Comprehensive review of hemolysis in ventricular assist devices
2020-10-31ChristosPapanastasiouKonstantinosKyriakoulisChristinaTheochariDamianosKokkinidisTheodorosKaramitsosLeonidasPalaiodimos
Christos A Papanastasiou, Konstantinos G Kyriakoulis, Christina A Theochari, Damianos G Kokkinidis,Theodoros D Karamitsos, Leonidas Palaiodimos
Abstract
Key words: Ventricular assist device;Hemolysis;Thrombosis
INTRODUCTION
Ventricular assist devices (VADs) have evolved into an essential therapeutic option for patients with end-stage heart failure as they are used as a bridge to transplant, a bridge to recovery or as a destination therapy[1-3].Despite their substantial benefits in this vulnerable group of patients, VADs have been associated with a great variety of adverse events, including but not limited to infection, bleeding and pump thrombosis[4].Recently, hemolysis has gained attention in the field of mechanical circulatory support as a side-effect that is associated with important clinical and prognostic information[5].
Although a low level of hemolysis exists by default in patients supported with VADs and may indicate that the device works well, clinically significant hemolysis constitutes a major complication associated with deleterious consequences and an eminent need for aggressive management[5].Unfortunately, the absence of unanimous criteria on the definition of clinically significant hemolysis remains its Achilles heel,blurring important epidemiological aspects of this clinical entity.In this review we discuss critical issues with regard to hemolysis from the perspective of long-term(durable) VADs, covering the entire clinical spectrum of this expected but potentially life-threatening complication.
DEFINITION
The laboratory markers lactate dehydrogenase (LDH), plasma free hemoglobin(pfHgb), haptoglobin, and bilirubin are traditionally used to detect hemolysis.However, most studies adopted different sets of “preferred” diagnostic criteria for hemolysis detection with dissimilar parameters and varying cut-off values for each biomarker.This inconsistency in the definition is also reflected in the contents of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS).The most recent INTERMACS definition allows the distinction of major from minor hemolysis by the presence of clinical signs, while cut-off values for LDH (2.5 times the upper limit of the normal range) and pfHgb (20 mg/dL) have been employed to indicate a hemolytic event[6].
INCIDENCE
By moving from the first to newer-generation devices, a significant reduction in the rates of most adverse events has been observed[7].The reported hemolysis rates were slightly higher in continuous-flow devices than in pulsatile devices[7].Even among newer-generation devices, there are considerable differences in hemolysis incidence associated mainly with mechanical factors (Table 1)[8].
Frictional heat generated at the contacting parts of most axial-flow VADs and shear stress created by the device impeller result in increased rates of hemolysis, ranging from less than 5% up to 18% for the landmark device Heartmate II (HMII), depending on the definition of hemolysis used across the studies[9,10].Paganiet al[9]defining hemolysis as two consecutive pfHgb values greater than 40 mg/dL and a LDH value greater than 1000 mg/dL, reported an incidence of 4% over a 6-mo period of support[9].The study by Slaughteret al[11], which evaluated HMII as destination therapy reported a similar hemolysis incidence[11].Another study by Ravichandranet al[10]used completely different parameters to define hemolysis (Hgb <10 g/dL, haptoglobin <8 g/dL and LDH >250 IU/L) and demonstrated a four to five-fold higher incidence(18%)[10].Along these lines, a recent study from Japan showed that hemolysis was the most frequent adverse event among HeartMate II receivers, of whom 14% developed a major hemolytic event[12].
The employment of non-contact bearings, which allows for rotation without wear,has significantly reduced the hemolysis rates in the third generation VADs[13].Slaughteret al[14]used laboratory markers (pfHgb >40 mg/dL) in combination with pertinent clinical signs to determine hemolysis rates for the centrifugal, continuousflow HeartWare device and reported lower hemolysis incidence (1.2% over a 36-mo period)[14].More impressive were the results of two small-sized studies for the Heartmate 3 LVAS, where no cases of hemolysis were observed[15,16].
ETIOLOGY
Several mechanisms have been proposed to account for hemolysis related to mechanical circulatory support[8,17].The commonly held hypothesis is that red blood cells undergo increased shear stress as they pass through the mechanical components of the device and become fragmented, a process ultimately resulting in hemolysis.This hypothesis was also supported by Yasudaet al[18]who performedin vitrotests in order to define the factors that contribute to hemolysis during blood flow through artificial organs.According to their results, shear stress and flow acceleration were the major factors responsible for the lysis of erythrocytes[18].These parameters are strongly associated with the hyperdynamic circulation, which can be caused by a variety of reasons, such as dehydration, aortic regurgitation, and increased pump speed.Of note,the restoration of blood flow into the ascending aorta post LVAD implantation, may affect the function of the aortic valve due to an increase in afterload creating a local circuit where blood leaks backward into the pump[19].As a consequence, red blood cells are exposed to high mechanical shear forces caused by the blood-pump interface and hemolysis becomes an unavoidable complication.
Additionally, the presence of hemolysis may be the reason behind pump thrombus,or malpositioning of the LVAD inflow cannula[17].Pump thrombosis and hemolysis are two complications linked in a bidirectional way.Virchow’s triad, modified and applied to the context of the VAD microenvironment (bioreactive surfaces, activated platelets, abnormal flow patterns) remains the key to understanding the fundamental principles of thrombogenesis in mechanical circulatory support[20].Βioreactive materials used in VADs set the stage for thrombus formationviatwo pathways:(1) By activating the intrinsic cascade of coagulation, when plasma proteins interact with the non-hemocompatible surface of a circulatory pump, and (2) By promoting platelet adhesion to the pump surface through adhesion proteins found on the metallic surface(e.g., fibrinogen, von Willebrand factor)[21].When platelets adhere to the surface, the secretion of their granule-stored mediators (e.g., adenosine diphosphate) further fuels the self-perpetuating processes of platelet aggregation and activation.The third component of Virchow’s triad plays a central role in platelet activation, as well.Shear forces have been implicated in triggering von Willebrand – platelet glycoprotein Ib interaction, leading to the formation of loose aggregates and activation of integrin aIIbβ3, hence allowing fibrinogen to bind to platelet membranes[22].Beyond these adhesion signaling pathways, Slepianet al[23]introduced mechanically-related aspects of shear-mediated platelet activation, according to which platelet porogenisity could be increased by membrane damage and shear-sensitive channels, allowing the influx of activating mediators[23].
The smaller size of newer VADs predispose them to thrombosis of the entire pump,facilitating the process of hemolysis through erythrocyte membrane injury[24].Consequently, byproducts released by lysed erythrocytes exert pro-thrombotic actions through various pathways.Cell-free hemoglobin scavenges circulating nitric oxide(NO) and erythrocyte arginase depletes L-arginine, the substrate for NO synthesis,limiting the ability of endothelial NO synthase to produce NO[25].Thus, NO bioavailability is reduced and its inhibitory actions on platelet adhesion and activation are abolished[26].Moreover, heme catabolismviaheme oxygenase-1 releases carbon monoxide and iron, two end-products with hypercoagulable and hypofibrinolytic features[27].Of note, iron overload has been associated with activation of blood coagulation and formation of fibrin-like dense matted deposits, which are more resistant to degradation, compared to thrombin-induced fibrin clots[28].Finally, a recent study demonstrated a direct relationship between hemolysis and VAD thrombosis,indicating that free hemoglobin inhibited ADAMTS-13, thus protecting active von Willebrand factor from degradation[30].
Despite significant breakthroughs in understanding the pathophysiological mechanisms of hemolysis and thrombosis in the setting of mechanical circulatory support, it is still unknown whether hemolysis or thrombosis is the first step on the cascade.It seems that these two inextricably related complications establish a new hematologic status, which is characterized by hemolytic and clotting diathesis,resulting in device dysfunction, heart failure or even death.

Table 1 Basic characteristics of different types of left ventricular assist devices
RISK FACTORS
Although the complications of mechanical circulatory support are well-described,there are few data on the risk factors that predispose patients to hemolysis.Three studies explored the differences in baseline characteristics between patients with and without hemolysis as a complication due to VAD[5,10,30].Katzet al[5]retrieved data from the INTERMACS registry and demonstrated that patients who received a continuousflow LVAD and suffered a hemolytic event were more likely to be younger and female[5].In another study, younger age, smoking and female sex were also found to have a significant association with the development of hemolysis in patients who were supported with the HMII device[10].Contrary to these findings, Cowgeret al[30]did not detect significant differences in any of the aforementioned risk factors[30].From a biomarker standpoint, higher LDH at the time of VAD implantation and lower INR were reported to be associated with higher rates of hemolysis[10,30].
MANAGEMENT
Although hemolysis is a potentially life-threatening complication of mechanical circulatory support, there is no consensus to date regarding the management of this clinical entity.Hemodynamic stability, surgical candidacy and the underlying cause of hemolysis should be carefully considered before initiating any treatment.The first step in the evaluation of an acute hemolytic event or worsening of pre-existing anemia should be the close monitoring of hemolysis-related biomarkers[24].Apart from hemoglobin/hematocrit and total/indirect bilirubin, emphasis should be given to serial changes in LDH[24].A non-significant and stable increase in LDH levels may be associated with a hyperdynamic circulation, caused by dehydration or increased pump speed.If a marked elevation in LDH levels is detected, further diagnostic studies may be necessary to specify the underlying cause of hemolysis.An echocardiography ramp test, in which left ventricular dimensions are recorded at increasing pump speeds, is suggested to detect device malfunction[31].Although pump thrombosis is the predominant reason for defective unloading of the left ventricular, in terms of increased pump speeds, graft malpositioning is an alternative possibility.In this case, a computed tomography scan can be used to reveal potential mechanical impairment, which should be corrected surgically.
Antithrombotic treatment should be immediately intensified, irrespective of the cause of hemolysis.Specific medication treatment strategies for device thrombosis include initiation of unfractionated heparin, either alone or in combination with antiplatelet agents or direct thrombin inhibitors, and thrombolytics[32].Although the optimal medical approach is not well established, a recent meta-analysis sought to shed light on this topic by evaluating the efficacy and complications associated with the aforementioned agents[33].No significant difference in thrombus resolution was found between thrombolytic and non-thrombolytic treatment, while the risk of major bleeding was higher in the thrombolysis group.Surgical therapy with device exchange has shown higher success rates in resolving pump thrombus, compared to medical treatment but is limited by its invasive nature and decreased long-term survival[34].As a result, indications are limited to patients with hemodynamic instability, persistence of hemolysis or progressive heart failure despite the initial medical treatment[35,36].
PROGNOSIS
Although limited, observational data exist regarding the prognostic role of hemolysis after VAD implantation, it seems that the development of a serious hemolytic event is associated with poor outcomes (Table 2).Katzet al[5]showed that survival was significantly attenuated in patients who suffered a hemolytic episode, compared to those who did not.Similarly, in another study, 1-year survival was markedly decreased in the hemolysis group as compared to the non-hemolysis group of patients(38.9%vs89.3%,P<0.001)[10].Finally, Cowgeret al[30]demonstrated that the hazard of death was four times greater in hemolytic patients than that observed in nonhemolytic patients (HR:4.3, 95%CI:2.1-8.9)[30].However, it should be highlighted that in these studies, only crude analyses were performed.Interestingly, in a recent study by Xiaet al[37], hemolysis or pump thrombosis were found to be significant predictors of poor long-term survival, even after adjusting for potential confounders[37].Whether hemolysis is associated with adverse outcomes in patients supported by VADs should be further investigated.
CONCLUSION
Hemolysis appears to be a relatively common complication of mechanical circulatory support that may serve as an early indicator of adverse events.Given the low specificity of the hemolysis-related biomarkers, patients supported with a VAD should be closely monitored for both clinical and laboratory signs of hemolysis.Future research studies should attempt to approach this interesting topic in a more standardized way, adopting a reliable and consistent hemolysis definition in order to elucidate further the risk factors and prognostic significance of this complication.The destruction of red blood cells may be just the tip of the iceberg of a disrupted hematologic profile in the setting of mechanical circulatory support.
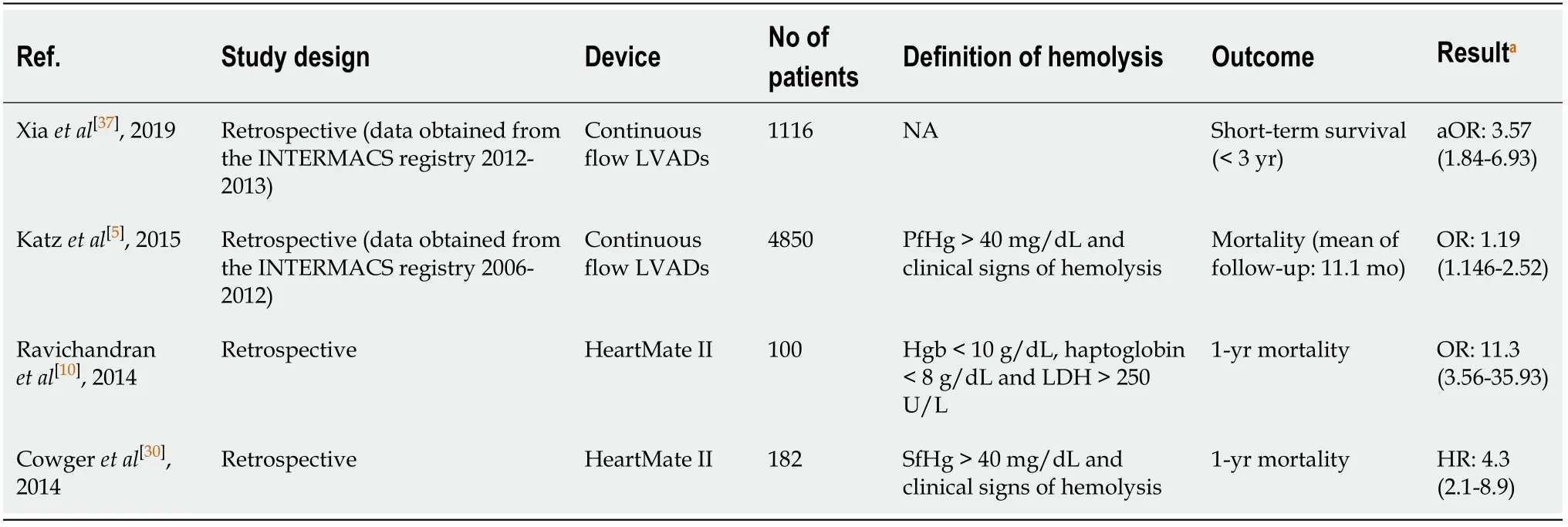
Table 2 Baseline characteristics, demographics and results of studies investigating hemolysis in left ventricular assist devices
