COVID-19: Off-label therapies based on mechanism of action while waiting for evidence-based medicine recommendations
2020-12-16MatteoScottoDiVettaMarcoMorroneSerafinoFazio
Matteo Scotto Di Vetta,Marco Morrone,Serafino Fazio
Matteo Scotto Di Vetta, Department of Internal Medicine, University of Naples, Naples 80100, Italy
Marco Morrone, Farmacia, Farmacia Morrone, Casoria 80022, Italy
Serafino Fazio, Department of Internal Medicine, Cardiovascular and Immunologic Sciences, Federico II University of Naples, Napoli 80100, Italy
Abstract
Key words: COVID-19; Evidence based medicine; Hydroxycloroquine; Azithromycine; Indomethacine; Doxycycline
COMMENTARY ON HOT TOPICS
At the end of 2019, some cases of pneumonia of unknown etiology were observed in Wuhan (China).A few weeks later, this disease was discovered to be due to a virus of the coronavirus family, that was named severe acute respiratory syndromecoronavirus-2 (SARS-Cov2), and the related coronavirus disease 2019 (COVID-19) was named accordingly.
COVID-19 embodies a high rate of disease transmission that has caused a critical hospitalization overload.By May 15, 2020, it had produced more than 4 million documented positive cases and about 280000 deaths all over the world.At the beginning, we underestimated and misunderstood COVID-19.Now, however, we have sufficient information of the disease pathophysiology, and it is clear that we are not dealing with just a respiratory disease.In fact, in many cases, if a prompt treatment is not undertaken, the infection may evolve towards a more severe disease and the occurrence of a cytokine storm with multi-organ damage[1-3].
Multi-organ damage should be investigated in patients recovered from moderatesevere COVID-19.Follow-up studies should be conducted to verify the higher risk of developing autoimmune diseases due to the uncontrolled and abnormal immune response to the virus.Finally, the possibility of developing neoplastic disease should be examined[4].
It is mandatory to promptly initiate a therapy at the onset of symptoms to stop the progression of the COVID-19 disease.This will ultimately reduce the risk of cytokine storm and, consequently, a hospitalization overload.
From the beginning of the pandemic, due to the lack of specific and approved therapies for the disease, the medical community was split among two currents of thought.The interventionists suggested that antiviral drugs, immunomodulators and low molecular weight heparin should be used off-label.In contrast, the evidence-based medicine (EBM) supporters preferred to wait for a treatment backed by scientific studies.
In only 4 mo, this pandemic has severely tested hospitals and health organizations all over the world, particularly under the absence of specific therapies.However, while waiting for EBM indications, we believe that during an emergency, even with a lack of specific approved therapies, it is not ethical to not at least try some therapies based on medical rationale.
Thus, we suggest a therapeutic scheme based on drugs that have an indication according to their mechanisms of action to treat patients in home health care at the onset of symptoms.This may allow for the avoidance of disease progression as well as hospital overload.
An important issue, to which we need to pay attention, is the great difference among the mechanisms of different antiviral drugs.Some drugs work by inhibiting the entry of the virus into the host cells and others by inhibiting the viral replication inside the host cells.The first will be efficacious if promptly administrated at the onset of symptoms, to avoid the entry of the virus, while the others are useful once the virus has penetrated.On the basis of these observations, it is not surprising that, in some studies the use of hydroxychloroquine, which is an inhibitor of virus entry, has been unsuccessful due to the late onset of the therapy.
FEASIBLE AT-HOME THERAPIES FOR COVID-19
At-home treatment for patients with beginning mild-moderate disease without risk factors, based on Italian experience (not published data)
Hydroxychloroquine 400 mg b.i.d.p.o.on first day and 200 mg b.i.d.after for 7 d: Hydroxychloroquine blocks viral internalization into host cells and it is also effective in terms of modulating the immune response to COVID-19 infection[5-9](Table 1).Azithromycin 500 mg, 1 tablet/d p.o.the first day and then 250 mg/d for 4 d: Azithromycin blocks viral internalization into host cells.It probably inhibits viral proteases and it’s also effective in terms of modulating the immune response to COVID-19 infection.Furthermore, it can protect patients from bacteria superinfections[7-9].
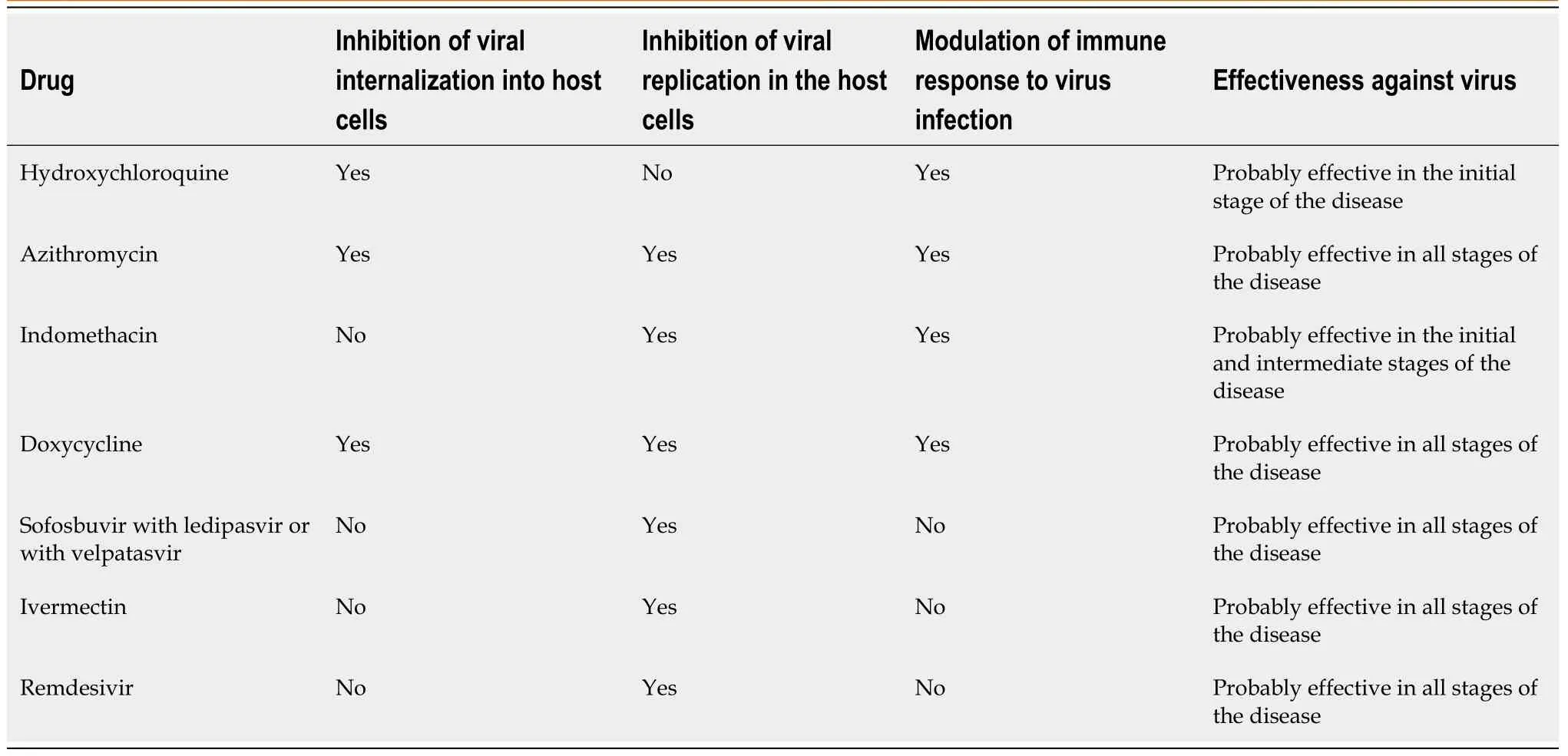
Table 1 Mechanisms of some anti-coronavirus disease 2019 drugs
Alternatives: When the above two drugs are contraindicated (particularly in patients with retinopathy, diabetes, or heart disease because of risk of prolonged QT tract), we suggest indomethacin as an alternative to hydroxychloroquine and doxycycline as an alternative to azithromycin.
Indomethacin should be given as 50 mg b.i.d.p.o.(associated with gastric protection) for 7-10 d.It acts by blocking viral RNA synthesisin vitroand this effect is independent of cyclooxygenase inhibition.In addition, indomethacin has antiinflammatory properties[10].
Doxycycline should be given as 100 mg 2 tablet/d p.o.on the first day and 1 tablet/d p.o.for another 6 d.It has antiviral properties based on chelating zinc compounds on matrix metalloproteinases which are important for COVID-19 survival and cell infiltration.Furthermore, it can block cell-to-cell adhesion and viral replication.Doxycycline also has anti-inflammatory properties and can protect the patients from bacteria superinfections[11].
Treatment for patients with beginning mild-moderate disease associated with risk factors and for patients with moderate disease
Low molecular weight heparin (4000 to 8000 u.i.b.i.d.) should be added according to the patient's weight and condition, in order to block intravascular coagulation correlated to the cytokine storm[12,13].
Antiviral drugs should also be added.In addition to the antiviral drugs currently being tested (lopinavir-ritonavir, remdesivir and favipiravir), there are other promising antiviral drugs not being tested.Antiviral drugs used successfully against hepatitis C virus (HCV) are sofosbuvir associated with ledipasvir or with velpatasvir[14-16].HCV is a single-stranded RNA+ virus, like the coronavirus; as such, the antiviral drugs against HCV (RNA-dependent RNA polymerase inhibitors and protease inhibitors) could be effective against the COVID-19.
Ivermectin (an anti-helminthic drug) has already shown antiviral properties against human immunodeficiency virus, Zika, Dengue and West Nile.It has also shownin vitroantiviral properties against the COVID-19, likely based on its nuclear transport inhibitory activity that stops the viral capacity to reduce the host cell’s antiviral response[7,17].
Recently, the Federal Drug Administration gave emergency-use authorization for remdesivir in adults and children hospitalized with severe COVID-19[18].Remdesivir is a nucleotide analogue, and the RNA-dependent RNA polymerase incorporates the active triphosphate form of remdesivir into viral RNA.Incorporation produces termination of viral RNA synthesis and inhibits viral replication[19].
Currently, among the antiviral drugs, the most used off-label is hydroxychloroquine, particularly in combination with azithromycin.But this combination seems controversial because of its arrhythmogenic risk.However, recently, a paper reported the results of a study in which were used data from the United States Food and Drug Administration Adverse Event Reporting System (on about 13 million total reports) and concluded that hydroxychloroquine use was not associated with safety signals, while azithromycin alone was associated with TdP/QT prolongation events and should be used with caution[20].To avoid the problem of additive cardiotoxicity of hydroxychloroquine plus azithromycin, we can replace azithromycin with doxycycline in at-risk patients.
It is also very interesting that in some countries, such as Peru, in which hydroxychloroquine and ivermectin are largely in use, the lethality of COVID-19 is among the lowest (2.7%).Perhaps this good result could be due to their different mechanisms of action, which are empowered one with the other.
CONCLUSION
The take-home message we want give is: while waiting a specific vaccine or EBM indications, upon which old or novel drugs will be certainly useful in the treatment of COVID-19, and on the basis of the large experience acquired in the first 4 mo of this pandemic, we think that a prompt treatment should be started at home in those patients with mild-moderate disease using off-label drugs with a medical rationale.In this way, we could avoid disease progression, hospital overload and, possibly, reduce mortality.
杂志排行
World Journal of Meta-Analysis的其它文章
- Learning and competence development via clinical cases – what elements should be investigated to best train good medical doctors?
- Immunotherapy in hepatocellular carcinoma: Combination strategies
- Combined endoscopy/laparoscopy/percutaneous transhepatic biliary drainage, hybrid techniques in gastrointestinal and biliary diseases
- Thrombopoietin-receptor agonists in perioperative treatment of patients with chronic liver disease
- Role of non-coding RNAs in pathogenesis of gastrointestinal stromal tumors
- Exclusive cigar smoking in the United States and smoking-related diseases: A systematic review
