微小RNA-22-3p负性调控人腹膜间皮细胞NLRP3的表达及功能*
2020-12-03伍军李相友封宝红李菊霜朱戈丽张艳霞毕智敏巩雪敏
伍军, 李相友, 封宝红, 李菊霜, 朱戈丽, 张艳霞, 毕智敏, 巩雪敏
微小RNA-22-3p负性调控人腹膜间皮细胞NLRP3的表达及功能*
伍军, 李相友△, 封宝红, 李菊霜, 朱戈丽, 张艳霞, 毕智敏, 巩雪敏
(武汉大学附属同仁医院,武汉市第三医院肾内科,湖北 武汉 430074)
探讨微小RNA-22-3p (miR-22-3p)对人腹膜间皮细胞核苷酸结合寡聚化结构域样受体蛋白3(NLRP3)表达及功能的影响。将基因的3'-非翻译区(3'-UTR)序列及其突变体克隆到双萤光素酶报告基因载体psiCHECK2中,构建野生型及突变型重组双萤光素酶报告质粒,与miR-22-3p mimic和miR-22-3p inhibitor共转染LPS预处理的人腹膜间皮细胞株HMrSV5,检测萤光素酶活性;HMrSV5细胞随机分为以下6组: miR-22-3p NC+LPS组、miR-22-3p NC+LPS+ATP组、miR-22-3p mimic+LPS组、miR-22-3p mimic+LPS+ATP组、miR-22-3p inhibitor+LPS组和miR-22-3p inhibitor+LPS+ATP组。RT-qPCR和Western blot检测NLRP3 mRNA和蛋白的表达,ELISA检测白细胞介素1β (IL-1β)的表达和caspase-1的活性,Western blot检测caspase-1 p20蛋白的表达。NLRP3-3'-UTR野生型与miR-22-3p mimic共转染HMrSV5细胞后,萤光素酶活性较对照组降低(<0.05);NLRP3-3'-UTR野生型与miR-22-3p inhibitor共转染HMrSV5细胞后,萤光素酶活性较对照组升高(<0.05)。与miR-22-3p NC+LPS组比较,miR-22-3p mimic+LPS组NLRP3的mRNA和蛋白表达、IL-1β和caspase-1 p20的水平均下降且caspase-1活性减弱(<0.05),而miR-22-3p inhibitor+LPS组NLRP3的mRNA和蛋白表达、IL-1β和caspase-1 p20的水平均上升且caspase-1的活性增强(<0.05)。与miR-22-3p NC+LPS+ATP组比较, miR-22-3p inhibitor+LPS+ATP组NLRP3的mRNA和蛋白表达、IL-1β和caspase-1 p20的水平均上升且caspase-1的活性增强(<0.05)。miR-22-3p可负性调控人腹膜间皮细胞NLRP3的表达及功能。
微小RNA-22-3p;核苷酸结合寡聚化结构域样受体蛋白3;人腹膜间皮细胞;腹膜透析;终末期肾脏病
腹膜透析是终末期肾脏病(end-stage renal disease, ESRD)患者的重要肾脏替代治疗方法,具备保护残余肾功能、早期生存率优势等优点[1-3]。腹膜间皮细胞是构成腹膜的主要细胞群体之一,其位于腹膜巨噬细胞和间皮下微血管之间紧密连接的关键部位。然而,在长期高糖腹膜透析液作用下,腹膜间皮细胞可产生活性氧簇(reactive oxygen species, ROS)、分泌炎症因子,使细胞长期处于慢性炎症状态,从而启动新生血管形成及腹膜组织纤维化过程[4-5],最终导致腹膜功能障碍,患者不得不退出腹膜透析。因此,及时减轻或缓解间皮细胞炎症反应有助于延缓新生血管形成以及腹膜纤维化的到来。核苷酸结合寡聚化结构域样受体蛋白3 (nucleotide-binding oligomerization domain-like receptor protein 3, NLRP3)炎症小体在机体炎症和免疫中发挥关键作用,其可被多种类型的病原微生物或危险信号所识别并激活,促进胱天蛋白酶1 (caspase-1)活化,活化的caspase-1切割位于炎症反应上游的白细胞介素1β (interleukin-1β, IL-1β)和IL-18前体,产生相应的成熟细胞因子,从而启动炎症反应[6]。我们既往的研究证实,高糖腹膜透析液作用于腹膜间皮细胞可提升细胞线粒体的ROS水平,而升高的ROS激活NLRP3-IL-1β,启动线粒体自噬清除受损线粒体,降低细胞ROS水平;应用ROS抑制剂则可削弱NLRP3-IL-1β活化,从而减轻腹腔慢性炎症反应,提示负性调控NLRP3可作为干预腹膜透析腹腔慢性炎症的靶点之一[7-8]。前期我们还应用生物信息学数据库预测了微小RNA-22-3p (microRNA-22-3p, miR-22-3p)与NLRP3可能的靶向结合位点,并构建NLRP3-3'-UTR野生型(wild-type, WT)及突变型(mutant, mut)双萤光素酶报告基因载体, NLRP3-3'-UTR野生型与miR-22-3p mimic共转染293T细胞后,萤光素酶活性降低,于是初步认为miR-22-3p可负性调控基因及功能[9]。本研究利用脂多糖(lipopolysaccharide, LPS)致敏腹膜间皮细胞,探讨miR-22-3p对三磷酸腺苷(adenosine triphosphate, ATP)诱导的NLRP3炎症小体活化的影响及机制。
材料和方法
1 材料
人腹膜间皮细胞株HMrSV5购自美国模式培养物集存库(American Type Culture Collection, ATCC)。
逆转录试剂盒(PrimeScript RT Master Mix)购自宝日医生物技术(北京)有限公司; qPCR Master Mix购自南京诺唯赞生物科技有限公司; DNA聚合酶(Taq DNA polymerase)购自天根生化科技(北京)有限公司;双萤光素酶报告基因载体psiCHECK2(包含海肾萤光素酶基因和萤火虫萤光素酶基因)、双萤光素酶报告基因检测试剂盒购自Promega; miR-22-3p mimic、miR-22-3p inhibitor及阴性对照购自吉玛基因;抗NLRP3抗体购自ABGENT;抗caspase-1 p20抗体购自Adipogen; IL-1β ELISA试剂盒购自联科生物科技有限公司; caspase-1活性检测试剂盒购自BioVision。
2 实验方法
2.1细胞转染体外培养取人腹膜间皮细胞株HMrSV5第5~10代用于实验研究,参考我们前期的报道[7],用含10%胎牛血清的DMEM培养基,于37℃、5% CO2培养箱中培养,取处于对数生长期,生长状态良好的细胞,以每孔5×105的细胞密度分别接种于6孔板, 37℃培养过夜;当细胞汇合70%左右时开始转染,转染前2 h换成无血清DMEM培养基,参照LipofectamineTM2000说明书进行转染。将细胞分为miR-22-3p NC+psiCHECK2组、miR-22-3p NC+psiCHECK2-NLRP3-3'-UTR-WT组、miR-22-3p mimic+psiCHECK2-NLRP3-3'-UTR-WT组、miR-22-3p mimic+psiCHECK2-NLRP3-3'-UTR-mut组、miR-22-3p inhibitor+psiCHECK2-NLRP3-3'-UTR-WT组和miR-22-3p inhibitor+psiCHECK2-NLRP3-3'-UTR-mut组。每组设3个平行孔,重复3次。
2.2双萤光素酶活性的检测转染后48 h吸尽细胞培养液加入细胞裂解液充分裂解细胞,(10 000~15 000)×离心3~5 min,取上清用于测定。按双萤光素酶报告基因检测试剂盒提供实验方案,计算相对萤光素酶活性。
2.3RT-qPCR和Western blot检测HMrSV5细胞NLRP3的mRNA和蛋白表达HMrSV5细胞分别转染miR-22-3p NC、miR-22-3p mimic和miR-22-3p inhibitor,转染后48 h吸尽细胞培养液加入细胞裂解液充分裂解细胞用于实验。将细胞分为miR-22-3p NC+LPS组、miR-22-3p NC+LPS+ATP组、miR-22-3p mimic+LPS组、miR-22-3p mimic+LPS+ATP组、miR-22-3p inhibitor+LPS组和miR-22-3p inhibitor+LPS+ATP组,各组中LPS的浓度为1 mg/L,与细胞作用时间为4 h,而ATP浓度为5 mmol/L,于LPS加入前作用细胞30 min。收集细胞,应用RT-qPCR和Western blot检测NLRP3的mRNA和蛋白表达。RT-qPCR所用引物序列见表1。

表1 RT-qPCR引物序列
2.4HMrSV5细胞IL-1β含量和caspase-1活性的检测实验分组同2.3,收集细胞上清,分别按IL-1β ELISA试剂盒说明书和caspase-1活性检测试剂盒说明书进行检测。
2.5Western blot检测HMrSV5细胞caspase-1 p20蛋白的表达实验分组同2.3,收集细胞上清,用Western blot检测细胞上清caspase-1 p20蛋白的表达,具体实验方法与本课题组之前的报道相同[5]。
3 统计学处理
全部数据采用SPSS 21.0统计软件分析。计量资料用均数±标准差(mean±SD)表示,根据方差齐性检验结果,多组间比较采用单因素方差分析(one-way ANOVA),组间多重比较采用SNK-检验,以<0.05为差异有统计学意义。
结果
1 双萤光素酶活性检测
与miR-22-3p NC+psiCHECK2-NLRP3-3'-UTR-WT组相比, miR-22-3p mimic+psiCHECK2-NLRP3-3'-UTR-WT组萤光素酶活性显著降低(<0.05),miR-22-3p mimic+psiCHECK2-NLRP3-3'-UTR-mut组萤光素酶活性无显著变化, miR-22-3p inhibitor+psiCHECK2-NLRP3-3'-UTR-WT组萤光素酶活性显著升高(<0.05), miR-22-3p inhibitor +psiCHECK2-NLRP3-3'-UTR-mut组萤光素酶活性无显著性改变(>0.05),见图1。上述结果提示, miR-22-3p可靶向调控HMrSV5细胞基因。
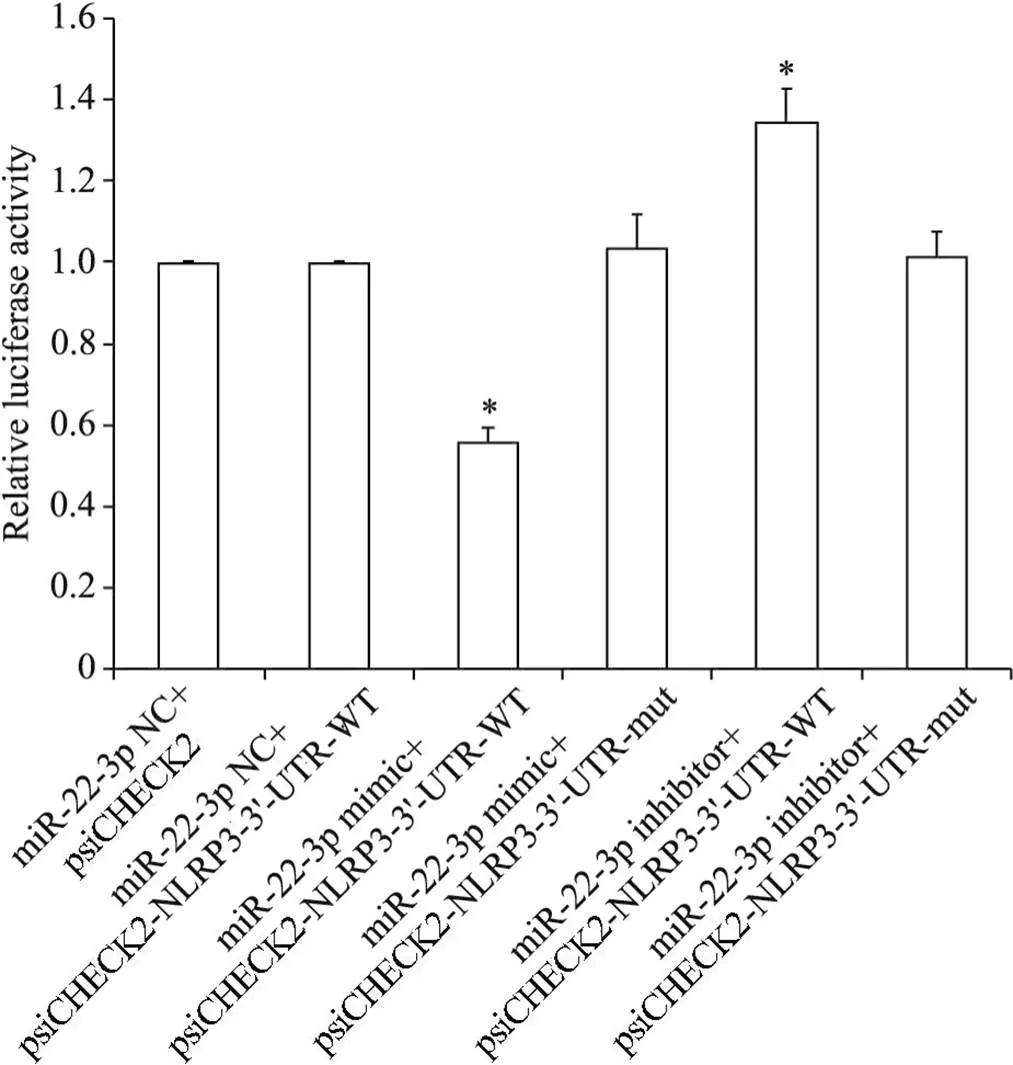
Figure 1. The dual luciferase activity in the HMrSV5 cells. Mean±SD. n=3. *P<0.05 vs miR-22-3p NC+psiCHECK2-NLRP3-3'-UTR-WT group.
2 HMrSV5细胞miR-22-3p表达的变化
HMrSV5细胞分别转染miR-22-3p NC、miR-22-3p mimic和miR-22-3p inhibitor后,RT-qPCR检测结果显示miR-22-3p mimic转染组miR-22-3p的表达较对照组明显升高(<0.05),miR-22-3p inhibitor转染组miR-22-3p的表达较对照组明显降低(<0.05),见图2。
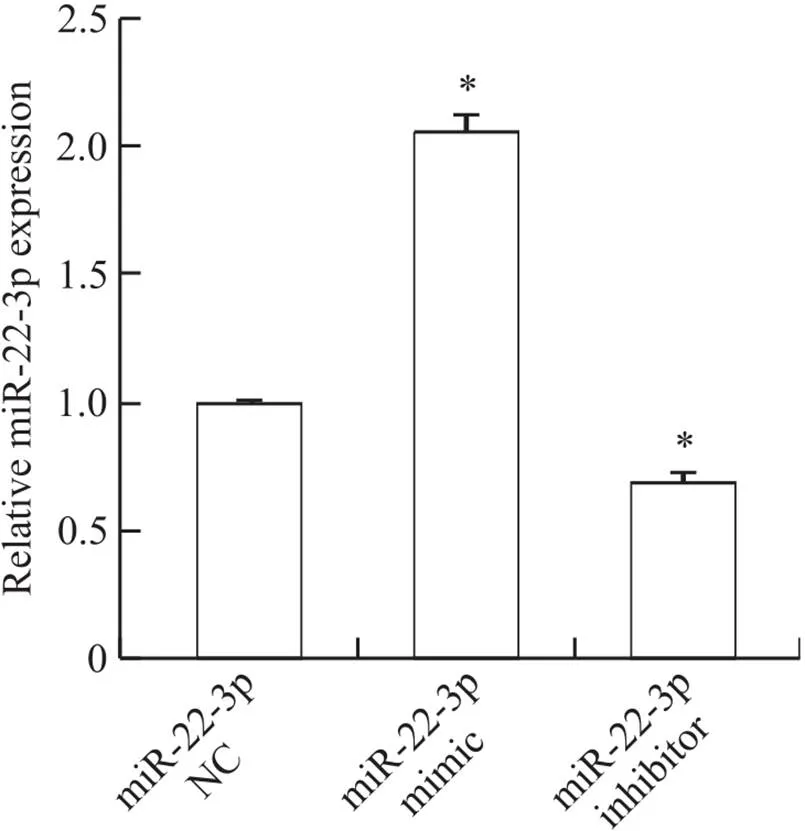
Figure 2. The expression of miR-22-3p in the HMrSV5 cells. Mean±SD. n=3. *P<0.05 vs miR+22-3p NC group.
3 miR-22-3p对HMrSV5细胞NLRP3 mRNA和蛋白表达的影响
经LPS预处理的HMrSV5细胞分别转染miR-22-3p NC、miR-22-3p mimic和miR-22-3p inhibitor后,检测NLRP3 mRNA和蛋白表达,结果显示,与miR-22-3p NC+LPS组比较, miR-22-3p mimic+LPS组NLRP3 mRNA和蛋白表达下降(<0.05),转染miR-22-3p inhibitor组NLRP3 mRNA和蛋白表达升高(<0.05); NLRP3激动剂ATP可有效上调NLRP3 mRNA及蛋白表达,然而,相较于miR-22-3p NC+LPS+ATP组, miR-22-3p mimic+LPS+ATP组NLRP3 mRNA及蛋白水平仍显著降低(<0.05),表明miR-22-3p可负性调控NLRP3的表达,见图3。
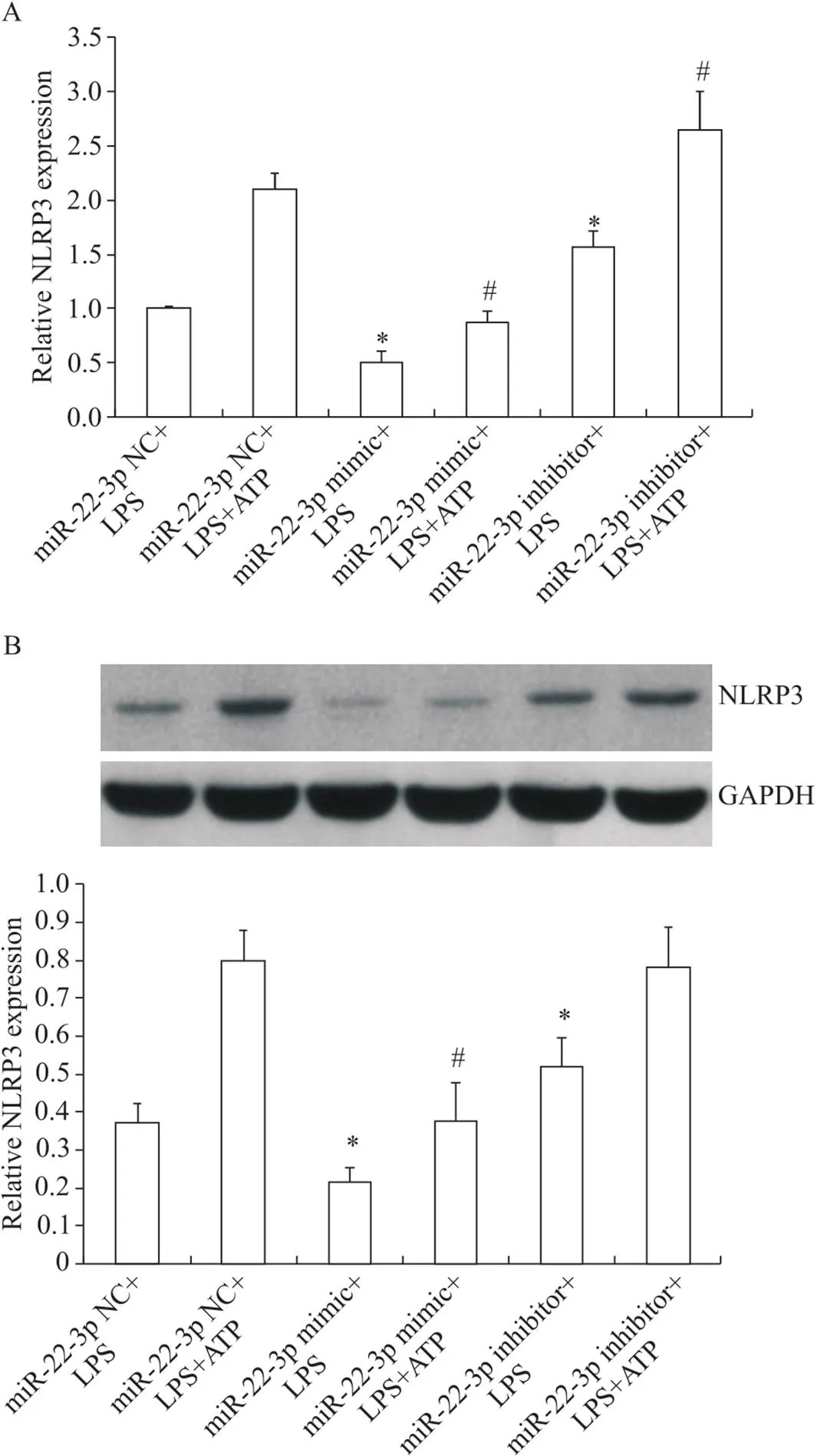
Figure 3. The effect of miR-22-3p transfection on expression of NLRP3 at mRNA (A) and protein (B) levels in the HMrSV5 cells. Mean±SD. n=3. *P<0.05 vs miR-22-3p NC+LPS group; #P<0.05 vs miR-22-3p NC+LPS+ATP group.
4 转染miR-22-3p对HMrSV5细胞IL-1β表达的影响
HMrSV5细胞分组处理后ELISA检测细胞上清IL-1β的含量。结果可见,与miR-22-3p NC+LPS组比较, miR-22-3p mimic+LPS组的IL-1β含量显著降低(<0.05), miR-22-3p inhibitor+LPS组的IL-1β含量显著升高(<0.05);此外, miR-22-3p mimic+LPS+ATP组IL-1β的含量较miR-22-3p NC+LPS+ATP组显著减少(<0.05),见图4。
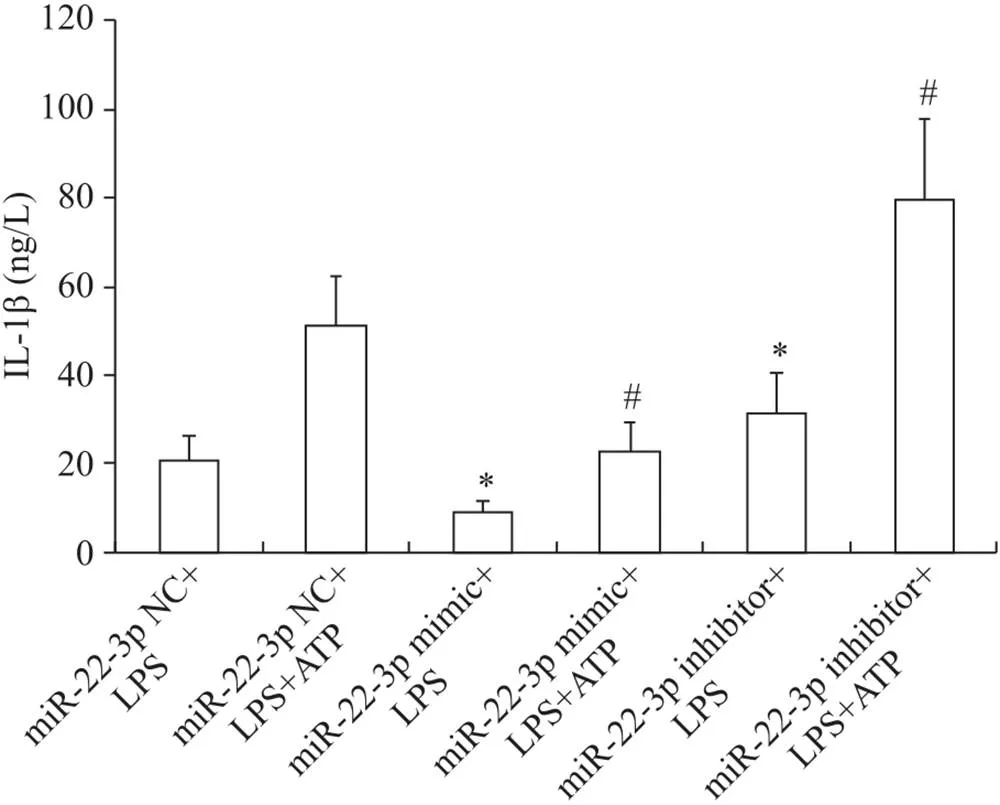
Figure 4. The effect of miR-22-3p transfection on expression of IL-1β in the HMrSV5 cells. Mean±SD. n=3. *P<0.05 vs miR-22-3p NC+LPS group; #P<0.05 vs miR-22-3p NC+LPS+ATP group.
5 转染miR-22-3p 对细胞caspase-1活性的影响
HMrSV5细胞分组处理后,检测细胞caspase-1活性。结果可见,与miR-22-3p NC+LPS组比较,miR-22-3p mimic+LPS组的caspase-1活性下降(<0.05),miR-22-3p inhibitor+LPS组的caspase-1活性升高(<0.05),此外,miR-22-3p mimic+LPS+ATP组caspas-1的活性较miR-22-3p NC+LPS+ATP组明显降低(<0.05),见图5。

Figure 5. The effect of miR-22-3p transfection on the activity of caspase-1 in the HMrSV5 cells. Mean±SD. n=3. *P<0.05 vs miR-22-3p NC+LPS group; #P<0.05 vs miR-22-3p NC+LPS+ATP group.
6 转染miR-22-3p 对HMrSV5细胞caspase-1 p20蛋白表达的影响
HMrSV5细胞分组处理后,检测细胞caspase-1 p20的表达水平。结果可见,与miR-22-3p NC+LPS组比较,miR-22-3p mimic组的caspase-1 p20表达下降(<0.05),miR-22-3p inhibitor+LPS组的caspase-1 p20表达升高(<0.05);此外,miR-22-3p mimic+LPS+ATP组caspas-1 p20的表达较miR-22-3p NC+LPS+ATP组明显减少(<0.05),见图6。
讨论
临床上长期应用高糖腹膜透析液所致的腹腔慢性炎症、腹膜纤维化是腹膜透析技术的瓶颈,是学者们一致重点关注的研究领域。NLRP3炎症小体是核苷酸结合寡聚化结构域(nucleotide-binding oligomerization domain, NOD)样受体(NOD-like receptor, NLR)家族成员之一,其可广泛识别细胞内外的应激和危险信号, NLRP3持续激活可导致广泛的组织损伤并参与许多急慢性炎症性疾病的发生[10]。前期体内外的实验研究证实:高糖腹膜透析液可通过ROS诱导NLRP3-IL-1β活化,启动线粒体自噬或者应用ROS抑制剂均可削弱NLRP3-IL-1β的活化,从而减轻腹腔慢性炎症,提示负性调控NLRP3可作为干预腹膜透析腹腔慢性炎症的靶点之一[7-8]。
研究证实,miR-22通过有效结合靶基因、阻断靶基因的表达在抑制肿瘤生长、转移、抗肿瘤治疗方面发挥重要作用[11-13]。那么, miR-22是否可以阻断基因的表达及功能,发挥对抗炎症的作用呢?
前期我们成功构建NLRP3-3'-UTR野生型及突变型双萤光素酶报告基因载体,在293T细胞中初步证实miR-22-3p可负性调控基因及功能[9],在此基础上本实验在腹膜间皮细胞中发现,miR-22-3p模拟物可抑制NLRP3-3'-UTR野生型萤光素酶活性、miR-22-3p抑制物可提升NLRP3-3'-UTR野生型萤光素酶活性;进一步的实验发现,LPS预刺激的腹膜间皮细胞转染miR-22-3p模拟物可下调NLRP3的mRNA和蛋白表达、miR-22-3p抑制物可上调NLRP3的mRNA和蛋白表达,上述实验提示miR-22-3p可负性调控腹膜间皮细胞基因的表达。那么,miR-22-3p是如何调控NLRP3功能的?为此我们进一步验证了NLRP3下游IL-1β和caspase-1 p20蛋白的表达以及caspase-1的活性,腹膜间皮细胞转染miR-22-3p模拟物可下调IL-1β和caspase-1 p20蛋白表达并减弱caspase-1活性、miR-22-3p抑制物可上调IL-1β、caspase1 p20蛋白的表达并提升caspase-1活性,加用NLRP3的激动剂ATP处理细胞并不影响miR-22-3p模拟物的作用,NLRP3的表达仍较miR-22-3p NC+LPS+ATP组降低。由此我们认为,miR-22-3p可负性调控人腹膜间皮细胞NLRP3的表达及功能。新近的研究发现,在胃癌组织中NLRP3表达异常升高,不仅启动胃粘膜上皮细胞的炎症反应还促使上皮细胞异常增生和胃癌的发生;miR-22可直接结合NLRP3-3'-UTR,抑制后者的高表达及生物学效应[14]。在口腔鳞状上皮细胞癌组织及细胞系中观察到miR-22表达明显低下,NLRP3的表达却明显升高;过表达miR-22可逆转NLRP3激活所致的促肿瘤效应[15]。除肿瘤领域外,研究者还发现,miR-22通过靶向抑制基因,减少冠心病大鼠心肌微血管内皮细胞凋亡及下调促炎症因子的表达,对大鼠心肌微血管内皮细胞发挥保护作用[16]。本研究与上述研究的结果类似,但本研究未能在动物模型中加之证实,这也是本研究的不足之处。下一步我们拟建立腹膜透析模型鼠,观察miR-22-3p腹腔内注射后对高糖腹膜透析液作用下NLRP3表达及功能的影响。
[1] Yu X, Yang X. Peritoneal dialysis in China: meeting the challenge of chronic kidney failure[J]. Am J Kidney Dis, 2015, 65(1):147-151.
[2] Li PK, Chow KM, Van de Luijtgaarden MW, et al. Changes in the worldwide epidemiology of peritoneal dialysis[J]. Nat Rev Nephrol, 2017, 13(2):90-103.
[3]伍军,阳晓,余学清. 腹膜透析与血液透析:哪种透析治疗方式具有更好的生存率?[J]. 中华肾脏病杂志, 2008, 24(12):931-933.
Wu J, Yang X, Yu XQ. Peritoneal dialysis and hemodialysis: which has better survival?[J]. Chin J Nephrol, 2008, 24(12):931-933.
[4] Yung S, Chan TM. Intrinsic cells: mesothelial cells -- central players in regulating inflammation and resolution[J]. Perit Dial Int, 2009, 29(Suppl 2):S21-S27.
[5]杨琼琼,叶任高,阳晓,等. 含糖透析液对慢性腹膜透析大鼠腹膜功能及间皮细胞形态的影响[J]. 中国病理生理杂志, 2004, 20(8):53-56.
Yang QQ, Ye RG,Yang X, et al.Effect of dislysate on the peritoneal membrane function and the mesothelial cell morphology in chronic peritoneal dialysis rats[J]. Chin J Pathophysiol, 2004,20(8):53-56.
[6] Schroder K, Tschopp J. The inflammasomes[J]. Cell, 2010, 140(6):821-832.
[7] Wu J, Li X, Zhu G, et al. The role of resveratrol-induced mitophagy/autophagy in peritoneal mesothelial cells inflammatory injury via NLRP3 inflammasome activation triggered by mitochondrial ROS[J]. Exp Cell Res, 2016, 341(1):42-53.
[8] Wu J, Zhang YF, Li JS, et al. The effect of high glucose-based peritoneal dialysis fluids on thioredoxin-interacting protein expression in human peritoneal mesothelial cells[J]. Int Immunopharmacol, 2019, 66:198-204.
[9]伍军,李相友,朱戈丽,等. NLRP3基因3'UTR双荧光素酶报告质粒的构建及miR-22-3p靶向调控的初步研究[J]. 广东医学, 2018, 39(23):3463-3468.
Wu J, Li XY, Zhu GL, et al. Construction of dual luciferase reporter plasmid of NLRP3-3'UTR and preliminary verification its tangeting miR-22-3p[J]. Guangdong Med J, 2018, 31(23):3463-3468.
[10] Abderrazak A, Syrovets T, Couchie D, et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases[J]. Redox Biol, 2015, 4:296-307.
[11] Zuo QF, Cao LY, Yu T, et al. MicroRNA-22 inhibits tumor growth and metastasis in gastric cancer by directly targeting MMP14 and Snail[J]. Cell Death Dis, 2015, 6:e2000.
[12] Jiang X, Hu C, Arnovitz S, et al. miR-22 has a potent anti-tumour role with therapeutic potential in acute myeloid leukaemia[J]. Nat Commun, 2016, 7:11452.
[13] Xu M, Li J, Wang X, et al. MiR-22 suppresses epithelial-mesenchymal transition in bladder cancer by inhibiting Snail and MAPK1/Slug/vimentin feedback loop[J]. Cell Death Dis, 2018, 9(2):209.
[14] Li S, Liang X, Ma L, et al. MiR-22 sustains NLRP3 expression and attenuates H. pylori-induced gastric carcinogenesis[J]. Oncogene, 2018, 37(7):884-896.
[15] Feng X, Luo Q, Wang H, et al. MicroRNA-22 suppresses cell proliferation, migration and invasion in oral squamous cell carcinoma by targeting NLRP3[J]. J Cell Physiol, 2018, 233(9):6705-6713.
[16] 胡波,张晓刚,李德才. miR-22靶向抑制NLRP3基因对冠心病内皮细胞炎症损伤的保护作用[J]. 安徽医科大学学报, 2018, 53(5):668-675.
Hu B, Zhang XG, Li DC. The protective effect of miR-22 on the inflammation induced injury of endothelial cells in coronary heart disease through targeting NLRP3[J]. J Anhui Med Univ, 2018, 53(5):668-675.
MicroRNA-22-3p negatively regulates NLRP3 expression and function in human peritoneal mesothelial cells
WU Jun, LI Xiang-you, FENG Bao-hong, LI Ju-shuang, ZHU Ge-li, ZHANG Yan-xia, BI Zhi-min, GONG Xue-min
(,,,430074,)
To investigate the role of microRNA-22-3p (miR-22-3p)in regulating nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3)expression and function in human peritoneal mesothelial cells.The 3'-UTR sequence ofgene and its mutants were cloned into the dual luciferase reporter plasmid psiCHECK2 respectively to construct a wild-type and mutant recombinant dual luciferase reporter plasmid. An SV40-immortalized human peritoneal mesothelial cell line HMrSV5 pretreated with lipopolysaccharide (LPS) was generated and transfected with recombinant dual luciferase reporter plasmid and miR-22-3p mimic or miR-22-3p inhibitor respectively, then the luciferase activity was detected. In addition, the HMrSV5 cells were randomly divided into 6 groups: miR-22-3p NC+LPS, miR-22-3p NC+LPS+ATP, miR-22-3p mimic+LPS, miR-22-3p mimic+LPS+ATP, miR-22-3p inhibitor+LPS and miR-22-3p inhibitor+LPS+ATP. RT-qPCR and Western blot were used to evaluate the expression of NLRP3. ELISA was used to measure the content of interleukin-1β (IL-1β) and the activity of caspase-1. The expression of caspase-1 p20 was determined by Western blot.Wild-type NLRP3 recombinant plasmid and miR-22-3p mimic co-transfection significantly decreased the luciferase activity compared with control group (<0.05), while wild-type NLRP3 recombinant plasmid and miR-22-3p inhibitor co-transfection significantly increased the luciferase activity compared with control group (<0.05). Compare with miR-22-3p NC+LPS group, the expression of NLRP3 at mRNA and protein levels, the content of IL-1β, the expression of caspase-1 p20 and the activity of caspase-1 were decreased in miR-22-3p mimic+LPS group (<0.05), while the expression of NLRP3 at mRNA and protein levels, the content of IL-1β, the expression of caspase-1 p20 and the activity of caspase-1 were increased in miR-22-3p inhibitor+LPS group (<0.05). Compare with miR-22-3p NC+LPS group, the expression of NLRP3 at mRNA and protein levels, the content of IL-1β, the expression of cspase-1 p20 and the activity of caspase-1 were increased in miR-22-3p inhibitor+LPS+ATP group (<0.05).miR-22-3p negatively regulates NLRP3 expression and function in human peritoneal mesothelial cells.
MicroRNA-22-3p; Nucleotide-binding oligomerization domain-like receptor protein 3; Human peritoneal mesothelial cells; Peritoneal dialysis; End-stage renal disease
R692.5; R363.2
A
10.3969/j.issn.1000-4718.2020.11.021
1000-4718(2020)11-2068-06
2019-11-19
2020-04-09
湖北省自然科学基金资助项目(No.2016CFB590);湖北省卫健委科研项目(No.WJ2019Q001);武汉市科技局应用基础前沿项目(No.2019020701011434);武汉市卫计委科研项目(No.WX16B09)
Tel: 027-68894905; E-mail: lixiangyou3@163.com
(责任编辑:林白霜,余小慧)
