AlCl3 exposure regulates neuronal development by modulating DNA modification
2020-12-22XueJunChengFuLaiGuanQianLiGongDaiHaiFengLiXueKunLi
Xue-Jun Cheng, Fu-Lai Guan,Qian Li, Gong Dai,Hai-Feng Li, Xue-Kun Li
Xue-Jun Cheng, Qian Li, Hai-Feng Li, Xue-Kun Li,The Children's Hospital, School of Medicine, Zhejiang University, Hangzhou 310052, Zhejiang Province, China
Xue-Jun Cheng, Qian Li, Xue-Kun Li,The Institute of Translational Medicine, School of Medicine, Zhejiang University, Hangzhou 310029, Zhejiang Province, China
Xue-Jun Cheng, Qian Li, Hai-Feng Li, Xue-Kun Li,National Clinical Research Center for Child Health, Hangzhou 310052, Zhejiang Province, China
Fu-Lai Guan, Gong Dai,School of Basic Medicine, Weifang Medical University, Weifang 261053, Shandong Province, China
Abstract
Key Words: Aluminum; DNA demethylation; 5-hydroxymethylcytosine; Neural stem cells; Neuron; Neuronal development
INTRODUCTION
Epigenetic modifications mainly include histone posttranslational modifications, DNA and RNA methylation and demethylation, and non-coding RNAs. Previous studies have indicated that epigenetic pathways play a critical function in diverse biological processes[1,2]. DNA methylation, mainly on the fifth carbon of cytosine [5-methylcytosine (5mC)] in mammalian, is established by DNA methyltransferases (DNMTs), including DNMT1, DNMT3A, and DNMT3B. In embryonic and postnatal neuronal development, the deficiency ofDNMTsaffects embryonic viability, cell survival, synaptic development, and learning and memory; however, neuronal activity could influence DNA methylation, suggesting that DNA methylation is important for normal neuronal function[3-5].
Recent studies have shown that 5mC can be further converted to 5-hydroxymethylcytosine (5hmC) by ten-eleven translocation (TET) proteins including TET1, TET2, and TET3[6,7]. 5hmC is significantly enriched in the brain relative to many other tissues and cell types, is acquired during postnatal neurodevelopment and aging, and displays spatial and temporal dynamics. Recent studies have shown consistently that Tet1 regulates neuronal activity, the formation and extinction of memory, and neurogenesis[8-13]. Recently, DNA N6-methyladenine (6mA) modification has been uncovered, which regulates gene expression and is involved in neuronal outcomes induced by environmental stress[14,15].
Aluminum is a neurotoxin and is associated with neuronal inflammation, memory impairment, and neurological disorders through different mechanisms[16]. AlCl3exposure (50-100 mg/kgin vivo) significantly exacerbates amyloid beta (Aβ) deposition, plaque formation, and tau phosphorylation; causes cognitive dysfunction and mitochondria oxidative; and therefore induces Alzheimer’s disease-like phenotypes in rats[17-19]. Aluminum exposure (25 mg/kgin vivoor 0.5 mmol/Lin vitro) also stimulates the expression of pro-inflammatory cytokines including TNF-α and IL-6, induces the production of reactive oxygen species (ROS), and then causes neuroinflammation and DNA damage[19-21]. However, it remains largely unknown whether aluminum has a neurotoxic effect by altering epigenetic states.
In the present study, we found that aluminum (AlCl3) skewed the differentiation of adult neural stem cells (aNSCs) toward glial cells and induced apoptosis of newborn neurons. Furthermore, aluminum inhibited the morphological development of neurons generated upon aNSC differentiation and hippocampal neurons. Finally, we found that AlCl3exposure differentially altered the level of DNA methylation and hydroxymethylation in aNSCs and neurons by regulating the expression of DNA methyltransferases and dioxygenases. In summary, our results suggest that AlCl3exerts a neurotoxic effect by modulating DNA modifications.
MATERIALS AND METHODS
Animals
Mice were housed in a standard condition of the Animal Center of Zhejiang University on a 12 h light/dark cycle with free access to food and water. All animal experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee of Zhejiang University.
Isolation and culture of adult neural stem cells
The isolation and culture of adult neural stem cells were performed according to an established protocol[9]. The aNSCs were cultured in DMEM/F-12 medium containing 20 ng/mL FGF-2 (Catalog No. 100-18B-B, PeproTech), 20 ng/mL EGF (Catalog No. 100-15, PeproTech), 2% B27 supplement (Catalog No. 12587-010, Thermo Fisher Scientific), 1% antibiotic-antimycotic (Catalog No. 15140-122, Thermo Fisher Scientific), and 2 mmol/L L-glutamine (Catalog No. 25030–149, Thermo Fisher Scientific) in a humidified incubator supplied with 5% CO2at 37 °C.
AlCl3 exposure
AlCl3was dissolved with nuclease free water to 50 mmol/L and applied to cells at a final concentration of 100 μmol/L or 200 μmol/L. The cells were collected at scheduled time-point forin vitroassay.
Proliferation and differentiation assays in vitro
Forin vitroproliferation assay, aNSCs were cultured on coverslips with medium supplied with 5 mmol/L BrdU for 8 h. Forin vitrodifferentiation assay, aNSCs were cultured on coverslips with proliferation medium, and then transferred into differentiation medium containing 1 mmol/L retinoic acid (Catalog No. R-2625, Sigma) and 5 mmol/L forskolin (Catalog No. F-6886, Sigma) for 48 h.
Isolation and culture of embryonic hippocampal neurons
Primary neurons were isolated from the hippocampus of E16-E18 mice and seeded in cell climbing slices (Corning, 354087) or plates that were coated with poly-D-lysine (5 μg/mL, Sigma, P0899-10). Approximately 1 × 105cells per well were seeded for a slice, while 1.5 million cells per well were seeded for a 6-well-plate. After growing in the plating medium for 4 h, which consisted of MEM (Gibco,11095-080), 10% FBS (Gibco,10091-148), 1% L-Glu (Gibco, 5030-149), 1% sodium pyruvate (Gibco, 11360-070), and 0.45% D-glucose (Amresco, 0188), the medium was changed to a maintaining medium that consisted of neurobasal (Gibco, 21103-049), 0.25% L-Glu (Gibco, 25030-149), 0.125% GlutaMax (Thermo, 35050061), and 2% B27 (Gibco, 17504-044). The medium was renewed half of the liquid volume every 3 d.
Immunofluorescence staining and quantification
To detect the function of proliferation and differentiation, cell samples were washed with PBS for 30 min followed by blocking with PBS containing 3% normal goat serum and 0.1% triton X-100 for 1 h at room temperature. Samples were incubated with primary antibodies overnight at 4 °C. For BrdU immunostaining, samples were treated with 1M HCl at 37 °C for 30 min before blocking. The following primary antibodies were used: GFAP (Catalog No. Z0334, DAKO), Tuj1 (Catalog No. G712A, Promega), Caspase 3 (Catalog No. AB3623, Millipore), and BrdU (Catalog No. ab6326, Abcam). The second day, after being washed with PBS for 30 min, sections were incubated with fluorophore-conjugated secondary antibodies for 1 h at room temperature. After final washes, samples were mounted onto glass slides and cover slipped with mounting medium. Images were captured using a Nikon invert microscope, and the numbers of BrdU+, Tuj1+, GFAP+, and Caspase3+Tuj1+cells were quantified with image J software (NIH).
Total RNA isolation, reverse transcription, and quantitative real-time PCR
Total RNA was extracted with TRIzol reagent (Catalog No. 15596018, Thermo Fisher Scientific) following the manufacturer’s protocol. The concentration was determined using a NanoDrop 2000 spectrophotometer, and 500 ng of total RNA was subjected to reverse transcription. All real-time PCR reactions were performed in triplicate using power SYBR Green PCR master Mix (Catalog No. Q71502, Vazyme), and the results were analyzed using the∆∆Ct method. The sequence of all the used primers can be found in Supplementary Table 1.
Western blot analysis
Cells were washed with PBS and resuspended in RIPA (Catalog No. ab156034, Abcam) containing 1 × protease inhibitor cocktail (Catalog No. 04693124001, Sigma). The samples were centrifuged at 4 °C for 20 min at 14000 rpm, and the supernatants were collected for further experiments. Protein concentrations of the samples were measured with a BioPhotometer, and 20 μg of each sample was used for electrophoresis after denaturation for 5 min at 95 °C. Samples were subjected to SDSpolyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The following primary antibodies were used: Anti-Tuj1 (Catalog No. G712A, Promega), anti-GFAP (Catalog No. 3670, Cell Signaling), and anti-GAPDH (Catalog No. AM4300, Thermo Fisher Scientific). Secondary HRP conjugated antibodies were applied for 1 h at room temperature. The signal was detected with the Tanon 5200 Detection system, and the relative level of signal intensity was normalized to that of GAPDH.
Genomic DNA extraction and DNA dot-blot
DNA extraction and DNA dot-blot were performed as described previously[9]. The following primary antibodies were used: 5mC (Catalog No. 61255, Active Motif), 5hmC (Catalog No. 39769, Active Motif), and 6mA (Catalog No. 61496, Active Motif).
Statistical analysis
All data are expressed as the mean ± SE. GraphPad Prism (GraphPad Software Inc.) was used for statistical analyses. Unpaired student’st-test was used to determine the differences between two groups with at least three replicates.P< 0.05 was considered statistically significant.
RESULTS
AlCl3 affects the differentiation of aNSCs and survival of newborn neurons
To determine the effects of AlCl3on the proliferation of aNSCs, aNSCs were exposed to AlCl3for 48 h, and BrdU was administered at 5 μmol/L for 8 h followed by immunofluorescence staining (Supplementary Figure 1A). The quantification results showed that the number of BrdU positive (BrdU+) cells did not show a significant difference between control and AlCl3exposure aNSCs (Supplementary Figure 1B), suggesting that AlCl3does not affect the proliferation of aNSCs.
To examine the effects of AlCl3on the differentiation of aNSCs, aNSCs were exposed to AlCl3for 2 d and then underwent differentiation induction. Immunostaining for neuronal cell marker Tuj1 and astrocyte marker GFAP was performed (Figure 1A). The quantification results of immunofluorescence staining showed that the number of neuronal marker Tuj1 positive cells was significantly decreased (Figure 1B), but the number of glial cell marker GFAP positive cells increased after AlCl3application (200 μmol/L) (Figure 1C). We also detected the expression levels of Tuj1 and GFAP by qRT-PCR and Western blot, and we found that the level of Tuj1 decreased, while the level of GFAP increased (Figure 1D-F). Taken together, these results suggest that AlCl3regulates the differentiation of aNSCs.
To determine whether AlCl3affects the survival of newborn neurons, we performed immunofluorescence staining for Tuj1 and caspase 3. Representative images (Figure 1G) and quantification results (Figure 1H) show that AlCl3at a dosage of 200 μmol/L significantly increased the number of Caspase3 positive cells, suggesting that AlCl3exposure induces apoptosis of newborn neurons.
AlCl3 inhibits the maturation of newborn neurons derived from aNSCs and hippocampal neurons
Next, we aimed to investigate whether AlCl3regulates neuronal development. We first analyzed the effects of AlCl3on the development of newborn neurons generated upon the differentiation of aNSCs. Immunostaining (Figure 2A) and Sholl analysis showed that AlCl3at a dosage of 200 μmol/L significantly decreased dendritic length and the number of intersections (Figure 2B-D).
Next, we isolated neurons from the hippocampal tissues of embryonic mice and examined the effects of AlCl3on the development of primary neurons. Immunofluorescence (Figure 2E) and Sholl analysis showed that AlCl3exposure at both 100 μmol/L and 200 μmol/L dosages significantly decreased the intersection number and dendritic length of hippocampal neurons (Figure 2F-H). Collectively, these results indicate that AlCl3exposure inhibits neuronal development.
AlCl3 regulates DNA methylation and demethylation of aNSCs and neurons
Previous studies have shown the important function of DNA modifications in neurogenesis and neuronal development[22,23]. To dissect the molecular mechanisms by which AlCl3regulates neuronal development, we first analyzed the effects of AlCl3on the DNA modifications of aNSCs and neurons. DNA dot-blots and quantification results showed that AlCl3exposure increased the global level of 5-hmC in proliferating and differentiated aNSCs, but AlCl3exposure decreased the global levels of 5-mC and 6mA (Figure 3A-F).
Next, we analyzed the effects of AlCl3on the DNA modifications in neurons. DNA dot-blot and quantification results showed that AlCl3exposure increased the global levels of 5-mC and 6mA but decreased the global level of 5-hmC in hippocampal neurons (Figure 3G-L). Taken together, these results indicate that AlCl3alters the epigenetic state in aNSCs and neurons.
AlCl3 differentially regulates the expression of DNA modification related genes in aNSCs and neurons
Next, we examined the expression of genes related to DNA modifications. We found that AlCl3exposure significantly increased the mRNA level ofTet2but did not affect the mRNA levels ofTet1andTet3in aNSCs (Figure 4A-C). Meanwhile, AlCl3exposure significantly decreased the mRNA level of DNA methyltransferasesDnmt1(Figure 4D) but did not affect the levels ofDnmt3aandDnmt3b(Figure 4E and F).
We then aimed to determine the effects of AlCl3on the expression ofTetsandDNMTsin neurons. We found that AlCl3exposure decreased the mRNA levels ofTet1andTet3while not affecting the level ofTet2(Figure 4G-I) in neurons. Furthermore, AlCl3exposure led to a decrease in hte mRNA levels ofDnmt1andDnmt3abut induced an increase inDnmt3b(Figure 4J-L). Taken together, these results suggest that AlCl3differentially regulates the expression of genes relating to DNA methylation and demethylation in aNSCs and neurons.
DISCUSSION
Previous studies have shown that DNA modifications play an important role in neuronal development and function and that dysregulation of DNA modifications is involved in neurological disorders[24-26]. Machineries of regulating DNA modifications have been identified. In the present study, we found that aluminum inhibits the differentiation of aNSCs and the development of neurons. Furthermore, aluminum can induce apoptosis of newborn neurons derived upon the differentiation of aNSCs. Mechanistically, aluminum affects the global level of 5mC, 5hmC, and 6mA in aNSCs and neurons by regulating the expression of DNA modification associated genes includingTetsandDNMTs. Taken together, our results reveal a novel mechanism for regulating adult neurogenesis.
In adult mammalian brain, two regions, the subventricular zone in the lateral ventricle and subgranular zone in the dentate gyrus of hippocampus, maintain the neurogenic capacity[27]. Adult neurogenesis is driven by aNSCs and regulated by multiple mechanisms including environmental stimuli, genetics, and epigenetics including DNA modifications[22,27,28]. Our results show that aluminum can affect the differentiation of aNSCs and induce apoptosis of newborn neurons. Therefore, our study reveals the roles of aluminum in regulating neuronal development and associated mechanisms.
DNA modifications are regulated by diverse factors, such as environmental stimuli and food nutrients. Nutrient Vitamin C can serve as a cofactor for Tet and improves the reprogramming and neuronal differentiation by enhancing the expression level ofTetsand therefore increasing the global level of 5hmC[29,30]. As one type of environmental pollution, the excessive intake of aluminum could induce inflammatory responses and oxidative stress, and then cause toxic effects on the neural, immune, and reproductive systems. Aluminum exposure also increases apoptosis and impairs learning and memory in adult rats[31]. These findings indicate the crosstalk between environmental signal and epigenetic modifications.
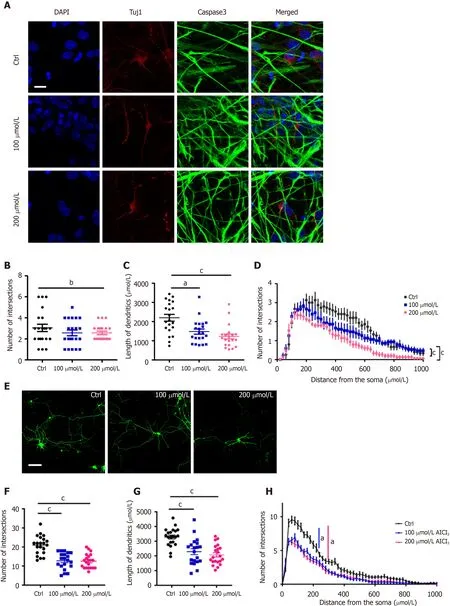
Figure 2 AlCl3 exposure inhibits neuronal development.
CONCLUSION
In summary, our findings show the neurotoxic effect of aluminum on neuronal development. One limitation of the present study is that the data were collectedin vitro. A further study should be performed to examine the effects of aluminum on neuronal development and DNA modificationsin vivo.
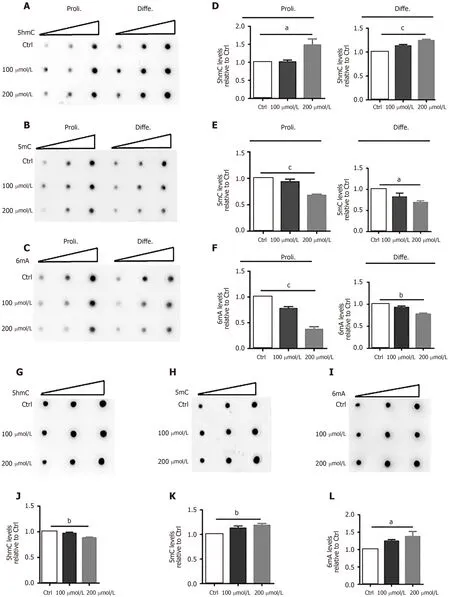
Figure 3 AlCl3 exposure alters the levels of DNA 5-hydroxymethylcytosine, 5-methylcytosine, and N6-methyladenine in adult neural stem cells and neurons.
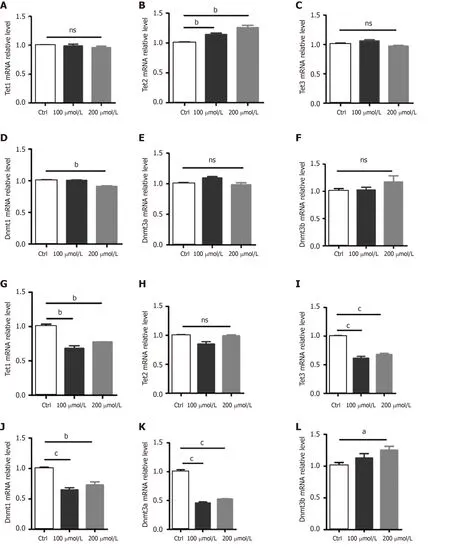
Figure 4 AlCl3 exposure regulates the expression of Tets and Dnmts at the transcriptional level in adult neural stem cells and neurons.
ARTICLE HIGHLIGHTS
Research background
With the industrial development of society, environmental pollution is becoming a serious challenge for humans. Previous studies have revealed the crosstalk between environment and epigenetics and consequent phenotypes.
Research motivation
Aluminum pollution is a common issue and its exposure induces neurotoxic effects and impairs neuronal development and cognitive function.
Research objectives
To study the effects of aluminum on epigenetics in the context of neuronal development.
Research methods
Neural stem cells were isolated from the brain of adult mice. Hippocampal neurons were isolated from the brain of embryonic mouse pups. The levels of DNA modifications were detected by dot-blot. The levels of DNA modification related genes were examined by qRT-PCR.
Research results
Our present findings uncovered the roles of aluminum in inhibiting neuronal development and promoting cell death. Our results also showed that aluminum exposure can display significant effects on DNA modifications.
Research conclusions
Our study indicated that aluminum exposure regulates neuronal development by modulating DNA modifications.
Research perspectives
Future studies should be performed to examine whether DNA modification could be a target for the treatment of neurological disorders induced by aberrant neuronal development.
ACKNOWLEDGEMENTS
We thank Dr. Dorazio RM for editing the manuscript. We thank Li YZ (Li Yanze) for narrating the core tip.
杂志排行
World Journal of Stem Cells的其它文章
- Acquired aplastic anemia: Is bystander insult to autologous hematopoiesis driven by immune surveillance against malignant cells?
- Glutathione metabolism is essential for self-renewal and chemoresistance of pancreatic cancer stem cells
- Effect of conditioned medium from neural stem cells on glioma progression and its protein expression profile analysis
- Immunophenotypic characteristics of multipotent mesenchymal stromal cells that affect the efficacy of their use in the prevention of acute graft vs host disease
- Isolation and characterization of mesenchymal stem cells in orthopaedics and the emergence of compact bone mesenchymal stem cells as a promising surgical adjunct
- New insight into dental epithelial stem cells: Identification, regulation, and function in tooth homeostasis and repair
