Eco-Friendly Fabrication of Selenium Nanoparticles by Solidstate Thermal Decomposition of SeCl4-L-Glutamine Precursor:Spectroscopic Characterizations
2020-11-04SattamAlOtaibi
Sattam Al-Otaibi
College of Engineering, Taif University, Al-Haweiah, Taif 21974, Saudi Arabia
Abstract In this article, spherical black spots-like selenium metal nanoparticles were synthesized. Accordingly, this experimental work proposed an innovative facile, green, one-step and solvent-free strategy to a large scale synthesis of Se-NPs via thermal decomposition of green precursor. The Se(Ⅳ) L-glutamine precursor was prepared by solid state grinding using selenium(Ⅳ) tetrachloride, SeCl4, and L-glutamine for 2 hr without using any organic solvent. It was characterized by infrared spectroscopy, and micro analytical. The solid precursor compound was subsequently annealed in the muffle furnace at 300 ℃ for 3 hr in static air. Selenium NPs was resulted and well characterized using X-ray powder diffraction (XRD), FT-IR spectroscopy, scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The FTIR and XRD data showed that the Se NPs is pure and has a good crystalline structure because no characteristic peaks of impurity were detected, while the SEM and TEM results showed that the obtained product is tiny, aggregated with spherical-like shape, narrow size distribution with an average size between 5~10 nm. Results show that the solid state thermal decomposition method is simple, eco-friendly, safe and suitable for preparation of SeNPs. This method can also be applied to synthesize nanoparticles pure metal and metal oxides.
Keywords Green precursor; Se NPs; Glutamine; FTIR; XRD; SEM; TEM
Introduction
Nanotechnology is able to monitor, measure, process and manufacture objects on a nanometer scale. The application of nanoparticles (NPs) can be related to many fields such as medical, food, environmental studies, electronics production, power generation and agriculture. Recently, many nanomaterials are produced with the help of this emerging technology that occupies an important place in scientific research[1-2]. The chemical composition, size, shape and morphology of nanoparticles are dealt with in the synthesis process which is a vital step in nanotechnology research[3]. Due to its unique physical and chemical properties, metallic NPs have attracted much attention. Their properties can be controlled depending on the preparation and grouping method. One of the main effects, which are enhanced by controlling particle size, is their antimicrobial action[4]. It is known that the antimicrobial activity of NPs is a function of surface area in contact with microorganisms. For this reason, high surface area NPs have effects on the surface of microorganisms, as they inhibit the vital functions of cells or cause cell death[5].
Selenium in metallic forms plays a vital functions in the human body by improving the action of enzymes such as glutathione peroxidase and selenium-enzymes that defend the body against diseases associated with immunity[6]. Moreover, it has higher work function (5.1 eV) compared to the commonly used metal and semiconductor material, therefore Se-NPs can play vital role in fabrication of electronic memory as storage element improving retention time[7]. As selenium semiconductor possesses optical properties, photocatalytic and catalytic character. It also has some uncommon properties such as effective chemical and biological functions compared to bulk materials[8]. Selenium may exist in many crystalline and amorphous forms, but the shape, size and structure of selenium nanoparticles depend on various factors such as the concentration, temperature, nature of the biomolecules, and the pH in the reaction mixture. The chemical preparation of selenium metal NPs utilize the reduction of a selenium salt using a reducing agent in the presence of stabilizing agent in order to block the clusters of selenium atoms to growing and to obtain stable NPs in colloidal suspension[9]. Many other synthetic techniques have been applied to isolated Se-NPs such as physical evaporation, hydrothermal, gama-radiolytic reduction and sonochemical processes[10-12]. On the other hand, the biological synthesis of Se-NPs is often achieved by reducing selenite/selenate in the presence of bacterial proteins or plant extracts containing phenols, flavonoids amines, alcohols, proteins and aldehydes[13]. More than sixteen different types of bacteria and viruses have been found to reduce colorless selenite and selenite to a red selenium metal with different shape and size[14-15]. Plants, fungi, and microbes may act as a product and protect the environment when used properly. Bacteria, plant extracts and other natural resources have been found to be an excellent alternative method of green synthesis, because this method does not use any toxic chemicals and also has many benefits, including environmental friendly, cost-effectiveness, and appropriateness of pharmaceuticals and biomedicine applications[16-17]. The thermal decomposition of mineral precursors in the presence of organic surfactants at high temperature is a widely used method due to the very easy production of highly crystalline NPs with size distribution control[18-19].
Herein, this present study demonstrate that preparation of Se-NPs. It was synthesized through thermal decomposition Se(Ⅳ) L-glutamine precursor at 300 ℃. The synthesized Se-NPs was characterized using FTIR, Raman, UV-Vis, XRD, SEM, and TEM. It exhibits a spherical shape with an average diameter between 5-10 nm, which was confirmed by TEM analysis.
1 Experimental
1.1 Materials and spectroscopic measurements
All materials for synthesis were commercially available and used as received without further purifications.
The micro analytical were measured using a Perkin Elmer CHN 2400. Infrared spectra were recorded on Bruker FTIR Spectrophotometer (4 000~400 cm-1). Raman laser spectra were measured on the Bruker FT Raman with laser 50 mW. The electronic spectra was scanned using UV2 Unicam UV/Vis Spectrophotometer. Scanning electron microscopy images were taken in Quanta FEG 250 equipment. X-ray diffraction patterns were recorded on X ’Pert PRO PANanalytical X-ray powder diffraction, target copper with secondary monochromate. Transmission electron microscopy images (TEM) were performed using JEOL 100s microscopy.
1.2 Synthesis of Se-NPs
SeCl4(1 mmole) and L-glutamine(2 mmole) were grinded separately for 10 min in an agate mortar. The fine powder reactants were mixed and grinded for 2 hr. The brown solid product was annealed in a muffle furnace at 300 ℃ for 3 hr in oxygen atmosphere. Se NPs were kept in a closed glass bottle until the beginning of the spectroscopic and physical analyses.
2 Results and discussions
Eco-friendly synthesis of Se-NPs was associated by a thermal decomposition method using the Se(Ⅳ) L-glutamine complexity as a green precursor. The nano-selenium was well characterized by FT-IR, UV-Vis spectroscopy, XRD, SEM and TEM techniques.
FTIR of Se(Ⅳ) L-glutamine precursor and Se-NPs spectra with frequency range from 4 000~4 000 cm-1were displayed in (Fig.1). Figure 1(a) refer to the FT-IR spectrum of Se(Ⅳ) L-glutamine precursor. This spectrum has three distinguish bands at 3 174, 1 589, and 1 110 cm-1attributed to —NH2frequencies[20]. On the other hand, two bands at 1 589 and 1 287 cm-1are assigned toνas(COO-) andνs(COO-), respectively[20]. The stretching frequency of —NH2group is shifted to lower wavenumbers due to involved in the complexity toward selenium metal ion. Se(Ⅳ) L-glutamine precursor has a stretching band at 1 678 cm-1corresponded to stretching vibration of carbonyl-amido group. The difference between [νas(COO-)-νs(COO-)] vibrations (Δ=302 cm-1), this data confirmed that the carboxylate group acts as monodentate contact to selenium metal ions[20]. The two new vibration bands at 499 and 419 cm-1are assigned to Se—O and Se—N stretching, respectively[20]. Figure 1(b) display the FTIR spectrum of synthesized Se-NPs. The spectrum of nano-selenium has a stretching frequencies at wavenumbers 3 130, 1 633, 1 385, and 809 cm-1corresponding to O—H groups in adsorbed water molecules on the surface of selenium metal (moisture). For Se-NPs produced by thermal decomposition of Se(Ⅳ) L-glutamine precursor, the Raman spectrum at the lower-frequency region (<600 cm-1) showed a single sharp band at 238 cm-1(Fig.2) which corresponds to the stretching of Se—Se mode[21].
The most noticeable characteristic of nanoparticles is their color change in different sizes. As the size changes, the color of the formed particles will also change; absorption in the visible area of the spectrum. Consequently, the UV-Vis spectrum is the most important method for identifying and characterizing nanoparticles[22]. The UV-Vis spectra in the region of 200~800 nm for the Se(Ⅳ) L-glutamine precursor and prepared Se-NPs are shown in Fig.3. There are number of reports were discussed the electronic spectra of selenium nanoparticles[23], which confirmed that the distinguish absorption peak of Se-NPs depends on chemical composition, shape, particle size and environment conditions[23]. In these reports, the peaks presence at 290 or 265 nm[24]. In the present article, the UV-Vis spectrum of synthesized Se-NPs
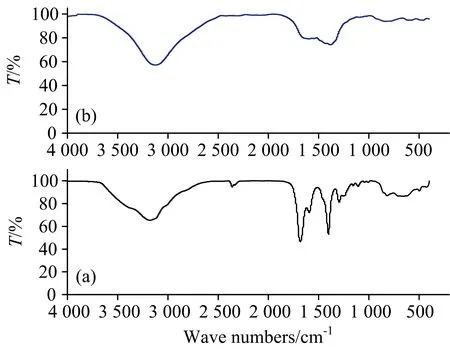
Fig.1 FTIR spectra of: (a) Se(Ⅳ) L-glutamine precursor and (b) as prepared Se-NPs

Fig.2 Raman spectrum of as prepared Se-NPs
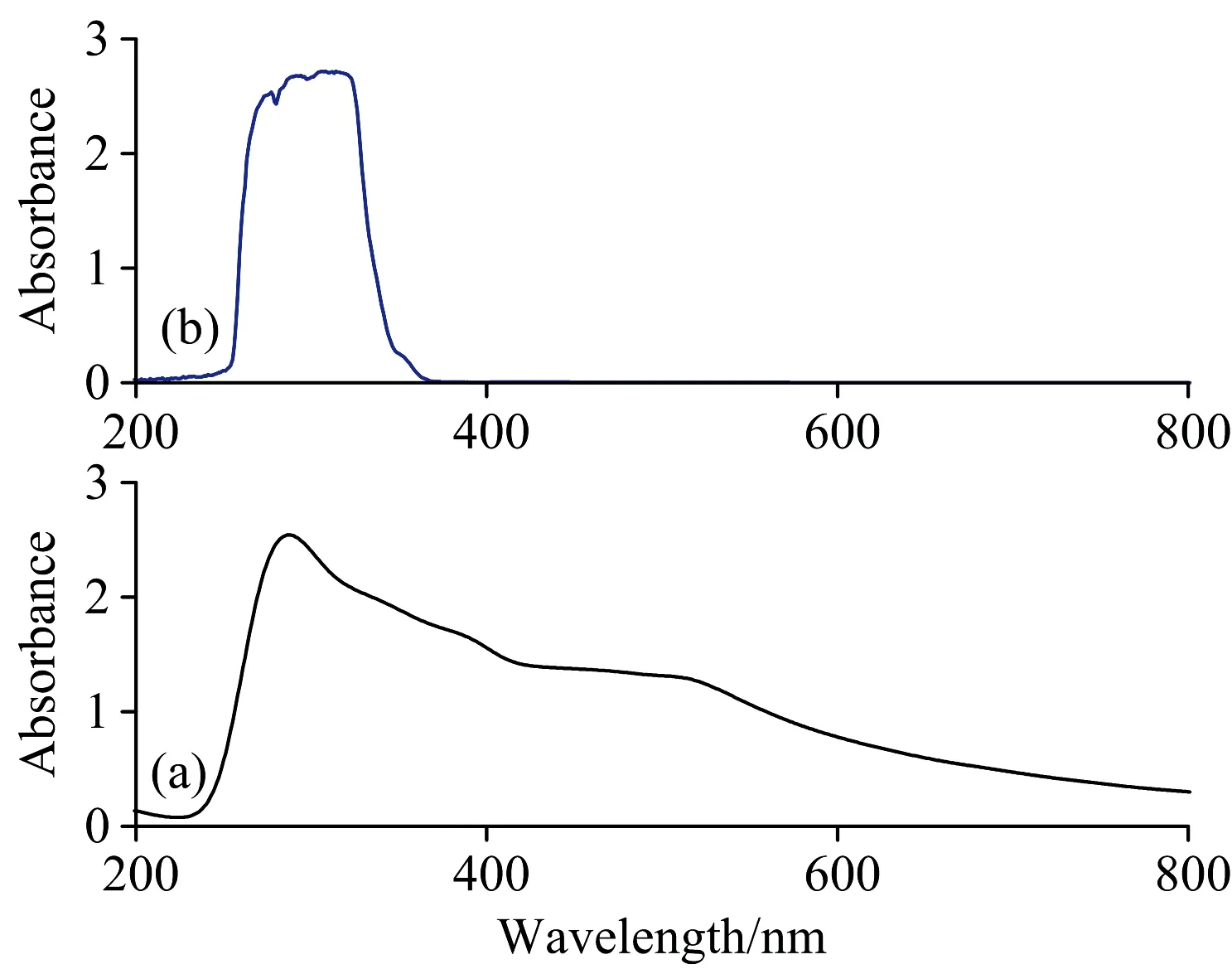
Fig.3 UV-Vis spectra of: (a) Se(Ⅳ) L-glutamine
through thermal decomposition Se(Ⅳ) L-glutamine precursor at 300 ℃ has a strong peak at 300 nm correspond to the nano-size of the prepared selenium metal.
Both XRD diffraction patterns of Se(Ⅳ) L-glutamine precursor and prepared Se-NPsare shown in Fig.4. The X-ray spectrum of Se(Ⅳ) L-glutamine precursor [Fig.4(a)] includes a number of peaks corresponding to both L-glutamine and also the characteristic peak of nano-selenium at 2θ=29° due to 101 crystal plane. The crystallinity nature of synthetic Se-NPs (Fig.4) was checked using X-ray powder diffraction patterns. The Se-NPs resulted by thermal decomposition of Se(Ⅳ) L-glutamine precursor has a crystalline shape and the distinguish diffraction patterns have been indexed as 23.36°, 29.58°, 41.18°, 43.47°, 45.28°, 47.88°, 51.33°, 55.89°, 57.05°, 61.29°, 65.06°, 67.67° and 70.94°, which assigned to 100, 101, 110, 012, 111, 200, 201, 003, 112, 013, 120, 211 and 113 crystal planes, respectively, accordingly with JCPDS 86-2246[10-19]. Based on the Scherrer’s relationship[25](Eq.1), the crystalline nature of synthetic Se(NPs was estimated. The crystallite size of synthetic Se-NPs was found to be 10 nm
D=0.94λ/βcosθ
(1)
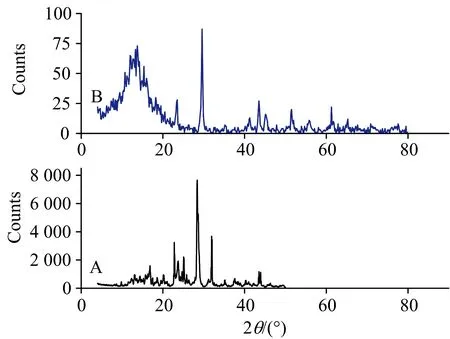
Fig.4 XRD spectra of: (a) Se(Ⅳ) L-glutamine precursor and (b) as prepared Se-NPs
Scanning electron microscopy technique is used for examining the surface morphology of prepared Se-NPs. SEM photo of synthesized nanoselenium show that it is a spherical shape, well distributed and homogeneous [Fig.5(a)]. Figure 5(b) show TEM image of chemically synthesized Se-NPs. This image describe that the shape of nano-selenium is spherical or semi-spherical with sizes presence between 5~10 nm, which is in agreement with the SEM analysis. Comparing this outcome data with those generated by other published articles[11-19], the particle size obtained in this study is smaller (5~10 nm). The L-glutamine compound allows particle size to be controlled and at the same time avoids its agglomeration and oxidation. This synthesis method depends on the high reactivity of Se(Ⅳ) L-glutamine precursor and agitation speed through the thermal decomposition reaction (scheme 1).

Fig.5 Micrographs (a) SEM and (b) TEM of Se-NPs obtained by thermal decomposition of Se(Ⅳ) L-glutamine precursor

Scheme 1 Chemical synthesized of Se-NPs by thermal decomposition of Se(Ⅳ) L-glutamine precursor
3 Conclusions
Herein, the pure and homogeneous spherical-like Se-NPs with an average size between 5~10 nm were successfully prepared by thermal decomposition of Se(Ⅳ) L-glutamine precursor at 300 ℃. This method is green, friendly, simple and conform for the preparation of nanoselenium.
