Hepatic and reproductive toxicity of sub-chronic exposure to dichlorvos and lead acetate on male Wistar rats
2020-11-03WahabAdekunleOyeyemiOoreoluwapoOloladeDaramolaAdeniranOluwadamilareAkinolaAdeoyeOyewoleIdrisIkponmwosaAikpitanyi
Wahab Adekunle Oyeyemi, Oore-oluwapo Ololade Daramola, Adeniran Oluwadamilare Akinola, Adeoye Oyewole Idris, Ikponmwosa Aikpitanyi
1Department of Physiology, School of Basic Medical Sciences, Igbinedion University, Okada, Edo State, Nigeria
2Department of Physiology, University of Medical Sciences, Ondo-City, Ondo State, Nigeria
3Department of Pharmacology and Physiology, Adeleke University, Ede, Osun State, Nigeria
ABSTRACT
KEYWORDS: Dichlorvos; Lead acetate; Androgen receptors;Pituitary testicular axis
1. Introduction
Environmental chemicals are well known to cause sexual dysfunction, reproductive disorders or infertility[1]. They also cause liver damage as a result of the vital role the organ plays in transforming, detoxifying and clearing chemicals. Lead and dichlorvos are among common chemicals that people are exposed to through agricultural and industrial activities, workplace or within the home environment[2].
Lead is a heavy metal found naturally in the environment as well as in manufactured products; it may be present as a pollutant in different sources such as contaminated food, lead hydrous piping,unsanitary preservation of food, industrial pollution, road traffic,paint, cosmetics and drinking water[3]. Lead has been recognized as one of ten environmental chemicals of primary public health distress. Similarly, lead exposure has been reported to cause over 1.06 million deaths worldwide, and the highest figure was in developing countries[2]. Several studies have reported that lead exposure caused impairment of spermatogenesis through disruption of hypothalamic-pituitary-testicular axis signaling and depletion of antioxidant reserves[4,5]. Studies have shown that lead may cause liver damage due to the increase in serum alanine aminotransferase(ALT) and aspartate aminotransferase (AST) they observed in Wistar rats after lead exposure[6,7].
Dichlorvos is a highly volatile organophosphate pesticide that has been in use for over 50 years to control pests/insects in households and agricultural stored products[8,9]. Although there is no reliable data on pesticide-related death, there is a rise in the cases using pesticides such as dichlorvos to commit suicide worldwide,especially in developing countries[10,11]. Exposure to dichlorvos could be accident, pollution, during its uses as insecticides or occupational activities. It may get into the body system through inhalation, direct dermal contact, drinking water or oral intake in children. It acts by inhibiting the cholinesterase enzyme activities in mammalian tissues. It has been experimentally suggested to bring about male infertility by causing alterations in spermatogenesis,sperm chromatin structure, testosterone synthesis and impairing the hypothalamic pituitary testicular axis[12], although Perry et al[13]reported that dichlorvos does not alter sperm counts, morphology and plasma testosterone in male Wistar rats.
Many people in developing countries are continuously exposed to a low amount of dichlorvos and lead due to uncontrol uses of insecticides, unsanitary preservation of food, industrial pollution,road traffic, paint, cosmetics, drinking water and illegal mining.Therefore, this study was designed to examine the hepatic and reproductive toxicity of sub-chronic exposure to dichlorvos and lead acetate in male Wistar rats.
2. Materials and methods
2.1. Chemicals
Dichlorvos and lead acetate used were products of Jubaili AgroTec LTD (Nigeria) and Sigma-Aldrich Chemical Corporation (St. Louis,MO, USA), respectively. All other chemicals and reagents used were of analytical grade.
2.2. Animals
Fifteen male Wistar rats aged 15-17 weeks (170-190 g) were obtained from the Igbinedion University animals’ house and housed in polypropylene cages with paddy husk bedding. They were fed with standard rat chow and had access to water ad libitium. The animals were housed and acclimated to the laboratory conditions of(22±3) ℃, (55±5)% humidity and a 12-hour light/dark cycle for 14 days. All procedures in this study conformed to the guiding principles for research involving animals as recommended by the Declaration of Helsinki and the Guiding Principles in the Care and Use of Animals.
2.3. Experimental design
Fifteen male Wistar rats were randomly assigned into three groups,with 5 rats in each group. Group 1 received 0.5 mL distilled water orally and served as the control group, while groups 2 and 3 were treated with 2 mg/kg body weight (b. w.) dichlorvos and 10 mg/kg b.w. lead acetate, respectively. The dosage of dichlorvos and lead acetate used were equivalent to 1/40 of their lethal dose[14,15]. The administration was done orally for 55 days to cover the spermatogenic cycle in rats.All the animals were anaesthetized on day 56, with 50 mg/kg sodium thiopental intraperitoneally[15]. Blood sample (3.0 mL) was obtained through cardiac puncture and centrifuged immediately for 5 min at 11 180 ×g to obtain the serum. Testes with epididymis and liver were harvested. The left testis and 2 g of the liver were fixed in Bouins’fluid, and 10% formalin respectively for immune-histochemical androgen receptors stain and histopathology assay.
Right testis (1 g) was homogenized in 4 mL ice-cold phosphate buffer saline. The homogenate was centrifuged at 10 000×g for 10 min. The testicular supernatant was used for the estimation of testicular 17β-hydroxysteroid dehydrogenase (17β-HSD), catalase and superoxide dismutase activities. ALT and AST activities were measured from the serum.
2.4. Serum biochemical assay
ALT and AST activities were evaluated with a commercially available enzymatic kit (Randox Laboratories Ltd). The serum (0.1 mL) was mixed with 0.5 mL of phosphate buffer(L-alanine) and (L-aspartate) for ALT and AST, respectively.The mixture was incubated for 30 min at 37 ℃ and then 0.5 mL of 2,4-dinitrophenylhydrazine was added and vortexed. The mixture was allowed to stand for 20 min at room temperature, then 5 mL of 0.4 mol/L sodium hydroxide was added, and the absorbance of the solution was read after 5 min at a wavelength of 546 nm.
2.5. Liver histology
Liver samples were fixed in 10% buffered neutral formalin and processed for embedding in paraffin. Sections of 5-6 μm thickness were stained with hematoxylin and eosin. The stained slides were examined under a light microscope (Olympus, Tokyo, Japan) ×400 magnification with attached Omax 10.0MP digital camera and liver micrographs were obtained.
2.6. Testicular malondialdehyde (MDA)
MDA concentration was measured according to the method reported by Alam et al[16]. Testicular supernatant (1 mL) of was mixed with 2.0 mL of trichloroacetic acid-thiobarbituric acidhydrochloric acid reagent. The mixture was heated in a boiling water bath for 20 min, then cooled and centrifuged. The absorbance of colour formed in the supernatant was assessed at 535 nm. The MDA concentration was evaluated with the extinction coefficient of the MDA-thiobarbituric acid complex, which was 1.56×105M-1cm-1,and the result was expressed as nM/mg tissue.
2.7. Testicular catalase activity
The catalase activity was evaluated according to the method reported by Alam et al[16]. The testicular supernatant (0.5 mL)was mixed with 0.5 mL of 30 M of hydrogen peroxide, 1 mL of 6 M H2SO4and 7 mL of 0.01 M of potassium permanganate. The absorbance of the mixture was read at 480 nm within 30 to 60 s against distilled water. The result was expressed as μM/mg protein.
2.8. Superoxide dismutase (SOD) activity
The estimation of testicular SOD activity was based on the principle of inhibition of epinephrine autoxidation in an alkaline medium at 480 nm in a UV spectrophotometer[16]. The SOD activity was stated in arbitrary units considering inhibition of autoxidation, as 1 unit of SOD activity (mU/mg tissue).
2.9. Serum follicle-stimulating hormone (FSH), luteinizing hormone (LH) and testosterone
Serum levels of FSH, LH and testosterone were assessed by using enzyme-linked immunosorbent assay. All the assays were performed according to the Calbiotech kit’s manual.
2.10. Testicular 17β -HSD activity
The testicular 17β-HSD activity was evaluated as described in our previous study[15]. The supernatant (1 mL) from homogenized testes was mixed with an equal volume of 440 μmol sodium pyrophosphate buffer (pH 10.2), 40 μL of testosterone (0.3 μmol)and 960 μL of 2.5% bovine serum albumin, to make a total of 3 mL incubation mixture. The 17β-HSD activity was evaluated after adding 1.1 μmol nicotinamide adenine dinucleotide to the incubated mixture in spectrophotometer cuvette at 340 nm against a blank (without nicotinamide adenine dinucleotide). A unit of enzyme activity was equal to a change in absorbance of 0.001/min at 340 nm.
2.11. Androgen receptors
Androgen receptors expressions were evaluated with the immunohistochemical stain as earlier described in our previous study[15]. A section of testes (5 μm) was mounted on a slide for androgen receptors immunohistochemical stain. The sections were dewaxed, rehydrated and autoclaved at 120 ℃ for 10 min in 10 mM citrate buffer (pH 6.0). The slides were washed and endogenous peroxidase was blocked with phosphate buffer saline and 0.3% hydrogen peroxide in methanol respectively for 15 min. Primary monoclonal and polyclonal antibodies for androgen receptors were added then incubated for 30 min. They were washed three times for 3 min each with phosphate buffer saline. The biotinylated polyvalent secondary antibody was added to the testes section and incubated for 30 min. The slides were washed in wash buffer for 3 min. For ease visibility of the stained testicular section, metal-enhanced 3,3’-diaminobenzidine substrate was added to the section and incubated for 10 min. The androgen receptor immunochemical stained slides were washed two for 3 min with wash buffer and counterstained with hematoxylin stain. The photomicrographs were taken under a light microscope at 200 magnifications with Omax 10.0 MP digital camera for the microscope.
2.12. Sperm analysis
Right caudal epididymis spermatozoa aliquot was used for sperm analysis (motility and viability). The spermatozoa aliquot (10 μL) was pipetted onto a pre-warmed microscope slide, and the slide portion with spermatozoa suspension was covered with a coverslip. The slide was examined with an optical microscope at ×400 magnifications,and progressive spermatozoa motility was estimated and expressed in percentage[17].
Spermatozoa aliquot and eosin-nigrosin staining solution of the same volume (10 μL) were placed on a microscope slide, and the slide was then covered with a coverslip. The stained slides were airdried. The dead and viable spermatozoa (i.e. stained and unstained respectively) were evaluated under a microscope at a magnification of 1 000. The viability was expressed in percentage[18].
Spermatozoa aliquot from the left caudal epididymis was used for evaluation of sperm count and morphology. The aliquot was diluted with sodium bicarbonate-formalin solution in ratio 1 to 20 and mixed properly. A drop of diluted spermatozoa was introduced into the improved Neubauer hemocytometer chamber. The spermatozoa were counted in 2 square mm under the microscope and expressed in million/mL[17]. A thin spermatozoa smear was made from two drops of spermatozoa aliquot on a slide and air-dried and stained with nigrosin-eosin. Abnormal spermatozoa morphology was assessed with a microscope at a magnification of 1 000 and expressed in percentage[17].
2.13. Statistical analysis
All the obtained data were analyzed by using one-way analysis of variance followed by Bonferroni post hoc test with GraphPad prism. The analyzed results were expressed and summarized as mean±standard deviation (mean±SD) and bar charts, respectively. Mean differences between groups were considered significant at P<0.05.
2.14. Ethical approval
This study was approved by the Igbinedion University Animal Ethics Committee (IUAC/18/0012) on August 21, 2018. All the animals used were handled and maintained according to the guidelines of the Committee.
3. Results
3.1. Serum biochemical
The rats exposed orally to dichlorvos and lead acetate showed a significant increase in the serum activities of ALT and AST (Figure 1A and B respectively) as compared with the control group (P<0.05).
3.2. Liver histology
The control group (Figure 2A) showed normal liver architecture,central venules and portal tracts appear normal (white arrow),the sinusoids seemed to be normal (slender black arrow) and also hepatocytes seemed normal morphology (blue arrow), while both dichlorvos (Figure 2B) and lead acetate (Figure 2C) groups showed poor liver architecture. There was a mild congestion seen in central venules and portal vein (white arrow). Mild portal triditis and mild periportal infiltration were seen; the sinusoids appeared very mildly infiltrated by inflammatory cells (slender arrow). Some hepatocytes showed mild cytoplasmic vacuolation (red arrow), piknotic and condensed nuclei (green arrow) while others appeared normal (blue arrow).
3.3. Testicular oxidant and antioxidant enzymes
Testicular MDA concentration was significantly increased in dichlorvos and lead acetate groups as compared with the control group (P<0.05). The testicular catalase activity was significantly reduced in both dichlorvos and lead acetate groups when compared with the control group (P<0.05). Dichlorvos significantly decreased superoxide dismutase activity in the testes when compared with the control group (P<0.05) (Table 1).
3.4. Hormones
The oral administration of dichlorvos and lead acetate significantly reduced FSH, LH and testosterone when compared with the control group (all P<0.01) (Table 2).
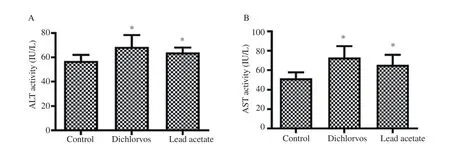
Figure 1. Effect of dichlorvos and lead acetate on serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in male Wistar rats. Bars represent mean±SD, n=5 in each group, *P<0.05: compared with the control group.
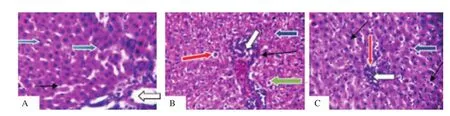
Figure 2. Effect of dichlorvos and lead acetate on liver histology of male Wistar rats (hematoxylin and eosin staining; magnification: ×400). A: The control group shows that liver architecture, central venules and portal tracts (white arrow), sinusoids (slender black arrow) and hepatocytes (blue arrow) seem normal. B and C: The dichlorvos and lead acetate groups show mild congestion in central venules and portal vein (white arrow), and there are mild periportal infiltration,infiltration of sinusoids with inflammatory cells (thin arrow), cytoplasmic vacuolation of hepatocytes (red arrow), pyknotic and condensed nuclei (green arrow).

Table 1. Effects of dichlorvos and lead acetate on testicular malondialdehyde, catalase and superoxide dismutase activities in male Wistar rats.
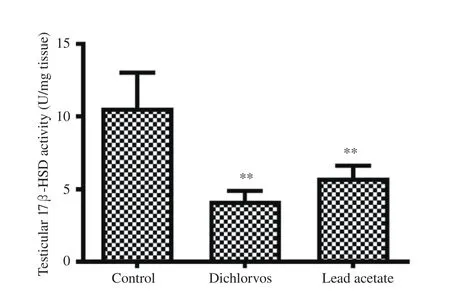
Figure 3. Effect of dichlorvos and lead acetate on testicular 17 β-hydroxysteroid dehydrogenase (17β-HSD) activity in male Wistar rats. Bars represent mean±SD, n=5 in each group, **P<0.01: compared with the control group.
3.5. Testicular 17β -HSD activity
A significant reduction in testicular 17β-HSD activity was seen in the orally treated male Wistar rats with dichlorvos and lead acetate when compared with the control group (P<0.01) (Figure 3).

Table 2. Effects of dichlorvos and lead acetate on serum follicle-stimulating hormone, luteinizing hormone and testosterone levels in male Wistar rats.
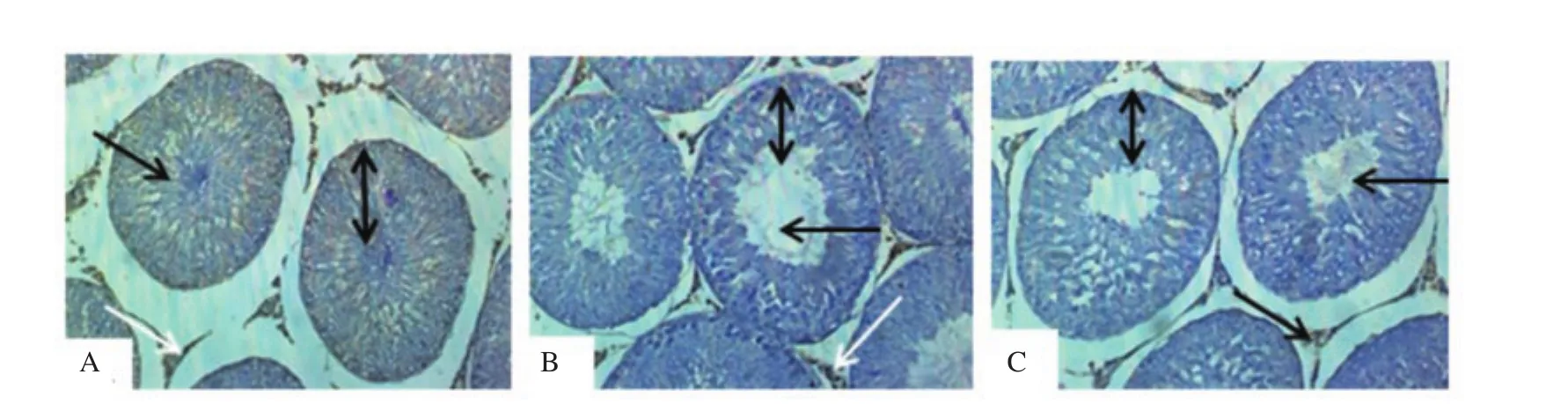
Figure 4. Effect of dichlorvos and lead acetate on testicular androgen receptors expression in male Wistar rats (hematoxylin staining; magnification: ×200). A: The control group shows normal seminiferous tubules with the presence of spermatozoa strand (black arrow), the normal stratification of germ cells layer (spanned arrow) and moderate testicular androgen receptors expression (white arrow); B and C: The dichlorvos and lead acetate groups show wide seminiferous lumens (black arrow) with a mild arrest of germ cells stratification layer (spanned arrow) and mild expression of androgen receptors expression (white arrow).
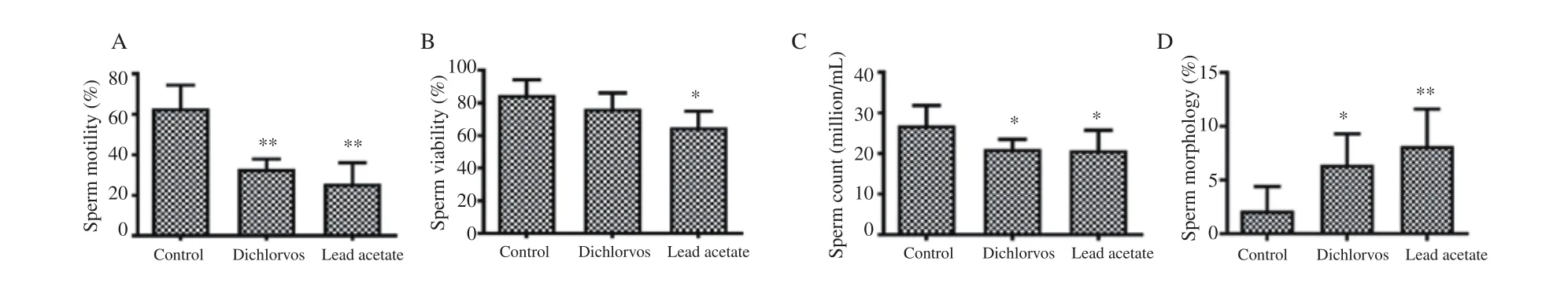
Figure 5. Effect of dichlorvos and lead acetate on sperm motility (A), viability (B), count (C) and morphology (D) in male Wistar rats. Bars represent mean±SD, n=5 in each group, *P<0.05 and **P<0.01: compared with the control group.
3.6. Testicular histology and androgen receptors
Compared with the normal seminiferous tubules with the presence of spermatozoa strand (black arrow) and the normal stratification of germ cells layer observed in the control group (Figure 4A), the testicular histology of dichlorvos (Figure 4B) and lead acetate(Figure 4C) groups showed wide seminiferous lumens (black arrow)with a mild arrest of germ cells stratification layer (spanned arrow).Moderate testicular androgen receptors expression (white arrow)was observed in the control group (Figure 4A) as compared with the mild expression seen in dichlorvos (Figure 4B) and lead acetate(Figure 4C) groups.
3.7. Sperm motility, viability, count and morphology
The sperm motility (Figure 5A) and count (Figure 5C) were significantly reduced in male Wistar rats orally treated with dichlorvos and lead acetate as compared with the control group(P<0.05). The sperm viability (Figure 5B) was significantly reduced in lead acetate treated group compared with the control group (P<0.05).The percentage of abnormal sperm morphology was significantly increased in male Wistar rats orally exposed to dichlorvos and lead acetate when compared with the control group (P<0.01) (Figure 5D).
4. Discussion
Liver enzymes AST and ALT are frequently used as biomarkers of liver injury because they are released by hepatocytes into the extracellular space[19]. We observed a significant increase in the serum levels of ALT and AST in dichlorvos exposed rats. This is in accordance with the earlier observations by Celik et al[6] and Kingsley et al[7] who reported the same increase in the level of AST and ALT in rats exposed to dichlorvos. Similarly, lead acetate caused a significant increase in the serum AST and ALT levels. Moreover,these results are in accordance with other studies by Haouas et al[20]and Shaymaa[21]. The significant increase in the serum AST and ALT recorded indicates that sub-chronic exposures to a low concentration of dichlorvos or lead acetate are hepatoxic.
From the histological perspective, both lead acetate and dichlorvos caused hepatic damage. The observed hepatic damage was evidenced in mild portal vein congestion and aggregation of inflammatory cells. Also, the sinusoids were mildly infiltrated by inflammatory cells with congestion in the central venules and portal vein. Some hepatocytes showed mild cytoplasmic vacuolation, pyknotic and condensed nuclei while others appeared normal. The observed hepatic damage due to exposure to lead acetate is similar to the report where sub-chronic exposure to lead acetate resulted in histological damage in the liver of the Wistar rats[22]. Also, chronic exposure of rats to dichlorvos has been reported to cause cytoplasmic vacuolization of liver cells[23].
Higher polyunsaturated fatty acid levels in the testes may subject it to oxidative stress and damage[24]. Studies have shown that the activities of catalase and SOD in tissue significantly decline after the administration of lead acetate[25,26], which is in agreement with this present study, indicating a significant decrease in the activity of catalase. However, no significant change was observed in the activity of SOD. This study showed that the concentration of MDA in the testes was significantly elevated in the lead treated animal which is in agreement with the study of Salawu et al[25] and Dorostghoal et al[26]. In comparison to the lead acetate treated group, there was a significant decrease in both the activities of catalase and SOD in rats exposed to dichlorvos. But no significant change was observed in the activity of SOD in the lead acetate group. Rat exposed to dichlorvos showed a substantial increase in the concentration of MDA. The observed increase in MDA concentrations in the testes of rats treated with dichlorvos and lead acetate indicates increased lipid peroxidation. The observed decrease in catalase activity, together with the increase in MDA concentration in dichlorvos and lead acetate exposed rats, serves as an indication of the generation of free radicals and oxidative stress[27].
In this study, there was a significant decrease in the level of FSH and LH after lead acetate treatment which is in accordance with the work of Oyeyemi et al[15], whose research was based on elucidating the mechanism of lead toxicity on the reproductive hormone in rats.The decline in the serum level of FSH and LH recorded in lead acetate group might have occurred through the disruption of the hypothalamic-pituitary axis[5]. A similar result was observed in the dichlorvos treated group, showing a significant decrease in the level of FSH and LH. Comparable results were reported in the Dirican and Yusuf’s study[14] where FSH and LH levels were decreased significantly at the end of the 4th-7th week in the dichlorvos treated group. Alteration in LH-releasing hormone gene expression and antagonist activity of organophosphate pesticides on androgen receptors[28] may be responsible for the observed reduction in serum LH levels in lead acetate and dichlorvos exposed Wistar rats.
Observation from this study after the sub-chronic exposure of rats to lead acetate showed a significant decrease in the level of testosterone, which follows with the findings of Amany et al[29]. In similarity to the lead acetate group, the administration of dichlorvos showed a decrease in the level of serum testosterone. The major signal for initiating/stimulating Leydig steroidogenesis is LH.The observed reduction in serum testosterone levels in the lead acetate and dichlorvos groups corresponded to a reduction in LH.Thus, it can be inferred that the decrease in the serum LH levels in Wistar rats exposed to lead acetate and dichlorvos may hinder the steroidogenesis in the Leydig cells and may be responsible for the reduction in serum testosterone levels.
The testicular histology results revealed mild testicular alteration in the Wistar rats exposed to sub-chronic dichlorvos and lead acetate.Some seminiferous lumens of the dichlorvos and lead acetate treated rats were wide with the mild arrest of germ cells stratification layer.This observation in the lead acetate group is similar to our previous study[15,30].
We observed a significant reduction in the testicular 17β-HSD activity of male Wistar rats treated with dichlorvos and lead acetate.A similar study has shown a significant decrease in the level of 17β-HSD after subjects were exposed to lead acetate[31,32]. The possible mechanism of dichlorvos and lead acetate in reducing 17β-HSD activity in the current study might be as a result of the decrease in LH, as reported earlier, and it is known that LH signals the initiation of steroidogenesis. Therefore, the reduction in testicular 17β-HSD activity in dichlorvos and lead acetate-exposed rats might play a role in the observed decrease in serum testosterone levels in this study[15].A significant reduction in the testicular androgen receptors expression was observed in dichlorvos and lead acetate exposed male Wistar rats. This observation suggests that sub-chronic exposure to dichlorvos or lead acetate at low concentration might have an antagonistic effect on androgen receptors expression. This result might be responsible for the reduction observed in FSH and LH in the dichlorvos and lead acetate groups. The organophosphate pesticides and lead acetate have estrogenic properties in male,which may be responsible for antagonizing androgen receptors expression[5,33]. Also, the down-regulation of testicular androgen receptors expression in lead acetate and dichlorvos groups may be accounted for by a parallel decline in serum testosterone levels since testosterone is known to regulate androgen receptors expression through 5α- reductase.
The lead acetate exposed rats showed a significant reduction of epididymal sperm count, viability, motility, and increase in abnormal sperm morphology, which are in agreement with the work of Anjum et al[32], Leiva et al[34] and Ekeh et al[35]. In comparison to the lead acetate group, there was a significant increase in the level of abnormal sperm morphology when treated with dichlorvos, which is in agreement with Faris’s report[36]. This study shows that sperm motility was significantly reduced in male Wistar rats treated with dichlorvos, which is in agreement with the work of Kemabonta and Akinhanmi[9], who also reported a significant decrease in sperm motility when adult mice were treated with dichlorvos. Dichlorvos caused a significant reduction in sperm count, which may occur due to its effect on the levels of sex hormones, especially testosterone,which play an essential role in spermatogenesis[14]. In contrast to the lead acetate group, there was no significant change in sperm viability of rat orally treated with dichlorvos. These adverse effects of dichlorvos and lead acetate treated groups on sperm parameters may be due to increasing generation of reactive oxygen species as indicated by increased concentration of the testicular MDA observed in the Wistar rats exposed to these two chemicals and reduction in FSH, LH and testosterone which is an indicator of possible disruption of the hypothalamic-pituitary-gonadal axis.It has been previously reported that dichlorvos and lead acetate increased generation of reactive oxygen species and disrupted the hypothalamic-pituitary-gonadal axis[14,15].
Despite the low concentration of the dichlorvos and lead acetate used in this study, the sub-chronic exposure of male Wistar rats to these chemicals disrupted the hepatic and reproductive function.Therefore, there is a need to focus and educate the populace of developing countries on the damage of sub-chronic environmental exposure to dichlorvos and lead. Also, the regulatory bodies should adequately monitor and regulate the sale of these chemicals and provide adequate information, surveillance and training on the uses of these chemicals.
The importation policy/banned of some chemicals and funding were the main limitations experienced in this study.
In conclusion, a comprehensive observation from this study suggests that lead acetate and dichlorvos may have similar negative influence on the testicular antioxidant, reproductive and hepatic functions. Hence, reproductive and hepato-toxicity activities of dichlorvos and lead acetate in male Wistar rats were similar.
Conflicts of interest statement
The authors declare that there is no conflict of interest.
Authors’ contributions
Wahab Adekunle Oyeyemi contributed to the conception, design and final approval of the version to be published. Oore-oluwapo Ololade Daramola, Adeniran Oluwadamilare Akinola andAdeoye Oyewole Idris contributed in term of performing the experiment, data analysis,interpretation and technical support. Ikponmwosa Aikpitanyi prepared and revised the manuscript.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Pregnancy-associated glycoproteins as a potential marker for diagnosis of early pregnancy in goats: A scoping reviewing
- Time-lapse videography reveals different morphokinetic profiles of human embryos displaying direct or reverse cleavage at different stages of development: A retrospective sibling embryo study
- The leaf extracts of Camellia sinensis (green tea) ameliorate sodium fluoride-induced oxidative stress and testicular dysfunction in rats
- Overexpression of tyrosine phosphorylated proteins in reproductive tissues of polycystic ovary syndrome rats induced by letrozole
- Vitamin D3 supplementation influences ovarian histomorphometry and follicular development in prepubertal albino rats
- Colonization of neonate mouse spermatogonial stem cells co-culture with Sertoli cells in the presence and absence soft agar
