policy,different packaging materials need to be bid separately.A unique procurement policy that wins the bid for each packaging material is adopted.For example,in 2019,Qinghai Province issued the Centralized Drug Purchasing Bidding of Qinghai Province in 2019,which marked the launch of volume purchases for key products and basic infusions.It separated tenders for plastic bags,soft bags and vertical soft bags for basic infusion.
(2) If the centralized procurement is carried out in a province,one department should be responsible for bidding,procurement and reimbursement(or payment),so as to realize the integration of volume and price.It is different from the traditional procurement method in which several departments are in charge.One department is responsible for price negotiation and others for the volume of the drugs.
(3) As to market share and purchase volume,price is not the most important factor in the selection of infusion packaging materials.The market share of soft aseptic bag with better clinical value is more,while glass bottle with the lowest clinical value has small market share.Therefore,the purchase of basic infusions with better clinical value can be given priority when bidding,thus achieving the two-in-one effect of reducing the price and improving the quality of basic infusions.
However,the government needs to consider the overall factors when purchasing basic infusions.This article only studies the clinical value of different basic infusion packaging materials.It does not fully consider other costs or factors that may occur during the procurement of basic infusions.Future research should take all the factors that can affect basic infusion procurement into consideration.
杂志排行
亚洲社会药学杂志的其它文章
- Empirical Study on Improving Customer Satisfaction of Retail Pharmacies by Using Quality Control Circle
- Research on Improving Customer Value by Using QCC in Drugstores Based on Customer Life Cycle
- Statistical Analysis of Defective Items of Pharmaceutical Wholesale Enterprises
- Current Effect of “4+7” Drug Centralized Volume Procurement and Suggestions for Future Improvement
- Research on Development Strategy of Online Pharmacies Based on Blockchain Technology
- Research on Risk ldentification of Supply Chain Management in Pharmaceutical Wholesale Enterprises
——Taking Enterprise A as an Example
