Molecular dynamics simulations of the interaction between OH radicals in plasma with poly-β-1-6-N-acetylglucosamine
2020-12-02ShuhuiYANG杨姝惠TongZHAO赵彤JingxianCUI崔静娴ZhiyunHAN韩智云LiangZOU邹亮XiaolongWANG王晓龙andYuantaoZHANG张远涛
Shuhui YANG (杨姝惠), Tong ZHAO (赵彤),∗ , Jingxian CUI (崔静娴),Zhiyun HAN (韩智云), Liang ZOU (邹亮), Xiaolong WANG (王晓龙) and Yuantao ZHANG (张远涛)
1 School of Electrical Engineering, Shandong University, Ji’nan 250061, People’s Republic of China
2 State Grid Shandong Electric Power Construction Company, Ji’nan 250061, People’s Republic of China
Abstract
Keywords: cold atmospheric plasma, molecular dynamics, reactive oxygen species, bacterial biofilm
1.Introduction
Cold atmospheric plasma (CAP) could be used directly on biological tissues due to its high efficiency, low energy consumption, high safety and reliability characteristics.Therefore, it has broad application prospects in biomedical fields.Experimental studies have shown that CAP could inactivate a variety of bacteria,fungi and viruses and exhibits a strong inactivation effect on both gram-positive and gram-negative bacterial biofilms [1-4].CAPs are mixtures mainly composed of reactive oxygen species(ROS; e.g.O, OH and H2O2), reactive nitrogen species (RNS;e.g.NO, NO2and ONOO-), ultraviolet (UV) light, electrons,ions and electric fields [5, 6].Among the various active substances produced in CAP,ROS and RNS might be the most important for biomedical applications [7].
A biofilm is the whole 3D structure composed of bacteria and matrix together with the aqueous environment, and attached to a surface.Because of its complex structure,coordinating ability and functionality, a biofilm possesses strong drug and host immune system resistance abilities.Therefore, the treatment of biofilms is more difficult than the inactivation of common pathogens [8, 9].Staphylococcus aureus(S.aureus)is a species of gram-positive cocci bacteria,which is considered to be one of the most common pathogens encountered in daily life.S.aureus has a high carriage rate and is a major hidden danger of infection and pathogenesis.The pathogenicity of S.aureus is closely related to its biofilm.Specifically, S.aureus can cause disease via the formation of biofilms attached to the surfaces of medical implants or tissues.Recently, biofilm-related diseases caused by S.aureus have increased annually due to the wide application of medical implants (catheters, artificial heart valves, artificial joints, etc) and are difficult to completely cure [10-12].In some cases,traditional clinical medicine methods might have difficulty in coping with biofilm infection, and curing such diseases has become a medical problem.
Researchers have found that CAP gradually shows a potent deactivation effect on various biofilms.In addition,the deactivation effects of CAP on biofilm are somehow not influenced by bacterial resistance and provide successful results and broad application prospects[13,14].Matthes et al used an atmospheric pressure plasma jet to inactivate an in vitro biofilm using argon/oxygen as the discharge gas and found that the gas composition, bacterial species and treatment time greatly influenced the inactivation effect [15].Alkawareek et al studied the inactivation of an in vitro microbial biofilm by a CAP jet with helium/oxygen as the discharge gas and found that all microorganisms were inactivated within 4 min using a discharge frequency of 40 kHz[16].These results indicate that plasma could possess a strong biofilm penetration ability, and the inactivation effect is not associated with biofilm resistance, which to some extent suggests that CAP has the ability to solve the global problem of the removal of difficult bacterial biofilms.
The effect of plasma on microbial cells is a combined effect from various active components.Researchers have speculated from different perspectives that the mechanism of the interaction between plasma and biofilms could be biochemical or physical.Under plasma irradiation,biofilms might be destroyed by a singular factor such as heat, UV light,charged particles or active substances(ROS,RNS,etc)or by a synergistic effect of several factors [17-21].Although plasma sterilization research has achieved some results,the sterilization mechanism of plasma, particularly the destruction mechanism of biofilms,has not yet been uniformly explained.To improve the understanding of the interaction mechanism between CAP and biofilms, however, good insight into the interaction mechanisms between the plasma and biochemically relevant structures is required.Due to the complex composition of CAP and these biochemical systems, it could be rather complicated and difficult to experimentally investigate the interactions of a specific reactive species with biofilms.Conversely, for example, some experimental studies have revealed that O and OH play a dominant role in inactivating bacteria and pathogens,whereas other plasma-generated components(e.g.UV photons,electric fields and heat) make a minor contribution [22-26].
However, the existing experimental detection methods are mainly used to characterize the killing effect of plasma on bacteria, fungi, viruses and bacterial biofilms, and explaining the underlying microscopic mechanism is a bit difficult.As a supplement to experimental research, the molecular simulation technique has been used more frequently in plasma biomedicine to study microphysical and chemical reactions between active particles and biological structures and has been increasingly widely recognized.Some studies have found that the reactive force field(e.g.ReaxFF)can be used in computer simulations to simulate the breaking and formation process of chemical bonds to explore the reaction mechanism between ROS and cell components [27-29].ReaxFF, which was proposed by van Duin, is designed to understand the dissociation and formation of chemical bonds in hydrocarbon compounds[30].Based on ReaxFF,Yusupov et al used reactive molecular dynamics (MD) simulations to observe the damaging effect of ROS on peptidoglycan and lipid A [31-33].In addition, MD simulations are used to explore the microscopic principles of the interaction of ROS with cell membranes, DNA and other organismal structures [34, 35].Therefore, MD simulations can be used to conduct theoretical research on the destruction mechanism of biofilms by CAP at the atomic scale.Furthermore,the results obtained from these studies can be mutually verified and complemented using macroscopic experimental phenomena.
This paper is designed to explore the chemical reaction pathways when OH radicals destroy biofilms.In a real-world situation,bacteria live in a humid environment,and when the working gas of plasma is oxygen, OH is the main active oxygen component of plasma in a liquid phase environment[36-38].In addition, studies have found that the charged species largely recombine or neutralize prior to diffusing into the liquid.The lifetimes of neutral radicals are much longer,so that these reactive species are able to diffuse into the water without significant losses [39-41].
2.Method principle and simulation settings
2.1.Principles of ReaxFF
In this paper, ReaxFF MD was applied to explore the interaction between OH radicals and a component of some bacterial biofilms with the aim of revealing the microscopic mechanism at the atomic level.Aiming to make practical the MD simulation of large-scale reactive chemical systems,van Duin et al first proposed ReaxFF in 2001[30].ReaxFF is a reactive force field that uses a general relationship between bond distance and bond order on the one hand,and between bond order and bond energy on the other hand that leads to proper dissociation of bonds to separated atoms.The bond order BO′ijis calculated according to the interatomic distance rij:

The parameters (pbo,1and pbo,2), (pbo,3and pbo,4) and(pbo,5and pbo,6) correspond to the δ bond, first π bond and second π bond,respectively.In each MD step,connectivity is updated as the interatomic distance changes, thereby achieving cleavage and formation of chemical bonds.ReaxFF not only retains the accuracy close to that of quantum mechanics,but has a small computational cost.This approach can also be used to describe systems composed of various macromolecules.The overall system energy in the ReaxFF is described as follows:
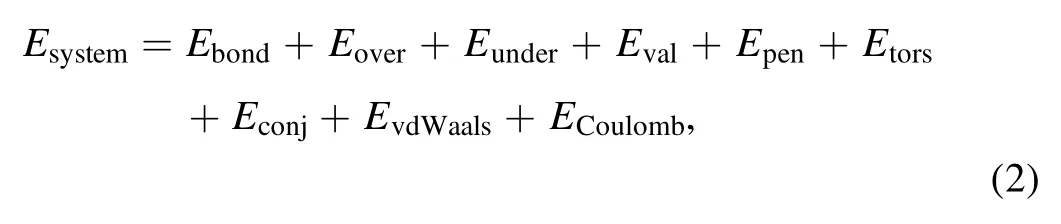
whereEbondis the bond energy,EoverandEunderare the atomic overcoordination and undercoordination terms, respectively,Evalis the valence energy,Epenis the penalty energy of two double bonds sharing one atom in a valence angle,Etorsis the torsion angle energy,Econjis the contribution of the conjugation effect to the molecular energy,EvdWaalsandECoulombrepresent the non-bonded van der Waals interactions, and Coulomb interactions, respectively.The energy of the entire system (Esystem) contains all relevant energy terms, ensuring that the chemical reaction process can be accurately described.
The ReaxFF was designed to describe the stability and geometry of nonconjugated, conjugated, radical-containing compounds and the dissociation and formation of chemical bonds in hydrocarbon compounds, while the accuracy of the ReaxFF has been validated by a lot of experiments.The results of the ReaxFF have been compared with an extensive set of literature on the heat formation and geometry data on the one hand and quantum chemical data on bond dissociation in various simple hydrocarbon molecules on the other hand.This comparison shows that the ReaxFF can indeed reproduce the energies associated with the nonreactive and reactive behavior of these compounds, and the commonly used ReaxFF is a reactive force field that has been expanded and improved a lot[42-46].In this paper,to study the interaction between plasma active particles and the biological cell structure, the ReaxFF potential comprising the C/H/O/N glycine/water parameters was used to simulate the reaction pathways between OH radicals with different concentrations and bacterial biofilm components [47].
2.2.Simulation model
Biofilms are film-like structures in which bacteria attached to the surface of the contact secrete extracellular polymeric substances (EPSs) to embed themselves and aggregate [48].The bacteria that form the biofilm survive in their secreted polymer, and this polymer constitutes their direct living environment.
In a mature bacterial biofilm, bacteria account for only approximately 10%-20% of the total biofilm volume,whereas the proportion of EPSs is as high as 80%.Studies have shown that the exopolysaccharide is the main component of EPSs.Under an electron microscope, exopolysaccharides are attached to the surfaces of cells and form a complex network structure composed of a large number of linear or branched molecules that support the wall morphology and maintain attachment of the bacteria to the contact surface [49, 50].Among them, poly-β-1-6-N-acetylglucosamine (PNAG) is a common exopolysaccharide and is found in many common pathogens, including S.aureus[51].The chemical structure of PNAG is shown in figure 1.PNAG is a polymer composed of glucosamine units linked together by β-1-6 bonds [52].The chemical structure and constructed model of PNAG used in those simulations are shown in figure 2.

Figure 1.Unit structure of PNAG.Here, n is an integer between 2 and 300,and the R group is selected from-NH2 and-NH-CO-CH3.
2.3.Simulation parameters
It should be mentioned that considering the simulation efficiency and intuitiveness, we performed the reactive MD simulations under ‘perfect’ vacuum conditions, that is, the PNAG structure was not surrounded by a water film.In reality, bacteria are typically coated by a water film, which can affect the behavior and stability of the plasma species.Many studies have investigated the behavior of ROS in a water film and demonstrated that certain ROS such as OH can penetrate the water film and be quite stable in the liquid [39, 40].Initially, this paper conducted in-depth research on the reaction between OH radicals with different concentrations and PNAG molecules and ignored the self-reaction of OH radicals.Due to space limitation, the interaction between other ROS(e.g.O atoms,H2O2,etc)and PNAG molecules was not covered, and that will be presented in the next study.

Figure 2.PNAG structure diagram used in the simulations.R group is either-NH2 or-NH-CO-CH3,which appears alternately in the PNAG molecular structure.Degree of polymerization is ten,and a total of ten monosaccharides and nine glycosidic bonds are included.(a)Chemical structure of PNAG.(b) Constructed PNAG molecular model.
In the practical application of plasma sterilization, it is well known that the working conditions of plasma would affect the sterilization efficiency, which might be related to the different kinds and content of reactive particles produced in plasma under different working conditions [53-55].Nimisha et al demonstrated that the generation of the OH radical is highly influenced by water fractions and the treatment time [55].Therefore, in this paper, OH radicals at different concentration levels (i.e.numbers of 5, 10, 20, 30, 40 and 50) were selected as reaction particles for addition to the model system.Although this density was a bit high, this degree of improvement was acceptable after extensive practice in the MD simulations to ensure that the system had a sufficient reaction efficiency.First, the geometry-optimized biomolecule PNAG was located in a 25 Å×25 Å×71 Å box, and periodic boundary conditions were adopted to maintain a constant density in the system.For our simulations,energy minimization based on smart minimizer (i.e.smart combination of steepest descent,conjugate gradient and Newton methods) was performed first to decrease the initial system energy of the model.After that,in order to minimize the adverse effect of system pressure fluctuations on the simulation results,the model was equilibrated in the canonical ensemble following NVT dynamics (i.e.constant number of particles, volume and temperature) at room temperature (300 K) for 100 ps.A Berendsen thermostat was employed to balance heat dissipation and keep the equilibrium temperature constant.The Berendsen thermostat is also a common method used in NVT dynamics simulations, and when using it for equilibrium of NVT dynamics, the system can be coupled with a heat bath with constant temperature.The model system after equilibrium was used as the initial state of the MD simulations, and the atomic directions were set to random.Finally,the ReaxFF based on the NVT ensemble was applied to the MD simulation program.The reaction temperature was set to room temperature (300 K),which was consistent with the CAP temperature.The time step was 0.1 fs,and the reaction lasted for 300 ps.To obtain a more general destruction mode,the simulations of each concentration were repeated 20 times for a total of 120 simulations.
3.Results and discussion
In the simulations, destruction of the bacterial biofilm components by the OH radicals is triggered by the hydrogen abstraction reaction.During the induction of PNAG molecular structure destruction, the OH radical attracts the hydrogen atom in PNAG,and the molecular structure is in an unstable state.This instability results in the subsequent series of C-O and C-C single bond cleavages and the formation of C=O and C=C double bonds, which would destroy the original monosaccharide and glycosidic bonds, leading to disintegration of the chain structure.These double bonds are relatively stable, and no further reaction of OH with the double bonds was found in the current study.In the simulations discussed in this paper, the addition reaction of OH to PNAG also occurred based on the hydrogen abstraction reaction.
3.1.Reaction pathway and data analysis of PNAG molecules destroyed by different OH concentrations
3.1.1.Basic destruction mode of PNAG molecules destroyed by different OH concentrations.Through simulations, we found 14 types of pathways by which OH radicals destroyed the PNAG structure.The destruction pathway that dominated at all concentrations (more than 75% of the total number of destructions) was defined as the basic destruction mode.The reaction pathway of the basic destruction mode is shown in figure 3 (the numbers in the figure represent the numbering for C atoms).In the monosaccharide structure, hydrogen abstraction occurred from two adjacent-OH groups at C3and C4by OH radicals, and thus the original C-O-H structure changed to a C=O structure, causing cleavage of the C3-C4single bond and destruction of the monosaccharide structural integrity.
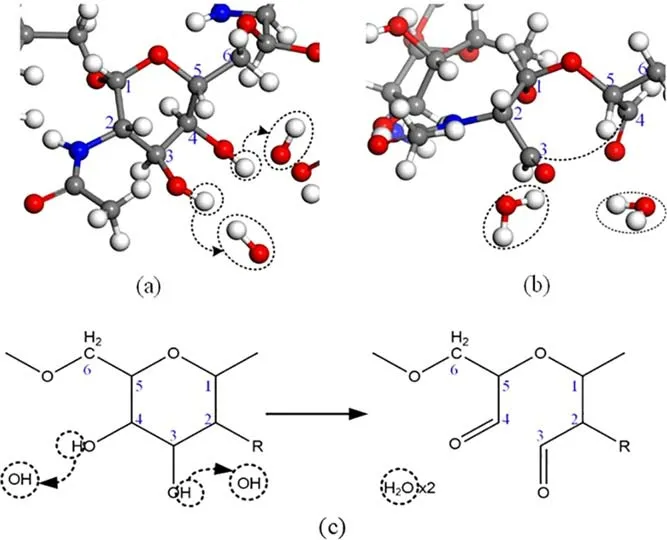
Figure 3.Basic reaction pathway for PNAG molecule destruction by OH.(a)OH triggers the hydrogen abstraction reaction.(b)Chemical bond cleavage of the monosaccharide.(c) Chemical structure diagram of the monosaccharide destruction mechanism by OH.
The basic destruction mode shown in figure 3 was the most frequent reaction pathway in the simulations of all OH radical concentrations and affected the integrity of the monosaccharide in the PNAG chain structure.The probability of destruction of each monosaccharide is called the monosaccharide destruction rate.Figure 4 shows the statistical analysis of the monosaccharide destruction rate caused by the basic destruction mode and all destruction modes at different concentrations.The relationship between destruction of the PNAG monosaccharide and the change in the OH concentration is shown in the figure.Between the five and 30 concentration levels, the proportion of the basic destruction mode to all destruction modes gradually increased to 97.1%.
Between the 30 and 50 concentration levels, the basic demonstration mode showed a slight downward trend, but still dominated and remained above 90%.Meanwhile, the results indicated that with the increasing OH concentration,the number of new destruction pathways appearing was also higher than those at the lower OH concentrations.
3.1.2.Stepwise reaction pathway of PNAG molecules destroyed by different OH concentrations.In the simulations of the six OH concentration levels (i.e.numbers of OH radicals of 5,10,20,30,40 and 50),whenever the OH number increased to the next concentration level, reaction pathways with destruction modes that differed from those in the low concentration appeared.This pattern was defined as a stepwise reaction pathway.The types of PNAG molecular destruction pathways increased with the increasing number of OH radicals added, and the new destruction mode also showed a great impact on the PNAG molecule.This section focuses on analysis of some significant destruction modes in the stepwise reaction pathway.
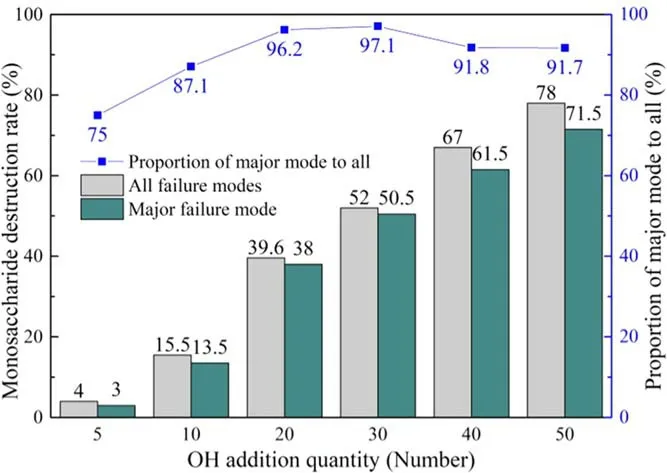
Figure 4.Statistics of PNAG monosaccharide structure destruction by different OH concentrations.
Due to space limitation,the reaction pathway diagram in the simulation software is not displayed in this section;only the chemical structures in the reaction are shown here.When the number of OH radicals added was ten, a stepwise reaction pathway occurred (as shown in figure 5), and hydrogen atom abstractions from C1and the -OH connected to C3by two OH radicals occurred to form two water molecules.At this time, the instability after hydrogen abstraction caused the C5-O and C3-C4single bonds to break.This reaction pathway not only destroyed the integrity of the PNAG monosaccharide structure, but caused cleavage of two chemical bonds to disintegrate the chain structure,affecting the supporting function of PNAG in the bacterial film structure.When the active particle concentration level reached 20,the destruction mode shown in figure 6 exhibited the highest frequency of occurrence of all of the stepwise reaction pathways.At this time, the hydrogen abstraction reaction initiated by OH occurred at C6,and the N atom of the R group attached to C2.This type of destruction can also cause breakage of the PNAG chain structure.
When the concentration of OH increases to 30 and 40,the destruction of PNAG molecules by OH is still concentrated on monosaccharides, and the reaction paths,similar to the above analysis, will not be repeated here.However, when the number of OH radicals added to the simulations reached 50, no new monosaccharide destruction mode occurred, but the OH radicals began to destroy the glycosidic bonds.Glycosidic bonds have higher stability than monosaccharide structures and are difficult to destroy using low concentrations of OH radicals.In the above monosaccharide destruction modes, two C-C single bonds in one monosaccharide structure must be broken simultaneously to achieve the effect of breaking the PNAG molecular structure.However, in the glycosidic bond structure, the occurrence of C-O or C-C single bond cleavage will lead directly to collapse of the PNAG chain structure.Therefore,studying the reaction concentration and reaction pathway of the OH radicals is important to achieve breakage of the glycosidic bond in PNAG.The OH radical destroying the glycosidic bond structure in PNAG only occurred in the simulations with the concentration level of 50.Figure 7 mainly shows two types of destruction pathways, and both frequencies of occurrence are 50%.These two destruction pathways destroyed the integrity of the monosaccharide structure and affected its biological function while causing cleavage of the glycosidic bond and collapse of the PNAG chain structure.
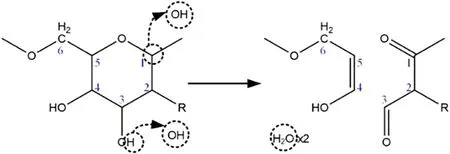
Figure 5.Stepwise reaction pathway for the destruction of PNAG molecules when ten OH radicals were added.

Figure 6.Stepwise reaction pathway for the destruction of PNAG molecules when 20 OH radicals were added.
In summary, OH radicals in ROS can damage the PNAG-S.aureus biofilm skeleton structure.In the experiment by Lee et al, the sterilization results evaluated by scanning electron microscopy showed that the S.aureus biofilm before plasma treatment formed a multi-layered and adhesive polysaccharide skeleton structure in which free bacteria aggregated and stabilized[4].After plasma treatment for 20 s, the biofilm was removed; biofilm reformation was difficult to achieve due to inference caused by erosion of the biofilm structure by ROS in the plasma [4].This finding is consistent with the conclusions arrived at in this paper in a certain sense.Therefore, the study described in this paper might be helpful to reveal the sterilization mechanism of plasma from the micro level.
3.1.3.Statistical analysis of PNAG molecules destroyed by different OH concentrations.The simulations showed that the degree of destruction of the PNAG structure gradually increased with the OH concentration.To more accurately explore the pattern of variation in the destructive effect of increasing the concentration of OH reaction particles on the PNAG molecule,the hydrogen abstraction and chemical bond cleavage data were statistically analyzed.Figure 8 shows the relationship of the number of occurrences and efficiency of the hydrogen abstraction reaction with the OH concentration.The number of hydrogen abstraction reactions was the average number occurring in each simulation.The number of triggered hydrogen abstraction reactions increased with the number of added OH radicals.However, an increase in the reaction concentration also allowed collision between the OH radicals to occur more easily, triggering the hydrogen abstraction reaction between them [32].As a result, the proportion of OH radicals involved in the induction of PNAG structure destruction decreased, reducing the OH reaction efficiency to some extent.During the plasma excitation process,increasing the OH concentration often requires more materials and energy, and the OH reaction efficiency decreases with the increasing OH concentration.Therefore,the pattern of variation in the OH reaction efficiency at different concentrations in figure 8 can provide some theoretical support for finding a relatively economical concentration for plasma sterilization.
In figure 9, the hydrogen abstraction reactions were divided into three categories according to the type of chemical bond cleavage(i.e.C-H,O-H and N-H bond cleavages),and the curves of the proportions of the three types of bond breaking as a function of the OH concentration were plotted.The proportions of the bond breaking is the ratio of the number of broken bonds in a particular type to all of the bonds in a PNAG molecule.The comparison of the three hydrogen abstraction reactions showed that at all reaction concentrations,the O atom in the structure was most prone to hydrogen abstraction by OH radicals, destroying the original O-H bond.Thus, the O atom was unstable, easily formed a C=O bond with the adjacent C atoms, and then initiated the subsequent bond cleavage.When the number of OH radicals added was 50,the proportion of the O-H bonds destroyed by hydrogen abstraction in the PNAG molecule was 86.75%,which was considerably higher than those of the other two hydrogen abstraction reactions.However,the destruction rate of the O-H bond increased with the OH reaction concentration, but the rate of the increase tended to be flat.Generally,the destruction rate of the N-H and C-H bonds showed a linear increase; the highest stability was found for the C-H bond, followed by the N-H bond.Thus, the hydrogen abstraction from the C atom by OH radicals was the most difficult reaction.What is more, the three chemical groups(O-H, C-H and N-H) do not exist alone, but as part of the molecular structure of PNAG molecules.Although there is a slight difference in bond energy among C-H bond,C-H bond and N-H bond, the results of MD simulation show that the molecular structure of PNAG molecule seems to have a greater influence on the broken probability of these three chemical bonds.Three important chemical bonds constitute the PNAG structure (i.e.C-C, C-O and C-N bonds).Cleavages of these three chemical bonds often indicate that the integrity of the PNAG structure is destroyed.Figure 10 shows the destruction of the C-C and C-O bonds by OH radicals at different reaction concentrations.Since the C-N bond cleavage data were insufficient, they had no universal significance, and no statistical analysis was performed.The number of chemical bond cleavages was the average value in each simulation.

Figure 7.Reaction pathway for destruction of the glycosidic bond when 50 OH radicals were added.(a) Destruction of glycosidic bonds started with the hydrogen abstraction reaction at C6 and C6′.(b) Hydrogen abstraction took place between OH radicals and -OH and C6′on C3.

Figure 8.Pattern of variation in the number of hydrogen abstraction reactions induced by the different OH concentrations and theirreaction efficiencies.

Figure 9.Pattern of variation in chemical bond cleavage during the hydrogen abstraction induced by different OH concentrations.

Figure 10.Pattern of variation in the important chemical bond cleavages of the PNAG structure induced by different OH concentrations of OH.
As shown in figure 9, the OH radical was most likely to destroy the O-H bond, and more C=O bonds were formed.At this time,the C atom was in an unstable state,causing the original C-C bond to be broken.In figure 10,the number and proportion of C-C bond cleavages were considerably higher than those of the C-O bond.The destruction rate of the C-C bond gradually increased and then gradually flattened,whereas the destruction rate of the C-O bond increased first gradually and then rapidly.Regarding the reason the C-N bond was barely destroyed by OH, we could infer from the structural characteristics of PNAG that the atoms adjacent to the C atom in the C-N bond were all C atoms.According to figure 9, hydrogen abstraction from C atoms was difficult,which accordingly increased the difficulty of forming a C=C bond to break the adjacent C-N bond and detach the R group in PNAG from the carbocyclic ring.
3.2.Microscopic pathway and data analysis of the addition reaction between different OH and PNAG concentrations
In this paper, we found that OH radicals could not only induce the destruction of PNAG molecules via the hydrogen abstraction reaction, but undergo the OH addition reaction with atoms in an unstable state after the hydrogen abstraction reaction to form new chemical bonds connected to the structure.This phenomenon did not appear in the previous simulations, therefore, an exploration of its effect on the interaction between OH radicals and PNAG was necessary.The previous study of the destruction process showed that the mode of destruction caused by OH was independent of the type of R group.However, during the addition process, the reaction site of OH within the structure was mainly located in the R group, especially -NH2.Furthermore, as the OH concentration increased, a phenomenon in which OH was added at the position of the glycosidic bond could occur.

Figure 11.Basic addition reaction of OH and the PNAG molecule.(a) Hydrogen abstraction reaction triggered by OH.(b) Addition reaction of OH with the structure.(c)Chemical structure diagram of the mechanism of the OH addition reaction.
The most common pathway for OH radical addition on the PNAG structure is shown in figure 11 and is defined as the basic addition mode.This addition mainly occurred on-NH2.After the hydrogen atom abstraction from -NH2by OH to form a water molecule, the -NH structure attracted an OH to replace the H, thereby forming a new relatively stable structure.This basic addition mode appeared in the simulations with an active particle concentration level of ten and above.
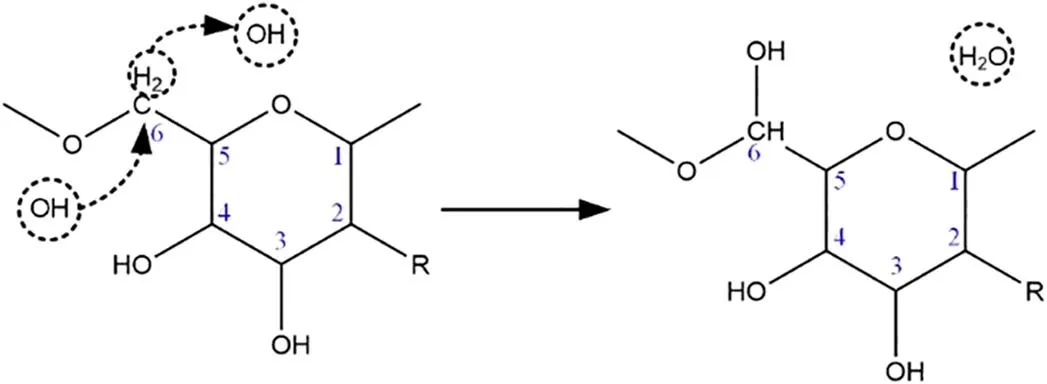
Figure 12.OH addition reaction pathway for the glycosidic bond of the PNAG molecule.
When the number of OH radicals added was five, an average of 87% of the OH radicals in each simulation was used for the hydrogen abstraction reaction (figure 8), but no OH addition reaction occurred.In addition, two types of OH addition reactions were found with the reaction site at -NH2,but due to the limited space, they will not be described here.Figure 12 shows the addition reaction occurring at the glycosidic bond when the number of OH radicals reached 40.After the hydrogen abstraction reaction occurred at C6,an OH radical was attracted to form a C6-OH single bond that was connected to the PNAG structure.
4.Conclusion
In this paper, the microscopic mechanism of interaction between different concentrations of OH radicals and the extracellular polymer molecule PNAG of the S.aureus biofilm was studied at the atomic level by ReaxFF MD simulations.The results showed that OH could damage different parts of PNAG (monosaccharides and glycosidic bonds)through the hydrogen abstraction reaction, and a new destruction pathway could be triggered as the concentration of the reaction particles increased, resulting in collapse of the biofilm structure.Meanwhile, the PNAG structure after hydrogen abstraction could form a new chemical bond through the OH addition reaction.
(1) Among the destruction pathways triggered by OH, the basic destruction pathway was dominant at all reaction concentrations, and the highest proportion was 97.1%.A stepwise reaction pathway occurred as the OH concentration increased, which also had a great influence on the destruction of the monosaccharide.When the OH concentration level reached 50, the destruction of glycosidic bonds occurred.
(2) The O atom in the structure was most prone to hydrogen abstraction by the OH radicals, and the original O-H bond was destroyed.Moreover, the destruction rate of the O-H bond in the structure increased with the OH reaction concentration, but the rate of the increase tended to be flat.The number and proportion of C-C bond cleavage were considerably higher than those of the C-O bond.The destruction rate of the C-C bond increased rapidly and then gradually stabilized, whereas the destruction rate of the C-O bonds increased first gradually and then rapidly.In contrast, the C-N bonds were barely destroyed by OH.
(3) OH could undergo an addition reaction with the PNAG structure,and the reaction site was mainly located at the R group, especially at -NH2.As the OH concentration increased, an OH addition reaction could occur at the glycosidic bond position.
Based on the simulation analyses,a possible microscopic mechanism of OH radical action on PNAG molecules in the S.aureus biofilm has been clarified and it seemed to be consistent with the phenomenon of plasma disintegration of the biofilm in experimental studies.At the atomic level, the reaction pathways and efficiency of the interaction between OH radicals and biological structures (PNAG) have been comprehensively explored.This study would, more or less,provide some theoretical support for exploration of the relationship between plasma physical properties and sterilization effects at the microscopic level, thereby guiding the application of plasma in biology in the medical field and promoting the development of plasma medical research to a certain degree.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No.11675095) and the Fundamental Research Funds of Shandong University (Grant No.2017JC017).
Appendix.The remaining destruction paths of PANG molecules
Through simulations, there were a total of 14 ways by which OH radicals destroyed the PNAG structure.The destruction paths of OH-mediated destruction of the PNAG molecules can be summarized into two types.The first type of destruction path is caused by hydrogen abstraction reaction of OH, as shown in figures 3 to 7 in section 3.The remaining three reaction paths for the first type of OH-mediated destruction of PNAG molecules are shown in figure A1 below.Because the destruction methods are all caused by the hydrogen abstraction reactions,leading to breakage of the chemical bonds, which is similar to the reaction path with the highest frequency, the reaction paths are not explained in detail.

Figure A2.Remaining four reaction paths for the second type of OH-mediated destruction of PNAG molecules.
Figures 11 and 12 in chapter 3 show the second type of destruction path, which is achieved through the addition reaction of OH molecules.Considering the other destruction paths with the lower frequency are somewhat contingent;only the most frequent and important reaction ways are listed in chapter 3, and the remaining destruction paths are shown as figure A2.The remaining destruction paths are not further explained in the appendix due to their similarity.
ORCID iDs
Tong ZHAO (赵彤) https://orcid.org/0000-0003-0523-4466
杂志排行
Plasma Science and Technology的其它文章
- The structure of an electronegative magnetized plasma sheath with non-extensive electron distribution
- The effect of forced oscillations on the kinetics of wave drift in an inhomogeneous plasma
- Numerical simulation of impact of supersonic molecular beam injection on edge localized modes
- Effect of background fluctuation on velocity diagnostics by Mach probe
- Mitigation of blackout problem for reentry vehicle in traveling magnetic field with induced current
- New method for rekindling the explosive waves in Maxwellian space plasmas
