Investigation of the major constitutes in Rehmannia glutinosa-Dioscorea opposite Thunb herb-pairs extract by high-performance liquid chromatography and UV-spectrophotometry methods
2020-11-06XIEYuleiZHUJinhuaLIUXiuhuaHUWeiping
XIE Yulei,ZHU Jinhua,LIU Xiuhua,HU Weiping
(Henan International Joint Laboratory of Medicinal Plants Utilization,College of Chemistry and Chemical Engineering,Henan University,Kaifeng 475004,Henan,China)
Abstract: The active ingredients of Dioscorea opposita Thunb and Rehmannia glutinosa as famous geoherbs in Henan province of China were researched,focusing on the influence of the component and content caused by the compatibility of the two species by forming herb pairs. The high-performance liquid chromatography (HPLC) method for the simultaneous determination of catalpol,5-hydroxymethyl-2-furaldehyde (5-HMF) and adenosine in the medicine pair of Dioscorea opposita Thunb and Rehmannia glutinosa with different proportions (3∶1,2∶1,1∶1,1∶2,1∶3,1∶5) and their single herb medicine was developed. The quantitative determination of polysaccharides content in the medicine pair with different proportions was conducted by using the phenol-sulfuric acid method,and the polyphenolics content in the medicine pair was assayed by using the Folin-Ciocalteu colorimetric method. The results show that the compatibilities of the herb pair can promote the dissolution rate of catalpol. But the compatibility of Dioscorea opposita Thunb and Radix Rehmannia Preparata has an inhibitory action to the dissolution rate of 5-HMF. The content of adenosine in the medicine pair has no obvious change with the variation of the proportions of medicine pair. The contents of polysaccharides and polyphenols in the medicine pair and their single herb medicine conducted by ultraviolet and visible spectrophotometry method indicate that the polysaccharides content in their herb pairs has an obvious increase in different proportions with respect to the theoretically calculated results. However,compared with the theoretical values,the polyphenols content in their herb pairs has no obvious change,showing a slight increase after compatibility. This study provides a good example for the rapid determination of major constituents in complex systems such as herbal extract or traditional Chinese medicine formula,and lays a good foundation for the activity study and clinical application in the future research.
Keywords: herb pair; HPLC; Rehmannia glutinosa; Dioscorea opposite Thunb; major constitutes
Receiveddate:2020-05-16.
Foundationitem:National Natural Science Foundation of China (21705033); Medical Interdisciplinary Cultivation Project of Henan University (CJ1205A0240016); First Class Discipline Cultivation Project of Henan University (2019YLZDYJ13).
Biography:XIE Yulei(1999-),female,engaging in the separation and analysis of active components in natural products.*Corresponding author,E-mail: huweiping@henu.edu.cn.
CLCnumber:O656.3Documentcode:A
ArticleID:1008-1011(2020)04-0318-10
高效液相色谱法和紫外分光光度法对地黄-怀山药“药对”提取物主要成分的研究
谢玉蕾,朱金花,刘绣华,胡卫平*
(河南大学 化学化工学院,河南省药用植物资源化利用国际联合实验室,河南 开封 475004)
摘 要:研究了河南省著名中药材怀山药和地黄的有效成分,并考察了怀山药和地黄配伍成“药对”对其成分和含量的影响.建立了同时测定不同配比(3∶1、2∶1、1∶1、1∶2、1∶3、1∶5)的怀山药和地黄药对及其单味药材中梓醇、5-羟甲基-2-呋喃甲醛(5-HMF)和腺苷的高效液相色谱法.采用苯酚-硫酸法对不同配比的“药对”中多糖含量进行了定量测定.用Folin-Ciocalteu比色法测定“药对”中多酚类物质的含量.结果表明,“药对”的配伍能提高梓醇的溶出速率.但怀山药和熟地黄的配伍对5-HMF的溶出度有抑制作用.“药对”中腺苷含量随药对比例的变化无明显变化.紫外和可见分光光度法对“药对”及其单味药中多糖和多酚的含量测定结果表明,“药对”中多糖含量与理论计算结果相比随不同比例明显增加.然而,与理论值相比,“药对”中多酚含量没有明显变化.但是复配之后会有一点提高.本研究为中药提取物或中药配方等复杂体系中主要成分的快速测定提供了一个很好的范例,为今后的活性研究和临床应用研究奠定了良好的基础.
关键词:药对;HPLC;怀山药;怀地黄;主要成分
Compatibility of Chinese medicinal herbs refers to the combination of two or more herbs with purpose in the light of the clinical requirement and medicinal properties and actions[1]. Unique combinations of the traditionally defined herbal properties of traditional Chinese medicine (TCM) are frequently used for achieving mutual reinforcement,mutual assistance,mutual restraint,mutual suppression or mutual antagonism[2-3]. The Rehmannia glutinosa-DioscoreaoppositeThunb herb-pair is a combination ofHuaishanyao[Chinese yam,the dried rhizome ofDioscoreaoppositeThunb] andHuaidihuang[Radix rehmanniae,the dried root of libosch (rehmannia glutinosa)]. It is an important multiherb remedy in TCM and first described by the famous Chinese physician ZHANG Xichun in his Zhongzhongcanxilu[4]. According to the traditional Chinese medical system,the herb-pair has been widely used to nourishing Yin and clearing heat and thirst[5].
On the basis of the chemical and physical properties of specific compounds,a number of different techniques including thermal analysis,spectrophotometry method,thin layer chromatography,high-performance liquid chromatography have been used for detecting the components in Rehmannia glutinosa orDioscoreaoppositeThunb[6-13]. Moreover,the determination of monosaccharide in Rehmannia glutinosa orDioscoreaoppositeThunb with HPLC method always coupled with evaporative light scattering detection. The HPLC with this kind of detector is very expensive. Recently,XU et al. developed a HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyranozolone to determine the monosaccharide in the medicine pair of Rehmannia glutinosa andDioscoreaoppositeThunb[14]. ZHANG et al. separated one compound fromDioscoreaoppositeThunb which had inhibitory activity againstα-glucosidase using high-speed counter-current chromatography (HSCCC)[15]. Compared to these methods,spectrophotometry method is simple and fast. But individual compounds could not be determined. HPLC methods can supply quantitative analysis of individual compounds at low amount of samples within a relatively short analysis time. The HSCCC method can also obtain relatively pure individual compound during the separation process.
Although chemical constituents of the individual herbs have previously been reported[10,16-21],little is known about the chemical composition ofHuaishanyao-Huaidihuangherb-pair in detail. Unlike chemical drugs,botanical products contain mixture compounds,the contents of which may be significantly affected by various factors. It was reported that there were certain amount of polysaccharide and polyphenol inHuaishanyaoandHuaidihuangwhich had good hypoglycemic activity[7-8,22-24]. 5-Hydroxymethyl-2-furfural (5-HMF) and catalpol (structures as Fig.1) are the indexes of compositions of Huaidihuang in the Chinese Pharmacopoeia[25]. Research provided the evidence that catalpol had the neuroprotective effect[13],5-HMF had been found to be endowed with diverse pharmacological and biological activities such as anti-oxidant activity,inhibiting sickling of red blood cells,ameliorate hemorheology and so on[26-30]. Adenosine exists both inHuaishanyaoandHuaidihuang,and it has diastolic blood vessels,lower blood pressure effect etc.[31].
Therefore,we selected 5-HMF,catalpol,adenosine,polysaccharide and polyphenol as the studied objectives,using a simple and robust HPLC method and a simple spectrophotometry method to determine the active constituents inHuaishanyao-Huaidihuangherb-pair with different proportions,and investigated the influence of different compatibility proportions ofHuaishanyaoandHuaidihuangon main ingredients. Our results proposed a good example for the rapid determination of major constituents in complex systems such as herbal extract or traditional Chinese medicine formula,and revealed the influence of different compatibility proportions on main chemical composition,which facilitated the study of the metabolic pathway of the herbs in the body to better understand the action mechanism.
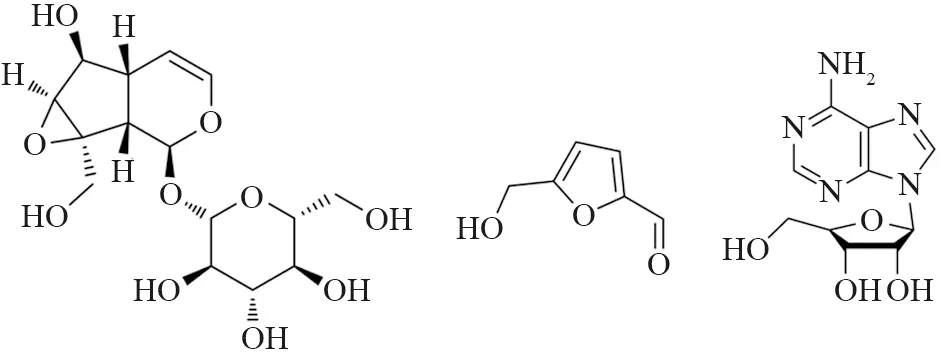
Fig.1 Chemical structures of analytes
1 Materials and methods
1.1 Chemicals and Reagents
Plant sample ofDioscoreaoppositaThunb (Chinese Yam,Huaishanyao) and Rehmannia glutinosa (Huaidihuang) was obtained from Wuzhi county (Jiaozuo,Henan province,China) where it is famous of plantingHuaishanyaoandHuaidihuangfor more than 1 000 years. Catalpol was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing,China). 5-HMF was obtained from Sigma-Aldrich Co. (St. Louis,MO,USA). Adenosine was purchased from Sinopharm Chemical Reagent Co.,Ltd. (Ningbo,China). Acetonitrile and methanol of HPLC grade were purchased from Beijing Chemical Factory (Beijing,China). Gallic acid,glucose and all other analytical chemical reagents were analytical grade (Tianjin,China). Deionized water was purified using a Milli-Q system (Millipore,Billerica,MA,USA); double-distilled water was used for all the preparations.
1.2 Apparatus
All chromatographic separations were performed using a 1100 Series HPLC instrument (Agilent,Waldbronn,Germany) coupled with a 1100 Series chromatography workstation. Separation was carried out on a reversed-phase column (Aglient Zorbax SB-C18,2.1 mm×150 mm i.d.,5m,Agilent,Waldbronn,Germany) connected to an Easy-Guard Kit C18(4 mm×2 mm,Grace,USA) guard column,with the column temperature set at 20 ℃. A TU-1900 UV spectrophotometer (Puxi Analytic Instrument Ltd. of Beijing,China) equipped with a 1.0 cm quartz cell was used to determine the contents of polysaccharides and polyphenolics in the medicine pair and their single herb medicine. A PHS-3B acidity meter (Shanghai Precision & Scientific Instrument Co.,Shanghai,China) was used for the pH measurement. The pH was adjusted with 1 mol/L NaOH or HCl.
1.3 Solutions and sample preparation
1.3.1 Standard solutions
SolutionforHPLC: Mixed stock standard solutions were prepared in methanol (MeOH) to give concentration of 800 mg·L-1for catalpol,500 mg·L-1for 5-HMF,72 mg·L-1for adenosine,respectively. The other standard solutions with different concentrations were prepared by diluting the stock standard solutions with MeOH. All the standard solutions were kept in dark and stored at 4 ℃ in a refrigerator. The mobile phase solution was prepared daily. All the solutions for HPLC were filtered through a 0.45m cellulose acetate membrane filter (Shanghai Xinya Purification Apparatus Factory,Shanghai,China) prior to use.
SolutionforUV-visible: A 40 mg·L-1Gallic acid and 1 000 mg·L-1glucose prepared in double-distilled water respectively were used as the stock standard solutions. The other standard solutions with different concentrations were prepared by diluting the stock standard solutions with double-distilled water. All the standard solutions were stored at 4 ℃.
1.3.2 Sample preparation
The samples were separated into five kinds: Chinese yam,raw radix rehmanniae,Rehmannia glutinosa,Chinese yam and raw radix rehmanniae with different mass ratio (3∶1,2∶1,1∶1,1∶2,1∶3,1∶5),and Chinese yam and Rehmannia glutinosa with different proportions (3∶1,2∶1,1∶1,1∶2,1∶3,1∶5). The weight of a single herb is equal to the weight of each medicine pair. For example,the Chinese yam is 2.0 g,the medicine pair of yam and raw radix rehmanniae (3∶1) is also 2.0 g just by mixing 1.5 g Chinese yam with 0.5 g raw radix rehmanniae as the sample. The others with different proportions were treated in the same way. Then they were extracted with different solvents for following use.
For HPLC experiments,water decoction extraction was used for sample disposed. 2.0 g samples of small pieced of Chinese yam were immersed in 40 mL distilled water and boiled for 1 h. This process was repeated for two times,and then the two extracting solutions were combined,centrifuged,filtered and concentrated to 60 mL. Then the solution was extracted with 60 mL n-butyl alcohol (saturated with water) for three times. Then-butyl alcohol phases were merged and concentrated to dry. The solute was dissolved in 10 mL MeOH and used as sample solution for Chinese yam. The sample solutions of raw radix rehmanniae,Rehmannia glutinosa,medicine pair of Chinese yam and raw radix rehmanniae with different proportions and medicine pair of Chinese yam and Rehmannia glutinosa were prepared as the same way.
For polyphenolic determination,the samples were disposed as reference[32]: The samples (1 g of pulverized each) were extracted with 30 mL ethanol/H2O (4∶1) at 70 ℃ for 3 h (×3),the supernatant was mixed and evaporated in a rotary evaporator to no ethanol existed,then the volume was fixed at 25 mL with distilled water and used as the sample solutions.
For polysaccharide determination,the samples were disposed as reference[22]: The driedD.oppositaThunb root powder and other four kinds of samples (1.0 g each) were extracted with 80% ethanol (30 mL×3) at 70 ℃ for 3 h to remove the pigments and free sugars,and the supernatant was removed. The residue was extracted with water (30 mL×3) at 80 ℃ for 3 h,the solutions were merged and evaporated to 25 mL and used as the sample solutions.
All the sample solutions were stored at 4 ℃.
1.4 Analytical methods
1.4.1 Determination of polysaccharides
The total sugars were determined by the phenol-sulphuric acid method[33-34]withD-glucose as standard. The reading of the absorbance was made at 490 nm. The polysaccharides content was evaluated from the absorbance value by the interpolation into calibration plot (A=40.875D-glucose (g·L-1)-0.015 6;r=0.999 8;n=7). The results (averaged from triplicates) were expressed as milligrams ofD-glucose equivalents per g dry weight of plant.
1.4.2 Determination of polyphenols
Total phenolic compound content was determined spectrophotometrically,according to the Folin-Ciocalteu method,which is using gallic acid as a standard. The reading of the absorbance was made at 760 nm. The phenolic content was evaluated from the absorbance value by the interpolation into calibration plot (A=0.121 0 gallic acid (μg·L-1)-0.022 8;r=0.999 5;n=7). The results (averaged from triplicates) were expressed as micrograms of gallic acid equivalents per g dry weight of plant (calibration range 1-200 mg·L-1).
1.4.3 HPLC method for determination of catalpol,5-HMF and adenosine
The mobile phase consisted of phosphate buffer of pH 6.5 and acetonitrile. The flow rate was 0.35 mL· min-1,the column temperature was set at 20 ℃. Simultaneous monitoring was performed at 210 nm and 260 nm. The injection volume for all samples was 2 μL.
2 Results and discussion
2.1 Optimization of the HPLC separation conditions
2.1.1 Selection of the optimum wavelength
The maximum absorption wavelength for catalpol,5-HMF and adenosine is 210,260 and 284 nm,respectively. The three wavelengths were investigated for the simultaneous determination of the three analytes. There is no absorption for catalpol at 260 nm,5-HMF has weak absorption at 210 nm,and adenosine has strong absorbance both at 210 and 260 nm. So the DAD detector was used,and two wavelengths were selected. For determination of catalpol,210 nm was used; for 5-HMF and adenosine determination,260 nm was used.
2.1.2 Selection of the mobile phase
Several mobile phases were investigated to obtain the optimum separation condition. Considering the migration time,resolution and other factors,acetonitrile -phosphate buffer (pH=6.5) with gradient elution was selected as the mobile phase. After optimization,the gradient programme was set as follows: 0.5% acetonitrile (0-12 min),3% acetonitrile (12.1 min). Under the optimum conditions,the typical chromatograms of standards were shown in Fig.2. From the chromatograms,it can be seen that the three analytes were completed separated and good peak shape was obtained. The elution sequence of analytes coincided with that of their polarities. The higher the polarities,the weaker interaction they had with the stationary phase. So,the shorter were the elution times.

Fig.2 Chromatograms of the standard mixture solutions at 210 nm (A) and 260 nm (B),respectively. Separation condition was as follows: column: Aglient Zorbax SB-C18,2.1 mm×150 mm i.d.,5 μm,20 ℃; mobile phase: acetonitrile-phosphate buffer (pH=6.5) with gradient elution (0.5% acetonitrile,0-12 min; 3% acetonitrile,12.1 min); The injection volume was 2 μL. 1,2 and 3 is for catalpol,5-HMF and adenosine,respectively. The concentrations of the standards were 300 mg·L-1 for 1,100 mg·L-1 for 2 and 15 mg·L-1 for 3 in MeOH,respectively.
2.2 Linearity,precision,stability,repeatability and recovery results of HPLC method
Standard solutions were performed by HPLC technique in order to determine the linearity of each compound,the precision,stability and repeatability of the method. The recovery results of three analytes in the sample were also determined. The results were shown in Table 1. As shown in Table 1,the results indicated that the calibration curves (based on the peak areas) were linear (r≥0.999 7) over the studied concentration range for HPLC method.
The precision,expressed by relative standard deviations (RSDs) of the peak areas of standard solution were studied at same conditions with five consecutive repeated injections at the same day. As can be seen in Table 1,theRSDsof the three analytes were below 1.54%.
Under the optimum separation conditions,the sample solutions were determined. The typical chromatograms were shown in Fig.3. Additionally,the stability and repeatability of the sample solutions were also investigated. The stability of the sample was studied by investigatingRSD(%) of the peak areas during 0-48 h’s storage. TheRSD(%) was<2.55%,which showed that the sample solution was stable at the experimental condition. The repeatability data were obtained by extracting 5 groups of the same sample parallelly,and investigated the peak area of the analytes. TheRSD(%) was 2.10%,1.25% and 1.79% for catalpol,5-HMF and adenosine,respectively. The result proved that this method was repeatable. The recovery of the method was determined with the addition of the standards in the real sample solution,with results from 96.8% to 99.2%. From the recovery results shown in Table 1,this method was accurate and reproducible,providing a useful quantitative method for the analysis of small molecule compounds in medicine pairs.
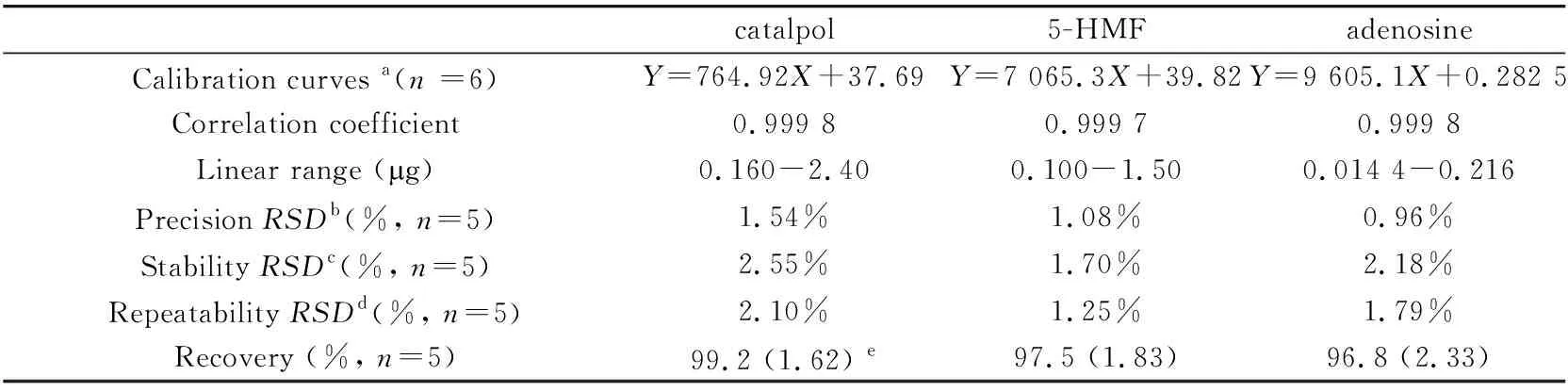
Table 1 Linearity,calibration graphs,precision,stability,repeatability and recovery result for HPLC method to determine catalpol,5-HMF and adenosine
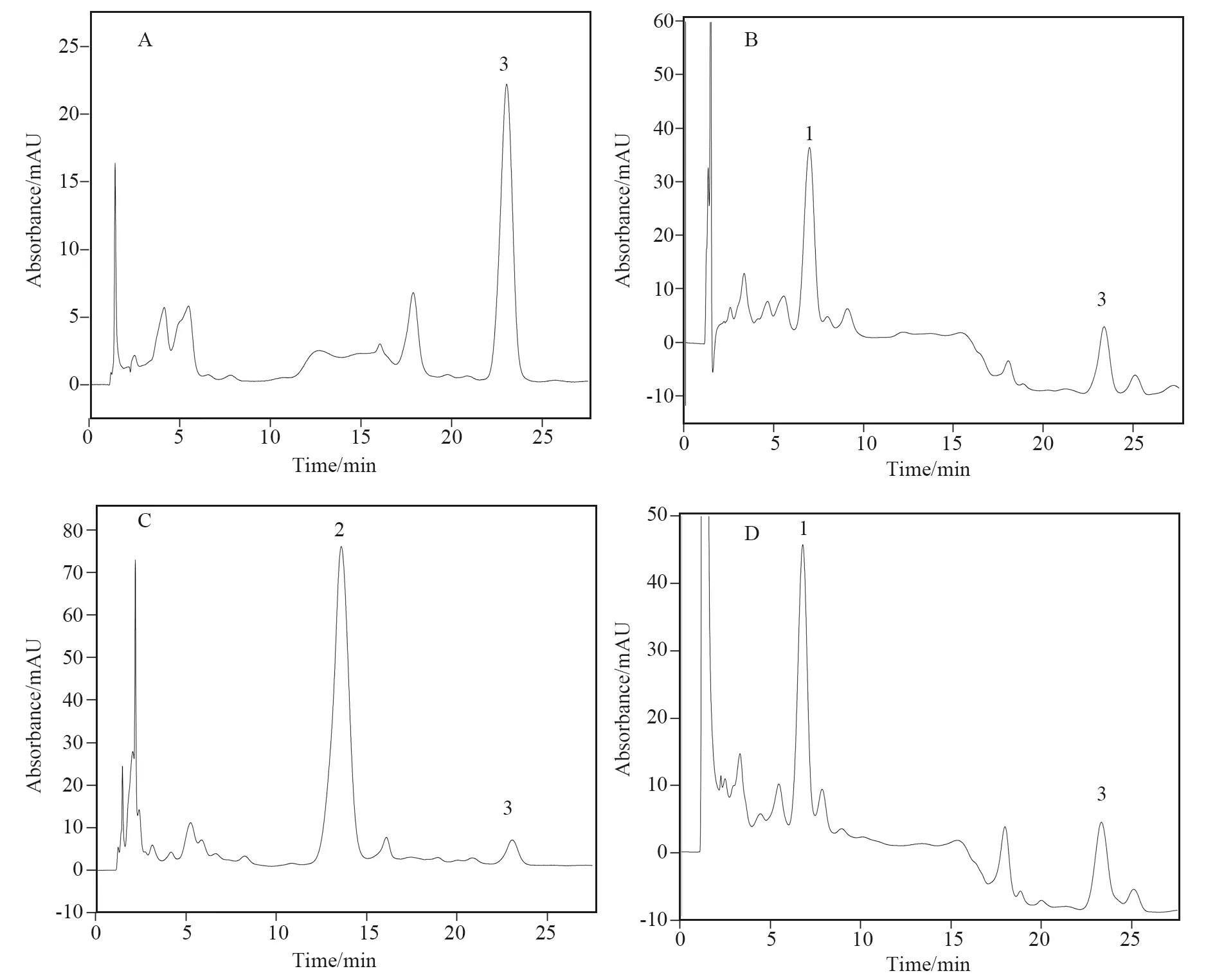

Fig.3 Chromatograms of the sample solutions: (A) Chinese yam at 260 nm; (B) raw radix rehmanniae at 210 nm; (C) Rehmannia glutinosa at 260 nm; (D) Chinese yam-raw radix rehmanniae (1∶1) at 210 nm; (E) Chinese yam-Rehmannia glutinosa at 260 nm. The other conditions and peak identification were as in Fig.2.
2.3 The content changes of the active components after compatibility
In order to investigate the effect of the compatibility on the main active components,the contents of polyphenols,polysaccharides and small molecule compounds of catalpol,5-HMF and adenosine in single herbs and their medicine pairs were determined using methods described above. The results were shown in Tables 2-5.
The variation of polyphenols content after combining Chinese yam with Rehmannia glutinosa was showed in Table 2. From Table 2,it can be seen that the polyphenols in Rehmannia glutinosa was much higher than that of in Chinese yam. The content of polyphenols increased with the increasing proportion of Rehmannia glutinosa in the medicine pair. However,there was no obvious difference between the experimental value and the theoretical value of polyphenols content. The results showed that the compatibility of Rehmannia glutinosa and Dioscorea opposite Thunb had not so much change on the content of polyphenols.

Table 2 Contents of polyphenols in herb-pair (n=4)
Table 3 showed the polysaccharides in medicine pair and its single medicine herb. Both polysaccharides Rehmannia glutinosa and Dioscorea opposite Thunb contained relatively high content of polysaccharides. The content of polysaccharides increased as the proportion of Rehmannia glutinosa increased in the medicine pair except the proportion of 1∶2. Furthermore,the experimental value obtained was higher than that of theoretical value calculated. The results suggested that the compatibility of Rehmannia glutinosa and Chinese yam had a positive effect on the polysaccharides dissolution. However,there was an abnormal experimental data existed in the medicine pair of Chinese yam-Rehmannia glutinosa with the proportion of 1∶2. In the proportion,the value measured was lower than the calculated result. The data were repeated several times,but they all had the same trend. The reason is not clear at present.
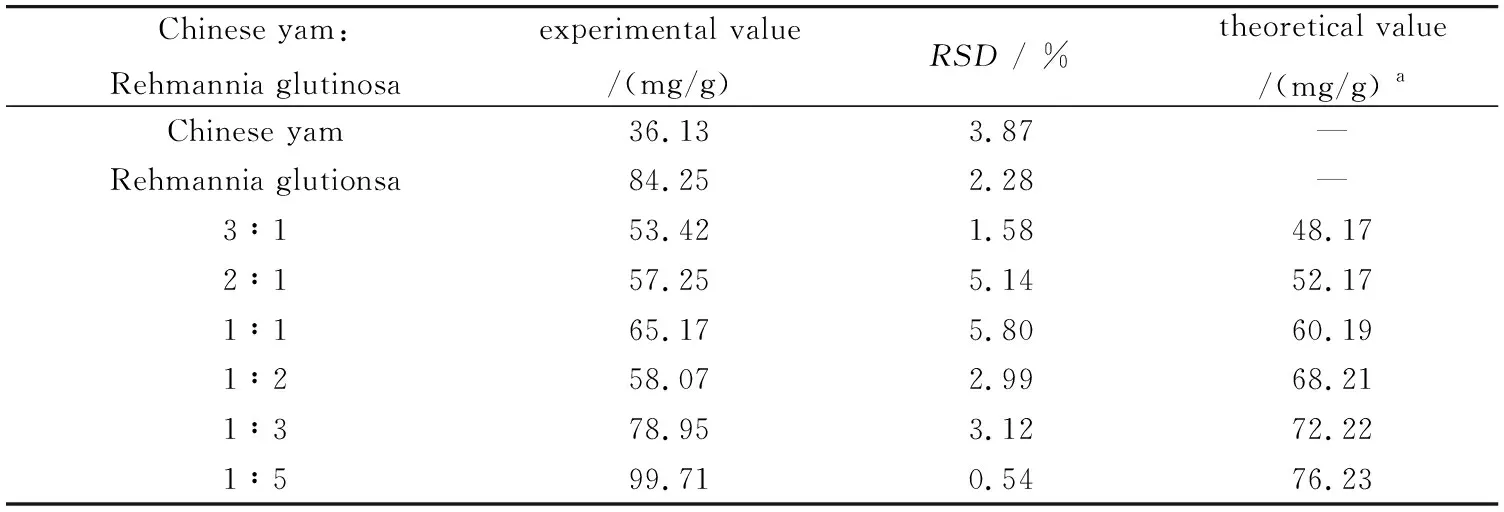
Table 3 The contents of polysaccharides inherb-pair (n=3)
Table 4 exhibited the contents of catalpol and adenosine in the medicine pair of Chinese yam and raw radix rehmanniae (Fig.3D). Catalpol content in radix rehmanniae was 2.160 mg/g. Catalpol was not detected in Chinese yam. However,the additions of Chinese yam in raw radix rehmanniae can increase the dissolution of catalpol,and the more yam,the more obvious the effect was. The reason may be that there may exist some ingredients in the yam which had solubilization effect on catalpol,so was the increase of catalpol dissolution. The content of adenosine did not change after compatibility. And different compatibility proportion had no effect on their content. The presumable reason was that adenosine existed both in Chinese yam and raw radix rehmanniae,and their contents had no obvious difference.

Table 4 The contents of catalpol and adenosine in the medicine pair of Chinese yam and raw radix rehmanniae (n=3)
Table 5 showed the variation of the ingredients in the medicine pair of Chinese yam and Rehmannia glutinosa with different proportion (Fig.3E). 5-HMF was not detected in yam which may be due to the different sample disposal method[35]. Yam pieces used in the experiment was cooled at oven at 40 ℃. They were not heated at high temperature or fried. So the content of 5-HMF was not detected. However,the compatibility of yam with Rehmannia glutinosa could decrease the dissolution of 5-HMF in Rehmannia glutinosa. The higher proportion,the more obvious suppression effect. The content of adenosine increased with the proportion of yam increasing in the herb pair. But there was not so much difference with theoretical value after compatibility. However,comprising the data in Table 4 with in Table 5,it was found that the content of adenosine changed tremendously. The content of adenosine in radix rehmanniae was 0.127 3 mg/g,while in Rehmannia glutinosa it was 0.066 5 mg/g. Heating caused the loss of adenosine during Rehmannia glutinosa preparation.
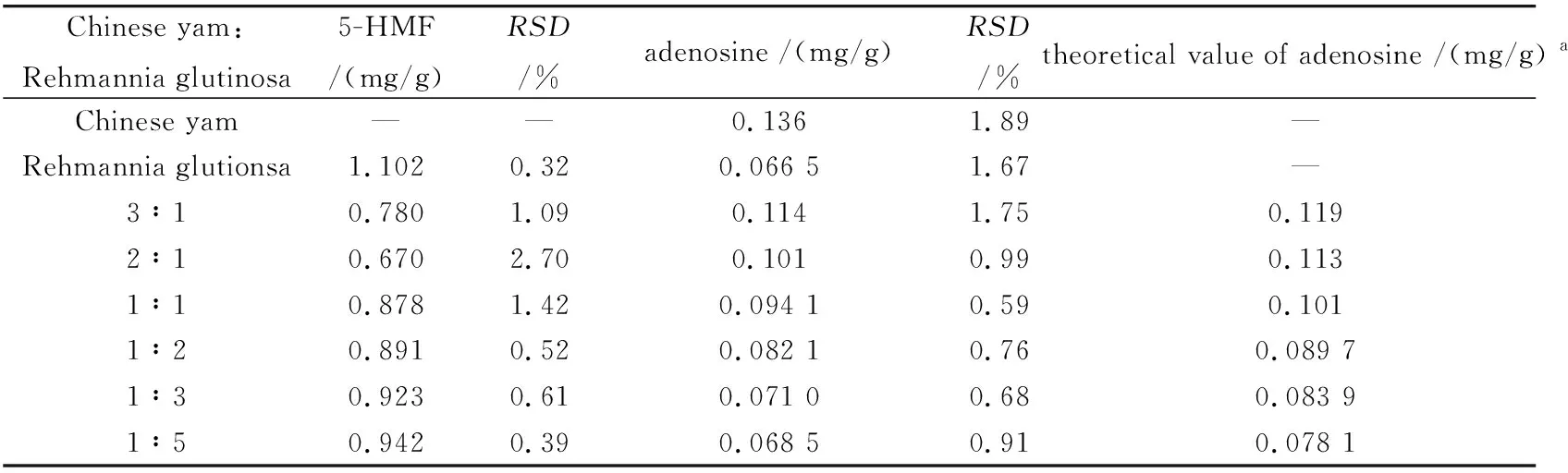
Table 5 The contents of 5-HMF and adenosine in the medicine pair of Chinese yam and Rehmannia glutinosa (n=3)
3 Conclusions
In this study,the HPLC and spectrophotometry methods were used to analyze the active ingredients ofDioscoreaoppositaThunb and Rehmannia glutinosa. The results showed that the compatibilities of the herb pair could promote the dissolution rate of catalpol. But the compatibility ofDioscoreaoppositaThunb and Radix Rehmannia Preparata had inhibitory action to the dissolution rate of 5-HMF. The content of adenosine in the medicine pair had no obvious change with the variation of the proportions of medicine pair. However,there was a great difference on adenosine between the Radix Rehmannia Preparata and the raw radix rehmanniae. The contents of polysaccharides and polyphenolics in the medicine pair and their single herb medicine showed that the polysaccharides content in herb-pair had an obvious increase in different proportions compared with the theoretical calculation results. However,the polyphends content in their herb pairs had no obvious change. But there was a little bit higher after compatibility. The experimental results analyzed the variation pattern of the active components in the medicine pair during the compatibility from chemical aspect. The results showed clearly that the function of two herbs after compatibility was not the simply additive of the two single medicines.
