Advance in metabolism and target therapy in breast cancer stem cells
2020-12-22XuGaoQiongZhuDong
Xu Gao, Qiong-Zhu Dong
Xu Gao,Department of Breast Surgery, Yiwu Maternity and Children Hospital, Yiwu 322000, Zhejiang Province, China
Qiong-Zhu Dong,Department of General Surgery, Cancer Metastasis Institute, Institutes of Biomedical Sciences, Huashan Hospital, Fudan University, Shanghai 200032, China
Abstract
Key Words: Breast cancer; Cancer stem cells; Metabolism; Oxidative phosphorylation; Tumor relapse; Target therapy
INTRODUCTION
Breast cancer is the most common cancer in women globally and also causes the greatest number of cancer-related deaths among women worldwide[1,2]. As a highly heterogeneous disease, breast cancer shows different morphological and physiological characteristics[3,4]. Therefore, it shows different clinical outcomes for different therapeutic strategies. Currently, assessment of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) status of the breast cancer specimens from the patients is considered a standard due to their predictive and prognostic implications[5-7]. Unfortunately, not all patients with ER or HER2 positive tumors respond to endocrine or anti-HER2 therapy[8,9]. Moreover, triplenegative breast cancer, negative for ER, PR, and excess HER2, is considered to be more aggressive and have a poorer prognosis than other types of breast cancer[10,11]. Therefore, we need to further understand the mechanism of breast cancer to develop more effective therapeutic strategies.
Cancer stems cells (CSCs), also known as tumor-initiating cells, are a subset of cancer cell groups that have the ability to renew themselves and promote tumor progression, recurrence, metastasis, and resistance to therapy[12,13]. CSCs have also been proposed as one of the determining factors contributing to tumor heterogeneity[14]. Therefore, the theory of CSCs provides a reasonable explanation for tumor heterogeneity. These subpopulations of cells are believed to be responsible for therapeutic resistance and tumor relapse[15,16].
With the deepening of research on breast CSCs (BCSCs), they are initially characterized by the expression of specific cell markers, such as high levels of cluster of differentiation 44 (CD44+) and low levels of cluster of differentiation 24 (CD24-)[17]. Similar to CD44+/CD24- cancer cells, aldehyde dehydrogenase (ALDH) is also one of the hallmarks of BCSCs[18]. Moreover, the prognosis of breast cancer patients with ALDH+ tumors was poorer than that of the ALDH- patients[19]. Interestingly, 500 ALDH+ BCSCs can form tumors in NOD/SCID mice[20]. The other markers mainly used to isolate and identify BCSCs in all types of breast cancer are CD133, EpCAM, CD166, LGR5, CD47, and ABCG2[21-23].
Emerging evidence shows that the metabolic properties of cancer cells are significantly different from those of normal cells[24,25]. Cancer cell metabolism is characterized by dysregulated glucose metabolism, fatty acid (FA) synthesis, and glutaminolysis, which lead to therapeutic resistance in cancer treatment[23,26,27]. Furthermore, a number of metabolic enzymes that are often upregulated in cancer cells may become novel targets for anticancer drug development[28,29]. Given the critical role of CSCs in tumors, targeting of the CSCs metabolism may provide new therapies to reduce therapeutic resistance and tumor relapse. Despite the importance of CSCs, there are few review articles summarizing therapeutic targeting of CSC metabolism, especially targeting of BCSCs metabolism. In this review, we summarize the important findings about therapeutic targeting of BCSC metabolism. First, we will describe the specific markers for identification and isolation of BCSCs. Then, the metabolic characteristics of BCSCs will be summarized. Finally, we will summarize the targeted therapies based on the metabolic characteristics of BCSCs.
BCSC MARKERS
CSCs were first identified by John Dick’s team in human acute myeloid leukemia in the late 1990s[30,31]. They provided the first evidence that human acute myeloid leukemia is organized as a hierarchy that originates from a hierarchy of leukemic stem cell classes harboring the potential of self-renewal, propagation, and differentiation. A subpopulation of leukemia cells that expressed surface marker CD34 but not CD38 (CD34+/CD38-) is capable of initiating tumors in non-obese diabetic, severe combined immunodeficient (NOD/SCID) mice that were histologically similar to the donor. In solid tumors, CSCs were first demonstrated in breast cancer. A small subpopulation of CD44+/CD24- cells (100 cells) enriched from human breast cancer tissue were able to generate tumors in NOD/SCID mice. Differently, CD44-/CD24+ cells do not exhibit tumor growth, even at a very high cell number[32]. Since then, the existence of CSCs has been confirmed in various cancers.
To better understand the functions of BCSCs, most of the studies in BCSCs have been focused on the identification of biomarkers on BCSCs. As a receptor for hyaluronic acid, CD44 can interact with other ligands, such as osteopontin, collagen, and matrix metalloproteinase[33]. Although CD44+/CD24- cells have been proved to have the characteristics of BCSCs, CD44+/CD24- cells are not present in all types of breast cancer cells[34-37]. Therefore, more BCSCs markers are required to be found in breast cancer cells.
Several other proteins have been then identified as important markers for BCSCs, including ALDH, CD326 (EpCAM), and CD133[13]. Alternatively, increased activity of ALDH detected by the Aldefluor assay in breast cancer cells was identified as another marker of BCSCs. ALDH is able to detoxify a variety of endogenous and exogenous aldehydes and is required for the biosynthesis of retinoic acid (RA) and other regulators of cell functional molecules[38]. Downregulation of ALDH expression is accompanied by a decline in BCSC characteristics[39]. Upregulation of ALDH expression is usually found in malignant BCSCs and positively correlated with poor prognosis[38,40]. Importantly, the subpopulation of breast cancer cells with a CD44+/CD24- phenotype and high ALDH enzymatic activity has become the “gold standard” signature for BCSCs, because only 20 positive cells can be tumorigenic[41-43].
Epithelial cell adhesion molecule (EpCAM), a transmembrane glycoprotein, has been implicated in multiple cellular functions including cell adhesion[44]. Knockdown of EpCAM was found to reduce the proliferation, migration, and invasion of breast cancer cells[45,46]. Moreover, clinical studies also showed that EpCAM is upregulated in breast cancer cells and is associated with a poor prognosis[45,47]. Recently, EpCAM has been identified as a marker of BCSCs and participates in promoting bone metastasis by enhancing tumorigenicity. BALB/c mice inoculated with EpCAM positive cells had a high bone metastasis potential, implying a possible target for the treatment of bone metastases with EpCAM in BCSCs[48,49].
CD133, transmembrane single-chain glycoprotein, is also identified as an important surface marker of BCSCs[35]. Importantly, CD133 is the most commonly used marker for isolation of CSC population from different tumors including breast cancer[50]. In normal breast tissue, CD133 is not a stem cell marker and plays an important role in morphogenesis. Upregulation of CD133 is accompanied by the increased malignancy and multidrug resistance by enhancing phosphatidylinositol-3-kinase (PI3K)-Akt signaling in breast cancer cells[51]. CD133 positive cells isolated from breast cancer cells display increased capability of tumorigenicity, self-renewal, proliferation, and differentiation into different types of cells[52]. Meanwhile, CD133 positivity means higher mortalities and poor prognosis among the breast cancer patients. CD133 expression was the highest in triple-negative breast cancer specimens[53]. Together, CD133 can be regarded as a useful surface marker for identifying BCSCs and a useful indicator for predicting prognosis in clinical practice.
In addition to the above mentioned markers of BCSCs, various potential markers for BCSCs were also identified from breast cancer cell lines. For example, activated leukocyte cell adhesion molecule (ALCAM/CD166) predicts response to adjuvant chemotherapy in breast cancer[54]. Meanwhile, CD166+ cells are enriched for ERα and possess a BCSC phenotype[55]. ATP-binding cassette sub-family G member 2 (ABCG2), which plays a key role in multi-drug resistance to chemotherapeutic drugs, also has been identified as an effective BCSC marker[56]. Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) has been identified as a BCSC marker by maintaining the stemness through activating Wnt-regulated target genes in breast cancer[57]. CD47, also known as integrin associated protein (IAP), was found upregulated in BCSCs[58]. A function-blocking CD47 antibody was proved to suppress the stemness in triple-negative breast cancer cells[59]. MUC1, a tumor antigen of breast cancer known as CA153, is also expressed on BCSCs. MUC1 positive cancer cells have multiple characteristics of BCSCs[60]. Although many BCSC markers have been found by flow cytometry (summarized in Table 1), more differentiated BCSC markers usually change according to different subtypes of breast cancer cells, histological stage, and internal heterogeneity[60]. Moreover, there is no universal marker that is specific for identification of BCSCs. These BCSC markers mentioned above are also used to isolateother types of CSCs. In patients with breast cancer accompanied by other primary tumors, the use of BCSC markers is limited. Therefore, further studies are still needed to determine the function and mechanism of BCSC markers in breast cancer cells.
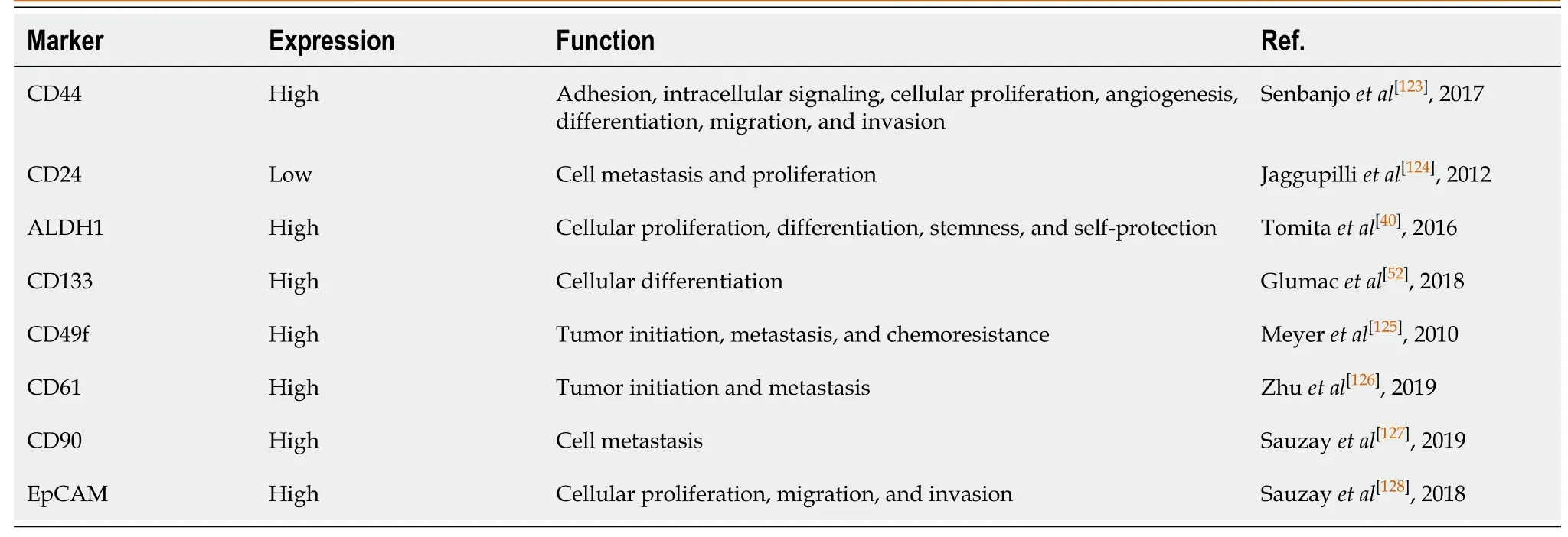
Table 1 Breast cancer stem cell markers
METABOLIC FEATURES OF BCSCS
Metabolic adaptation is one of the hallmarks of cancer cells. Cancer cell metabolism is characterized by dysregulated glucose metabolism, FA synthesis, and glutaminolysis to provide constant support for the increased division rate[61-63]. The unique metabolic characteristics of cancer cells provide an effective strategy to treat cancer with metabolic targeted therapy. Cancer is a highly heterogeneous disease with different kinds of morphological and physiological characteristics[64]. Due to this characteristic of cancer cells, different clinical results are associated with different treatment strategies. Unfortunately, most studies do not consider the cellular heterogeneity present in cancer cells. CSCs are considered one of the determinants of cellular heterogeneity in cancer cells. Therefore, the strategy of switching cancer cell metabolism to CSC metabolism may solve the problem of heterogeneity of cancer cells during metabolic targeted therapy. Here, we summarize the studies about therapeutic targeting of BCSC metabolism. First, it is necessary to summarize the metabolic characteristics of BCSCs.
Due to the small number of studies on the metabolism of CSCs, especially BCSCs, the metabolic features of CSCs remain largely unknown[65]. Nonetheless, limited studies show that the metabolic characteristics of CSCs are different from those of normal cells and cancer cells. Glucose is an essential nutrient for CSCs like that of cancer cells. More specifically, CSCs (including BCSCs) were more glycolytic than other differentiated cancer cells[66]. CSCs show increased glucose uptake, lactate production, and ATP content compared with other differentiated cancer cells[67]. Several studies have supported that BCSCs showed enrichment of glycolytic proteins (such as PKM2 and LDHA), as well as increased pyruvate kinase and lactate dehydrogenase activities[68,69]. Meanwhile, the levels of glycolysis intermediates, such as fructose 1,6-diphospate, pyruvate, lactic acid, and ribose 5-phosphate were found to be significantly higher in BCSCs[70]. Thus, the inhibitors of glycolysis are expected to be used to treat cancer with metabolic targeted therapy. Reports have demonstrated that inhibitors of glycolysis reduce the proliferation of BCSCs[21,69].
Although studies have shown that BCSCs are dependent on glycolysis, other studies have shown that BCSCs may also depend on mitochondrial oxidative phosphorylation (OXPHOS) metabolism[71-73]. Compared to their differentiated progeny, BCSCs are more dependent on OXPHOS[73,74]. BCSCs produces less lactate and lower levels of reactive oxygen species (ROS) by OXPHOS[69]. Meanwhile, BCSCs have more functional mitochondria, and have higher ATP content by OXPHOS[75]. Interestingly, emerging evidence shows that BCSCs have a preference for OXPHOS metabolism in the proliferative state[69,72,76]. In the quiescent state, BCSCs exhibit a higher glycolytic rate[10,75]. Moreover, metabolism of BCSCs is plastic. The different metabolic patterns of BCSCs might be due to their distinctive molecular characteristics[72,77,78]. It was demonstrated that the metabolic switch from OXPHOS to aerobic glycolysis was essential for the maintenance of CD44+/CD24-/ EpCAM+ BCSCs in response to the decreased ROS levels[79]. It has been observed that BCSCs display two interchangeable states: Quiescent mesenchymal-like (M) state and proliferative epithelial-like (E) state[80,81]. M-BCSCs are characterized by elevated ALDH activity and proliferative capacity. Differently, E-BCSCs exhibit CD44+/CD24- cell surface marker expression and quiescent state[78,82].
FA is the metabolic intermediates of various nutrients in cells, which are essential for maintaining the structure and function of cell membranes, energy storage, and signal transduction[83]. In addition to glucose metabolism, cells also generate energy by breaking down FA by FA oxidation (FAO)[84]. FAO is also important for cancer cell survival and chemotherapy resistance[26]. CSCs needs to metabolize FA through FAO to generate energy to maintain survival[85]. It was reported that CD44+/CD24- BCSCs contain high levels of lipid droplets and the number of lipid droplets correlates with the stemness of breast cancer cells. Small molecule chemical inhibitors targeting lipid metabolism directly impact the mammosphere formation of BCSCs. It was demonstrated that JAK/STAT3 signaling regulates lipid metabolism, which promotes BCSC stemness and breast cancer cell chemoresistance. Inhibiting JAK/STAT3 signaling or blocking FAO can prevent BCSC maintenance and breast cancer cell chemoresistance. Drug-induced inhibition of mitochondrial FAO with etomoxir impairs NADPH production and increases the levels of ROS. Importantly, etomoxir significantly inhibits tumorsphere formation of radiation-derived BCSCs. Promyelocytic leukemia (PML) gene was enriched in triple-negative breast cancers. It was reported that PML acted as a potent activator of PPAR signaling and fatty acid oxidation. Recent studies have demonstrated the critical role of PML in CSCs. Therefore, BCSCs from triple-negative breast cancers may depend on FA oxidation. Until now, there are few studies on the metabolic characteristics of BCSCs. Thus, welldefined features of BCSC metabolism still need to be depicted.
METABOLIC TARGETED THERAPY OF BCSCS
In view of the metabolic features of BCSCs, we summarize the current alternative therapeutics that target BCSC metabolism. BCSCs have more efficient mitochondria, and thus they are more efficient in performing OXPHOS[74]. Therefore, targeting OXPHOS may be an effective strategy to eliminate BCSCs. Transcription co-activator peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC1α) is a key transcriptional regulator of several metabolic pathways including oxidative metabolism and lipogenesis[86]. PGC1α was found to mediate mitochondrial biogenesis and OXPHOS in cancer cells to enhance metastasis[87,88]. Upregulated PGC1α expression was found in breast cancer metastases both in breast cancer xenografts and patients[89]. Some studies showed that the plasma concentration of PGC1α is higher than that of the healthy control group[86]. Meanwhile, there is a positive correlation between high expression of PGC1α and poor prognosis in breast cancer[89]. Estrogenrelated receptor α (ERRα) is a cofactor of PGC1α, which is also required for the transcription of nuclear mitochondrial genes and mitochondrial biogenesis[90]. XCT790, an ERRα-specific inverse antagonist, functions as a specific inhibitor of the ERRα-PGC1α signaling pathway that is involved in the control of mitochondrial biogenesis[91]. XCT790 has been found to reduce the stemness of BCSCs with a CD44+/CD24- phenotype and is accompanied by the inhibition of several signal pathways related to the maintenance of BCSCs[18].
Given that XCT790 significantly reduces OXPHOS and stemness of BCSCs, other mitochondrial inhibitors should also be considered, especially some drugs that have been proven safe through clinical trials[92]. For instance, the antibiotic doxycycline is widely used to treat infections such as chest infections, skin infections, rosacea, dental infections, and sexually transmitted infections (STIs), as well as many other rare infections[93]. Doxycycline, as a non-toxic inhibitor of mitochondrial OXPHOS, was reported to effectively reduce bone metastasis in anin vivopre-clinical murine model of human breast cancer[94]. Importantly, a phase II clinical trial for the use of oral doxycycline in early breast cancer patients shows that doxycycline can selectively eradicate BCSCs in breast cancer patients, which is consistent with a decrease in CD44 and ALDH expression[94].
Niclosamide, a clinically approved drug used to treat tapeworm infestations, is known to uncouple mitochondrial OXPHOS during tapeworm eradicating by diminishing the potential of the inner mitochondrial membrane to inhibit OXPHOS[95]. Niclosamide has been found to inhibit the proliferation of cancer cells with little toxicity to nonmalignant tissues[96]. Moreover, niclosamide can effectively inhibit the proliferation, migration, and invasion of human breast cancer cells at low concentrations, and induce significant apoptosis at high concentrations[97]. Importantly, it was showed that niclosamide treatment resulted in decreased self-renewal signaling pathway activity of BCSCs and increased cytotoxicity against BCSCs with a CD44+/CD24- or ALDH+ phenotype[98].
Another FDA-approved drug tri-phenyl-phosphonium (TPP), a non-toxic and biologically active molecule, can be delivered to and accumulated within the mitochondria of living cells by inhibiting OXPHOS metabolism and activating glycolysis[99]. Interestingly, TPP, applied in combination with vitamin C and berberine, was proved to inhibit the propagation of BCSCs[99,100]. Both vitamin C and berberine are natural compounds. Vitamin C enables the inhibition of glycolysis, while berberine can inhibit the OXPHOS metabolism.
Disulfiram (DSF), an FDA-approved small molecule used in the treatment of chronic alcoholism, can prevent the re-expression of stemness genes and the appearance of BCSC properties in breast cancer cells after radiation[101]. DSF was also demonstrated to disturb the function of mitochondria and inhibit the OXPHOS metabolism[102,103]. Due to the characteristics of targeting BCSCs, DSF has also been shown to successfully reverse the resistance and cross-resistance of acquired paclitaxel-resistant triple-negative breast cancer cells[104,105]. As a drug that has been clinically applied, we should comprehensively evaluate whether DSF can be re-used as a BCSC inhibitor to reverse acquired pan-chemical resistance.
In summary, BCSCs mainly depend on OXPHOS metabolism to provide energy, which results in increased mitochondrial membrane potential (Δψm)[106]. Disruption of mitochondrial metabolic function is one of the effective ways to eliminate BCSCs. This can be also used to increase the delivery of drugs to the mitochondria of BCSCs.
CD44 is the most typical BCSC marker. CD44 can be cleaved by proteolytic cleavage and releases the intracellular domain (CD44ICD). Subsequently, CD44ICD interacts with various stemness factors, including SOX2, NANOG, and OCT4, in breast cancer cells. Meanwhile, CD44ICD promotes the glycolysis pathway of BCSCsviaenhancing PFKFB4-mediated glycolysis by binding and activating the promoter region ofPFKFB4[107]. Therefore, inhibition of CD44 may provide potential therapeutic target against CD44+ BCSCs. It was reported that ablation ofCD44can induce glycolysis-to-OXPHOS transition through modulation of the c-Src-Akt-LKB1-AMPKα pathway in breast cancer cells[108]. Thus, the small molecule chemical inhibitor of CD44 may be more effective than CD44 monoclonal antibody in targeting BCSCs.
ALDH is one of the effective hallmarks of BCSCs and is an independent prognostic marker to predict metastasis and poor patient outcome in breast cancer[35,108]. As a mitochondrial methylmalonate semialdehyde dehydrogenase, ALDH is also one of the functional regulators of BCSCs and therapeutic resistance[19,21]. It has been shown that the HER2+/CD44+/CD24- subpopulation of breast cancer cells display enriched ALDH activity, tumorigenic potential, and radio-resistance[109]. Suppressing ALDH activity can prevent the expression of BCSC related genes and the BCSC properties in breast cancer cells after radiation[110]. In addition, ALDH can metabolize chemotherapeutic drugs into non-toxic compounds[111]. The characteristics of ALDH are responsible for the drug resistance of cancer cells, thus providing a potential target for the targeted therapy of BCSCs.
The progressive growth of solid tumors results in abnormal blood vessels to supply defective oxygen to tumor cells[112,113]. Recent reports show that BCSCs are stimulated in a hypoxic tumor microenvironment[114,115]. The undifferentiated phenotype of breast cancer cells strongly correlates with tumor hypoxia[116]. Under normoxic condition, stem cells lose their stemness characteristics[117]. Although the mechanism by which hypoxia promotes the generation of CSCs is unknown, doxycycline mentioned above as an OXPHOS inhibitor was reported to inhibit hypoxia-induced BCSCs[82]. Therefore, hypoxia-induced BCSC generation may be achieved by altering cell metabolism.
In addition to glucose metabolism, FA and cholesterol metabolism plays an important role in maintaining the stemness of CSCSs[118]. FA is metabolic intermediates of various nutrients in cells, which are essential for maintaining the functions of the cells[83]. FAO is also important for cancer cell survival and promotes chemotherapy resistance[85]. CSCs metabolize FA through the FAO catabolic pathway to generate energy to maintain survival[119]. Besides, increased cholesterol biosynthesis was proved as a key feature of BCSCs that affects patient outcomes[118,120]. It was demonstrated that simvastatin, an inhibitor of 3-hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase, specifically inhibits endogenous cholesterol synthesis in all of the animal cells[121]. Simvastatin has been shown to inhibit mammosphere formation and growth of BCSCs from patient-derived xenograft (PDX) tumors derived from triple-negative breast cancer[120]. Bisphosphonate, a drug widely used to reduce the incidence of breast cancer bone metastases, has been shown to significantly eliminate the formation of BCSCs[122]. Until now, there are few studies on the metabolic characteristics of BCSCs. Thus, well-defined features of BCSC metabolism still need to be depicted.
CONCLUSION
Breast cancer is a highly heterogeneous disease, and this high heterogeneity is a challenge to targeted treatment for breast cancer and other cancers. BCSCs have been considered to be an important cause of tumor heterogeneity and responsible for therapeutic resistance and tumor relapse. Therefore, the selective elimination of BCSCs at the root of breast cancer cells provides an effective therapeutic strategy for cancer treatment. Targeting the specific metabolic characteristics of BCSCs can promote the differentiation of BCSCs to reverse drug resistance and tumor metastasis in tumor therapy (Figure 1). BCSCs have been demonstrated to rely on OXPHOS metabolism for energy supply. Meanwhile, FA and cholesterol syntheses also contribute to BCSC maintenance. Thus, dual inhibition of metabolic pathways may be a better way to eliminate heterogeneous BCSCs.
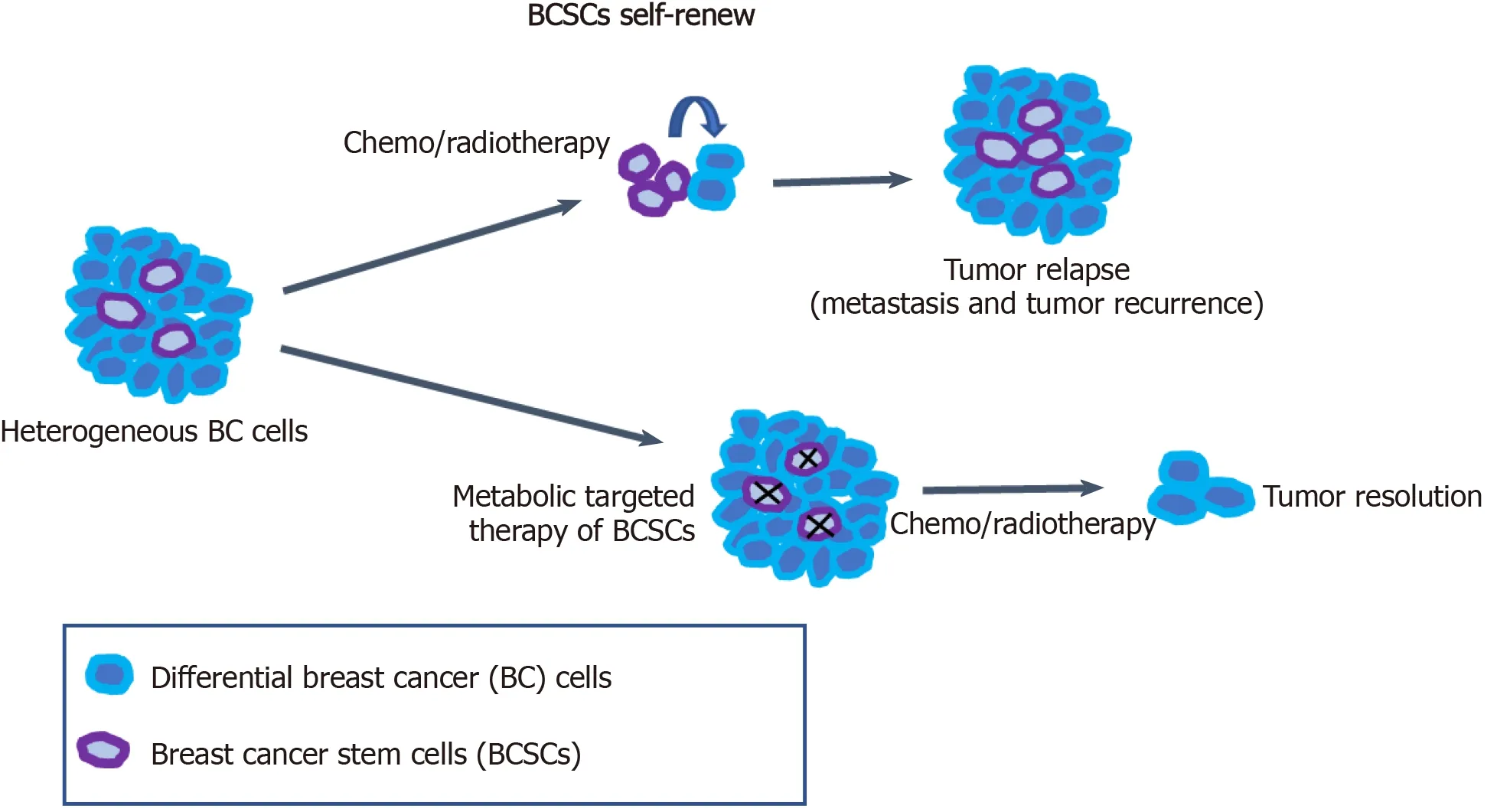
Figure 1 Metabolic targeted therapy of breast cancer stem cells.
杂志排行
World Journal of Stem Cells的其它文章
- Acquired aplastic anemia: Is bystander insult to autologous hematopoiesis driven by immune surveillance against malignant cells?
- Glutathione metabolism is essential for self-renewal and chemoresistance of pancreatic cancer stem cells
- Effect of conditioned medium from neural stem cells on glioma progression and its protein expression profile analysis
- Immunophenotypic characteristics of multipotent mesenchymal stromal cells that affect the efficacy of their use in the prevention of acute graft vs host disease
- AlCl3 exposure regulates neuronal development by modulating DNA modification
- Isolation and characterization of mesenchymal stem cells in orthopaedics and the emergence of compact bone mesenchymal stem cells as a promising surgical adjunct
