Modification Mechanism of Coconut Husk Activated Carbon Using FeSO4 and Fe(NO3)3
2020-10-26HUANGBangfu1LIULanpeng1WANGDefu1LILu李露
HUANGBangfu1,2*,LIULanpeng1,2,WANGDefu1,2,LILu(李露)1,2
1 Department of Metallurgical Engineering, Kunming University of Science and Technology, Kunming 650093, China2 Clean Metallurgy Key Laboratory of Complex Iron Resources, University of Yunnan Province, Kunming 650093, China
Abstract: Modification conditions determine the surface topography and the active material phase composition of a catalyst. To study the influence of modification on a carbon-based sorbent, coconut husk activated carbon (AC) which was activated using HNO3 and modified by FeSO4 and Fe(NO3)3 was examined. The pore textures and surface chemical characteristics of the carbon materials were examined by scanning electron microscopy(SEM), Brunner-Emmet-Teller(BET), X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy. The surface topography, the pore structure, active materials, and functional groups of AC, AC modificated by HNO3 (HNO3/AC for short), and AC modificated by FeSO4 and Fe(NO3)3 (Fe/AC for short) were systematically studied. Subsequently, the mechanism of modifying the conditions for the carbon materials was determined. Results showed that the surface micro topography of HNO3/AC became unsystematic and disordered. After modification with FeSO4, the ferriferous oxide was mainly present as a near-spherical crystal. Ferriferous oxides from Fe(NO3)3 modification mainly exhibited a plate shape. HNO3 modification could enlarge the pores but decrease the specific surface area of AC. FeSO4 modification resulted in a new net post structure in the pore canal of AC. Fe(NO3)3 modification caused the pore space structure to develop in the interior, and a higher calcination temperature was useful for ablation. The ash content of the AC was substantially reduced upon HNO3 modification. Upon FeSO4 modification, α-FeOOH, α-Fe2O3 and γ-Fe2O3 coexisted under the condition of a lower concentration of FeSO4 and a lower calcination temperature, and a higher FeSO4 concentration and calcination temperature generated more α-Fe2O3. The same Fe(NO3)3 modification and a higher calcination temperature were beneficial to the minor chipping formation of γ-Fe2O3. A higher Fe(NO3)3 loading produced a lower graphitization degree. HNO3 modification formed various new oxygen-containing functional groups and few nitrogen-containing groups. Based on the cover, FeSO4 and Fe(NO3)3 modification could decrease the oxygen-containing and nitrogen-containing functional groups. These results could optimize the modification condition and improve physical and chemical properties of carbon-based sorbents.
Key words: HNO3; FeSO4; Fe(NO3)3; coconut husk activated carbon; characterization; modification mechanism
Introduction
Activated carbon (AC) is a good catalyst carrier, and it presents with a large specific surface area and many pores[1-2]. The surface of AC exhibits many oxygen-containing and nitrogen-containing functional groups. AC demonstrates stable chemical properties, and is acid-resistant, alkali-resistant and renewable. Thus, as an adsorbing material, AC is widely used in gas cleaning, catalysis, medicine and chemical engineering fields[3-5]. Coconut husk AC exhibits favorable adsorptive properties, a high mechanical strength, and stable chemical properties. The adsorption capacity and catalytic performance of coconut husk AC are determined by the pore structure and surface functional groups. Unmodified coconut husk AC exhibits limited adsorptive properties compared with modified coconut husk AC. As a result, coconut husk AC should be modified by materials that demonstrate a high catalytic activity to increase its physicochemical adsorption capacity.
The previous study[6]has shown that AC modified by nitric acid possesses appropriate acidity-basicity and increases the oxygen-containing functional groups, which destroys the pore structure and increases Brønsted acid sites and Lewis acid sites. The main metallic modification compounds include FeOx, CuOx, MnOxand CeOx[7-10], and these provide AC with low-temperature catalytic activity. In particular, the active metal oxide, FeOx, is an iron-based catalyst with a broad active temperature window, good selective catalytic reduction (SCR) capability, high N2selectivity, high low-temperature water resistance and high acidic active sites[11-12]. Therefore, AC modified with FeOxexhibits good physicochemical properties. However, the preparation conditions determine the Fe phase composition and physicochemical properties of the catalyst.
To study the influence of the modification conditions, coconut husk ACs activated with HNO3and modified using FeSO4and Fe(NO3)3were examined, respectively. The pore texture and surface chemical characteristics of the carbon materials were examined via scanning electron microscopy (SEM), Brunner-Emmet-Teller (BET), X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy. The physicochemical properties of AC, AC modified through HNO3(HNO3/AC) and AC modified through FeSO4and Fe(NO3)3(Fe/AC) were systematically studied.
1 Experiments
1.1 Catalyst preparation
Coconut husk AC (Chongqing Huaxi Cativated Create Factory, Chongqing, China) was used as the raw carbon material. The coconut husk AC was washed with distilled water and soaked in a water bath for 2 h. Then, the AC sample was dried in an oven at 100 ℃. The AC sample was immersed in 4.0 mol/L HNO3at 80 ℃ for 2 h. Then, HNO3/AC was obtained by drying the sample in an oven at 100 ℃.
The HNO3/AC sample (10 g) was first immersed in FeSO4liquor (100 mL), and the mixture was treated with supersonic at 60 ℃ for 2 h and stewed for 12 h. Afterwards, FeSO4liquor was added to 2 mol/L ammonia and 10% H2O2,and then the Fe(OH)3precipitate was obtained by stirring at 55 ℃ for 1 h. The Fe(OH)3precipitate was suction filtrated and dried at 55 ℃ for 12 h. The dried sample was calcined in a vacuum tube furnace. The catalyst samples were washed with distilled water three times and dried at 55 ℃ for 12 h. Details of the FeSO4modification conditions are provided in Table 1.

Table 1 Catalyst preparation conditions
Based on the water absorbing capacity of HNO3/AC, 10 g HNO3/AC was first immersed in isometric Fe(NO3)3and stewed for 24 h, and then the mixture was dried in an oven at 100 ℃ for 4 h. Afterwards, the dried sample was calcined in a vacuum tube furnace in N2atmosphere for 3 h. Details of the Fe(NO3)3modified conditions are provided in Table 1.
1.2 Materials characterization
To study the changes in the surface topographies of AC, HNO3/AC and Fe/AC, the microstructures of the carbon materials were observed by SEM (VEGA 3 SBU/VEGA 3 SBH, Tescan Orsay Holding, China). The pore diameters affected the physical adsorption capability. The BET surface areas of the samples before and after modifications were measured using the N2adsorption isotherm through the nitrogen adsorption desorption tester (QuadraSorb SI,Quantachrome Instruments, America). Volumes of total pores and micropores were obtained using the Barclay-James-Harvest (BJH) method. The characteristic structural parameters, including the pore-size distributionD, the volume of total poresVtotal, the volume of microporesVmicro, the specific surface area of total poresStotaland the specific surface area of microporesSmicro, were obtained by analyzing the adsorption tendency. The Fe phase composition was observed through XRD (TTR III, Beijing Advance Science & Technology Corporation, China). The species and the amounts of surface functional groups affected the surface chemical properties. To study the changes in the surface functional groups of AC, HNO3/AC and Fe/AC, FTIR spectra were recorded using a FTIR spectrometer (Nicolet iS10, Thermo Fisher Scientific, China).
2 Results and Discussion
2.1 SEM characterization
The surface micro topographies of the samples before and after modifications with HNO3are shown in Figs. 1(a) and (b). Figure 1(a) shows that AC develops a pore structure, a distinct boundary, a smooth wall of holes and a faviform surface structure. Figure 1(b) shows that the surface micro topography of HNO3/AC becomes unsystematic and disordered, and the same area presents more ditches and air void structures compared to that of AC. Meanwhile, some gully-like pores are formed on the surface of HNO3/AC.
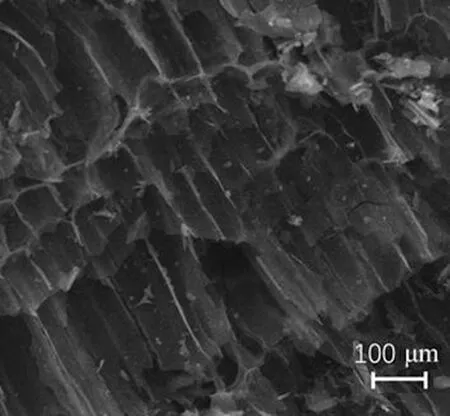
(a)
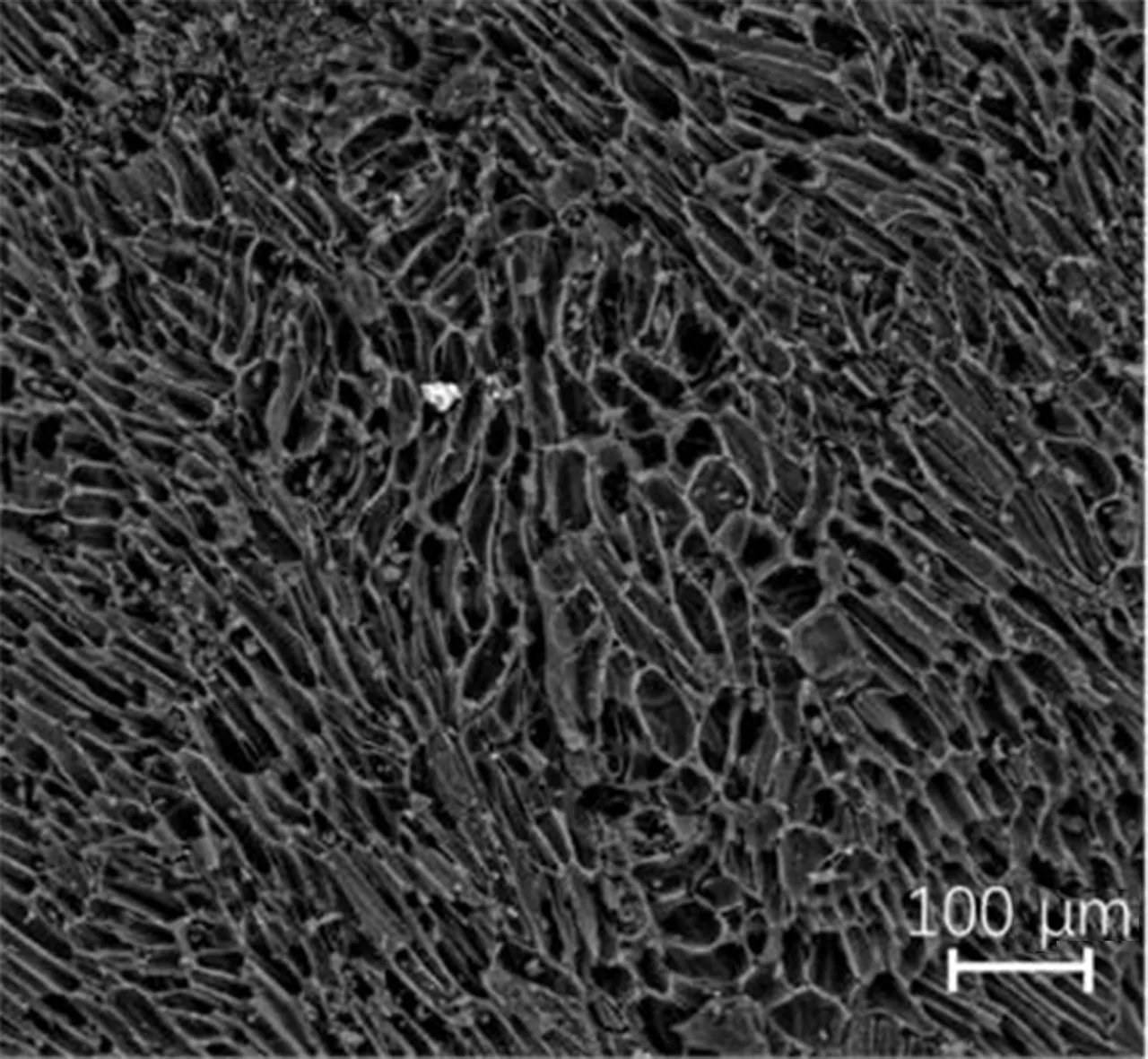
(b)
The SEM images for Fe/AC-1, Fe/AC-2 and Fe/AC-3 are shown in Fig. 2.

(a)

(b)
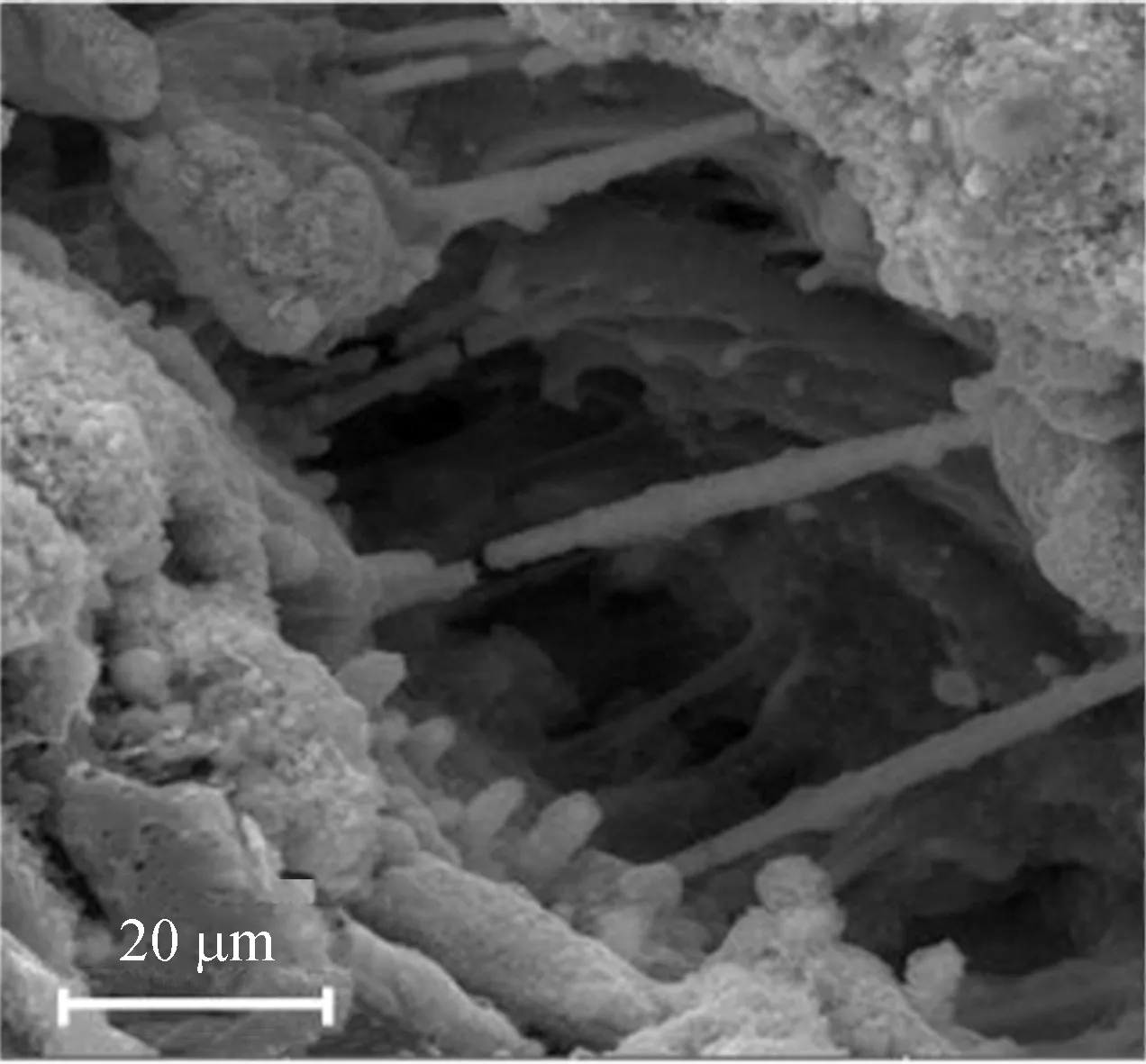
(c)
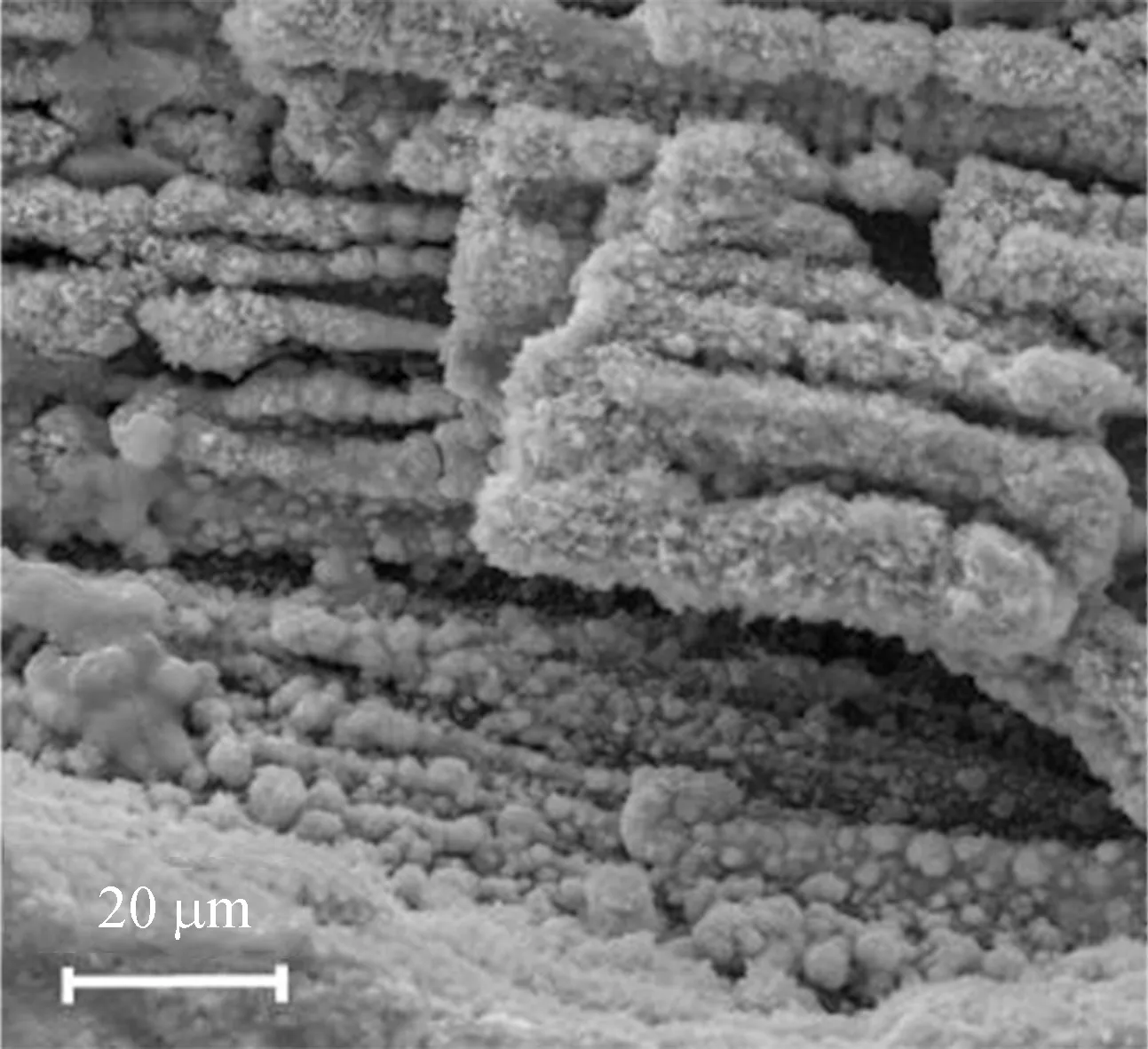
(d)

(e)

(f)
For Fe/AC-1 in Figs. 2(a) and (b), the ferriferous oxide shows a serious birdnesting phenomenon upon Fe(OH)3modification at 200 ℃ for calcination. The birdnesting phenomenon is as follows: a birdnesting lump attaches to the hole wall of AC with poor dispersivity; a birdnesting object accumulates on the surface of AC in a random manner and blocks part of the pore canal; the active material of ferriferous oxide coagulates in lumps and exhibits a sharp nonuniformity.
Figures 2(c) and (d) of Fe/AC-2 show that reticulate and prismatical crystals develop between the pore space of AC. Some of the crystals connect the top and the bottom pore walls, whereas others show a continuous growth. The reticulate and prismatical crystals of ferriferous oxide form a new pore path structure, which makes the inner space of AC be fully utilized. Therefore, Fe/AC-2 exhibits a larger specific surface area compared with AC and HNO3/AC. Meanwhile, on the surface of Fe/AC-2, the serious birdnesting phenomenon which is obvious in Fe/AC-1 disappears, and crystal grains replace the birdnesting phenomenon,present globosity, and are oval in shape. Thus, the good dispersivity of the crystal grains on the surface also increases the specific surface area of Fe/AC-2. For Fe/AC-2, there is no serious plug-hole phenomenon on the surface or in the pore space, and it could supply a larger specific surface area and more active adsorption sites.
For Fe/AC-3, as shown in Figs. 2(e) and (f), the surface of Fe/AC-3 becomes sunken, smashed and rough when the density of FeSO4increases and when the calcination temperature is 250 ℃. The ferriferous oxide of Fe/AC-3 adheres on the surface and accumulates in the pore space compared with Fe/AC-1 that accumulates on the surface. Moreover, most ferriferous oxide shows graininess, and few ferriferous oxide shows bond lumps and adheres on the surface of the pore path to create blockages.
The surface morphologies of Fe/AC-4, Fe/AC-5 and Fe/AC-6 modified with Fe(NO3)3isometric impregnation and high-temperature calcination are shown in Fig. 3.

(a)

(b)

(d)
For Fe/AC-4 in Figs. 3(a) and (b), the surface exhibits a certain thickness and lumps of ferriferous oxide, and many cracks in the ferriferous oxide exist. This may be due to the formation of NO2and O2when Fe(NO3)3begins to decompose upon high-temperature calcination. Moreover, some of the powdery accumulation and graininess of the irregular crystals of ferriferous oxide accrete on the hole surfaces of the AC, and less lumps of Fe2O3are present in the holes.
In comparison with Figs. 3(a) and (b), the pore structure of Fe/AC-5 is highly visible as the calcination temperature increases. This may be because the lumps of ferriferous oxide split into fine-grained chippings that spread out on the surface and the pore structure of AC. Furthermore, part of the fine-grained chippings inlays the pore holes. Consequently, the specific surface area and the micropore area are enlarged, which are favorable for increasing the adsorptive property.
The surface of Fe/AC-6 becomes crude and messy as the loading amount increases at a constant calcination temperature. Compared with Fe/AC-4, the ferriferous oxide of Fe/AC-6 is in big lumps attached to the surface of AC. Some minor micropores on the surface of the big lumps exist, and the shape of catalysts is irregular. Many ferriferous oxide crystals go into the pore structure of AC and create more pore paths.
Thus, ferriferous oxide from Fe/AC-1, Fe/AC-2 and Fe/AC-3 is mainly present as a near-spherical crystal, which forms a new net post structure in the pore canal. At the same loading concentration of FeSO4, the higher calcination temperature causes more scattered ferriferous oxide, which supplies a larger specific surface area and more adsorption active sites. For the same calcination temperature for FeSO4, a higher loading amount causes ferriferous oxide to become sunken, smashed and rough. Ferriferous oxide in Fe/AC-4, Fe/AC-5 and Fe/AC-6 is mainly present as a plate shape and adheres on the surface of Fe/AC. When the same loading amount of Fe(NO3)3is used, a higher calcination temperature causes minor chippings of ferriferous oxide, which spreads out on the surface and pore space. When the same calcination temperature is used for Fe(NO3)3, a higher loading amount results in more bulk mass ferriferous oxide, which forms more pore canals.
2.2 BET characterization
The BET characterization results for AC, HNO3/AC, and Fe/AC are shown in Table 2.
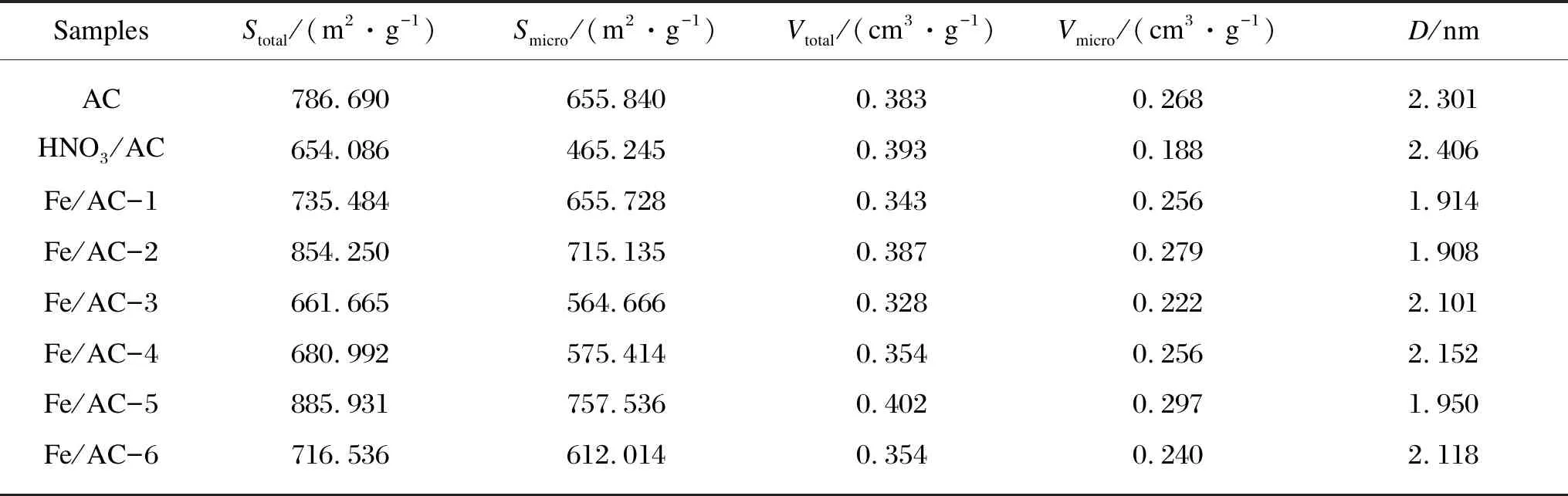
Table 2 Specific surface areas and pore structure parameters
For AC, the diameters of the micropores are almost 2 nm, and the developed micropore structure exhibits an immense specific surface area[13]. Upon calculating, the micropore volume accounts for 69.97% of the total pore volume, and the micropore specific surface area accounts for 83.37% of the total specific surface area. Therefore, mesopores and macropores are rarely present. For HNO3/AC, the average pore diameter and the total pore volume increase slightly after HNO3modification. This is because many closed pores are opened owing to the reaction between HNO3and carbon, which causes some of the micropores to disappear and the carbon skeleton to topple. Therefore, the micropore structure is destroyed, the micropore specific surface area decreases to 190.595 m2/g, and the total specific surface area decreases to 132.604 m2/g. Thus, HNO3modification enlarges the pores but decreases the specific surface area of AC as shown in Fig. 1(b).
The nitrogen isothermal adsorption-desorption curves and pore size distributions of Fe/AC-1, Fe/AC-2 and Fe/AC-3 are shown in Fig. 4. The adsorbed volumes of N2for Fe/AC-1, Fe/AC-2 and Fe/AC-3 rapidly increase during the initial stage in the low relative pressure, which means that many micropores are present and can be defined as type I according to International Union for Pure and Applied Chemistry (IUPAC) classification[13]. As shown in Figs. 4(a) and (b) and Table 2, the micropore contents are in the following order: Fe/AC-2 > Fe/AC-1 > Fe/AC-3. The adsorbed volume of N2for Fe/AC-1, Fe/AC-2 and Fe/AC-3 also rapidly increases at the high pressure. The reason for this may be that in the narrow free space, capillary condensation occurs under the medium pressure and the high pressure; thus, micropores and mesopores are present. This tendency can be defined as type IV. Condensation and evaporation of capillaries do not occur at the same pressure, which leads to a circular hysteresis ring. The mesopore amount of Fe/AC rapidly increases as the calcination temperature increases. Thus, the high temperature can change the micropores to mesopores; however, a larger increase in micropores is observed. Therefore, the average pore diameters of Fe/AC-1, Fe/AC-2 and Fe/AC-3 decrease to various degrees compared with those of AC and HNO3/AC.
One possible reason is the large amount of gas prior to the opening up of the inner pore space when the Fe(OH)3calcines and generates gas. Thus, the ferriferous oxide crystals attach to the macropore wall and continuously accumulate. Then, more micropores are formed in the pore canal, which makes the best use of the inner pore space. On the surface, the ferriferous oxide crystals form round-like crystals and enlarge the specific surface area of AC, especially for Fe/AC-2. Because there are more micropores in Fe/AC-2 than in Fe/AC-1 and Fe/AC-3, the pore volume and the specific surface area of Fe/AC-2 exhibit the highest increase; therefore, it can be concluded that the Fe/AC-2 has the strongest adsorption capacity.

(a)

(b)
The nitrogen isothermal adsorption-desorption curves and pore size distributions of Fe/AC-4, Fe/AC-5 and Fe/AC-6 are shown in Fig. 5.
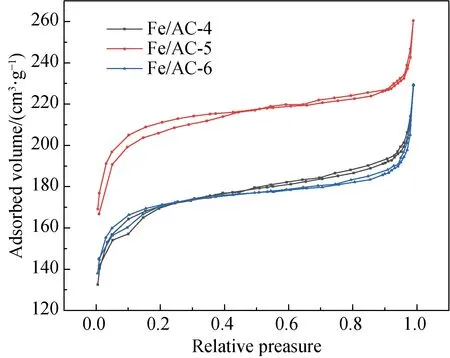
(a)
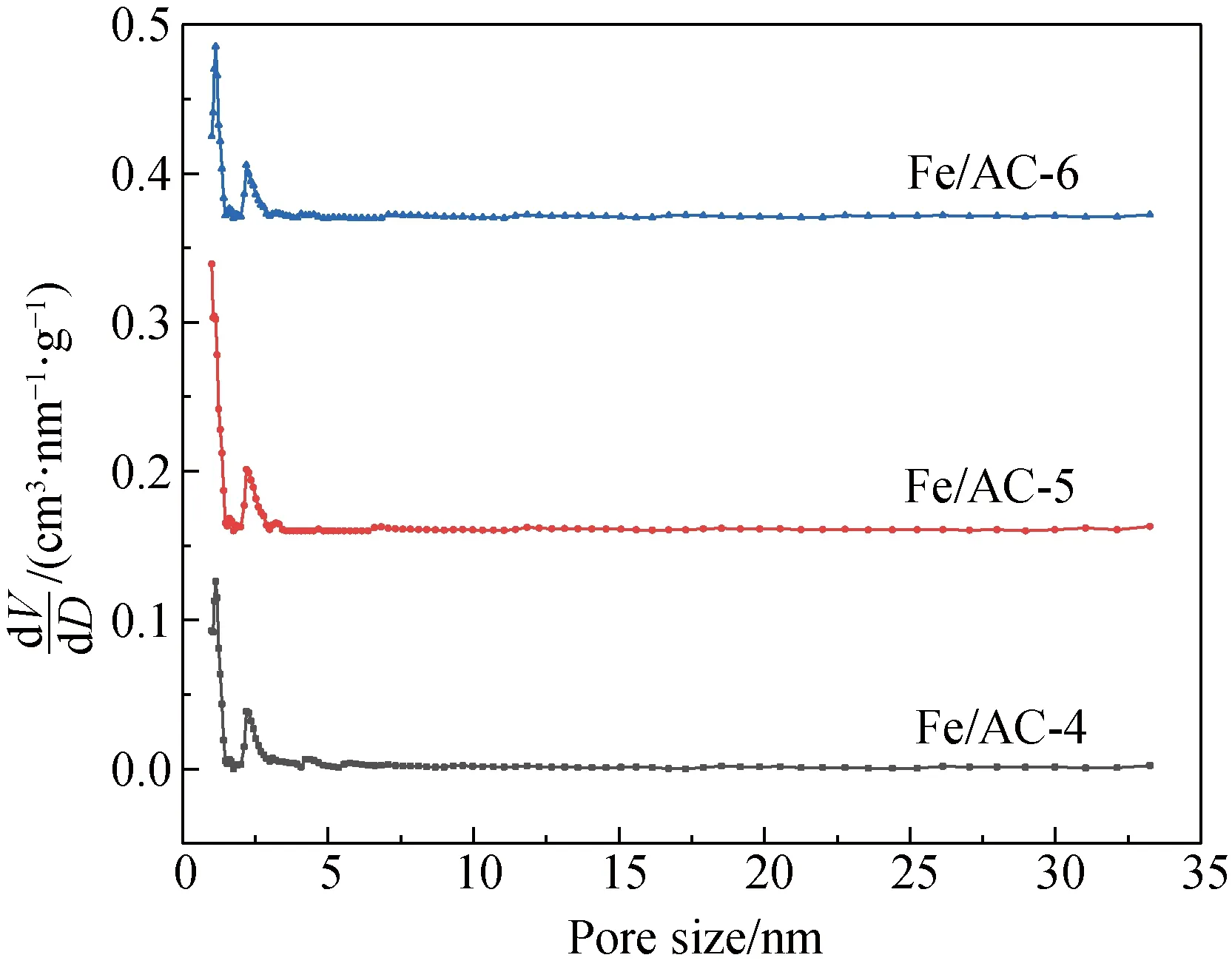
(b)
As shown in Fig. 5(a), the adsorbed volumes of N2for Fe/AC-4, Fe/AC-5 and Fe/AC-6 also rapidly increase at the high pressure during the initial stage; thus, many micropores are present. As shown in Table 2, from high value to low value, the specific surface areas are Fe/AC-5 > Fe/AC-6 > Fe/AC-4. In addition, some mesopores and narrow spaces are formed by numerous flaky particles. From Table 1, the mesopore number in Fe/AC-4 and Fe/AC-5 rapidly increases as the calcination temperature increases. Thus, the high temperature can change micropores to mesopores, but a larger increase exists in micropores than in mesopores. Therefore, the average pore diameters of Fe/AC-4, Fe/AC-5 and Fe/AC-6 decrease compared to those of AC and HNO3/AC.
The main reason for the pore diameter change is that the shrinkage of the AC skeleton makes the pore diameter and the channel decrease upon high-temperature calcination and nitrogen protection. The Fe ions could enter the pore space interior of AC via an immersion process. When Fe2O3forms during high-temperature calcination, the crystal positions become a point of heat accumulation and cause the reaction of Fe(NO3)3with the volatile component. Therefore, the pore space structure of AC can develop in the interior, and part of AC is ablated by Fe(NO3)3decomposition. Based on a loading amount of Fe2O3of 5% and a calcination temperature of 500 ℃, Fe/AC-5 is abundantly ablated with the minimum pore diameter, the pore volume and the specific surface area are the maximum, while the adsorption capacity is also the maximum. When the calcination temperature is 400 ℃, the parameters for Fe/AC-4 and Fe/AC-6 are the same. The micropore volume of Fe/AC-6 is less than that of Fe/AC-4, and the micropore specific surface area of Fe/AC-6 is larger than that of Fe/AC-4. This could be attributed to ferriferous oxide supplying more micropores and the specific surface area as the loading amount increases.
2.3 XRD characterization
The graphite crystal is the main part of AC. The phase composition of ferriferous oxide loading into AC mainly includes paramagnetismα-Fe2O3and ferromagnetismγ-Fe2O3. To analyze the change in the graphite crystal and the Fe phase composition, the XRD spectra of AC, HNO3/AC and Fe/AC are measured, as shown in Fig. 6.
As shown in Fig. 6(a), a graphite peak is found in the XRD spectra of AC and HNO3/AC, and there are two characteristic peaks of broad dispersion at 23.813° and 43.467° (100 crystal face). Thus, AC is composed of amorphous carbon. The diffraction peak due to the decompacting and tiering graphene layer is at a 2θof 23.813°. The diffraction peak from the graphite crystal is at a 2θof 43.467°. The diffraction peak from the graphite crystal face of 002 is at a 2θof 26.468°[14]. Thus, the diffraction peaks of the graphene layer and the graphite crystal become keen-edged peaks when AC is modified via nitric acid oxidation. This could be attributed to that the ash content of AC substantially reduced when it is modified with nitric acid, which makes the diffraction peak keen-edged.
As shown in Fig. 6(b), the diffraction peaks for Fe/AC-1 are at 35.632° and 63.070°, which belong to 311 and 440 crystal faces ofγ-Fe2O3, respectively, according to Joint Committee on Powder Diffraction Standards(JCPDS) card No. 39-1346[15]. The diffraction peaks forα-FeOOH are at 33.116° and 41.223°, which belong to 130 and 140 crystal faces ofα-FeOOH, respectively, according to JCPDS card No. 29-0713. In addition, the diffraction peak forα-Fe2O3is at 33.116°. Thus, the strength of theα-FeOOH peak is likely weak owing to the low content and the unordered structure[12]. Fe(OH)3could not totally transform intoγ-Fe2O3, and at a calcination temperature of 200 ℃,α-FeOOH coexists withα-Fe2O3andγ-Fe2O3.

(a)
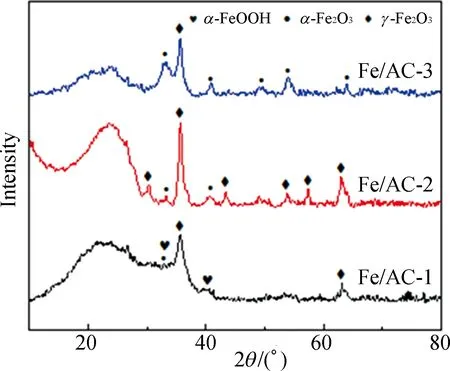
(b)

(c)
The diffraction peaks for Fe/AC-2 are at 30.214°, 35.679°, 43.317°, 53.720°, 57.276° and 62.872°, which belong to 220, 311, 400, 422, 511 and 440 crystal faces ofγ-Fe2O3, respectively. Although the diffraction peaks are comparatively large, the peak shapes are sharp and symmetrical. Theγ-Fe2O3crystal grain is loaded well; however, the diffraction peaks forα-Fe2O3are still at 33.243° and 40.815°[16].
For an increased loading amount and the same calcination temperature compared with Fe/AC-2, the diffraction peak forγ-Fe2O3for loading in Fe/AC-3 is at 35.547°, and the diffraction peaks forα-Fe2O3are at 33.243°, 40.881°, 49.638°, 53.856° and 63.860°, which belong to 104, 113, 024, 116, and 300 crystal faces, respectively, according to theα-Fe2O3standard JCPDS card No. 33-0664. The proportion ofα-Fe2O3increases as the loading amount increases. Thus,α-FeOOH orγ-Fe2O3is transformed intoα-Fe2O3[17].
He gathered slowly, moving backwards4 and forwards through the bushes, but always keeping up with her. After a while, he came back to the path. She answered his questions - the children, her job, her parents - but always skirted round the main issue.
The XRD spectra of Fe/AC-4 are shown in Fig. 6(c), and the diffraction peaks forα-Fe2O3are at 24.123°, 35.569°, 40.791°, 49.378°, 53.988°, 57.409°, 62.381° and 63.929°. The diffraction peaks forγ-Fe2O3are at 30.109°, 33.088° and 43.216°. There is considerableα-Fe2O3and lessγ-Fe2O3loaded in AC when the loading amount of ferriferous oxide is 5% and the calcination temperature is 400 ℃. The ferriferous oxide is mainly present as a plate shape and adheres on the surface of Fe/AC-4.
As shown in the XRD spectra of Fe/AC-5, the diffraction peaks forγ-Fe2O3are found at 30.378°, 35.735°, 43.412°, 53.879°, 57.732° and 62.991°. The diffraction peak forα-Fe2O3is at 33.236°. The diffraction peak is keen-edged with symmetry at 35.735°. Thus, the active material is mainlyγ-Fe2O3crystals with good crystallization behaviors that are well distributed when the loading amount of ferriferous oxide is 5% and the calcination temperature is 500 ℃. Theγ-Fe2O3crystals exhibit good crystallinity and dispersivity, and result in minor chippings of ferriferous oxide.
As shown in the XRD spectra of Fe/AC-6, the diffraction peaks forα-Fe2O3are at 24.182°, 33.170°, 35.645°, 40.861°, 43.334°, 49.460°, 54.055°, 57.553°, 62.409° and 63.979°. The diffraction peak forγ-Fe2O3is at 30.255°. The graphite peak for Fe/AC-6 is broader and lower in amplitude compared with that of Fe/AC-4. Muchα-Fe2O3attaches to the surface of AC, which reduces the graphitization degree of AC. The ferriferous oxide of Fe/AC-6 appears in a large area bulk on the surface.
Thus, for FeSO4modification, to obtain moreγ-Fe2O3, the FeSO4concentration should be 0.5 mol/L, and the calcination temperature needs to be at 250 ℃. Additionally, Fe(NO3)3is favorable forα-Fe2O3formation under the condition of 400 ℃ calcination, andγ-Fe2O3is formed via 500 ℃ calcination. Therefore, the crystal occurrence of Fe(NO3)3after calcination is determined by the calcination temperature. The loading amount of ferriferous oxide determines the graphitization degree, and a higher loading amount results in a lower graphitization degree.
2.4 FTIR characterization
The surface chemical properties of AC are determined by the types and the amount of surface functional groups. The surface functional groups of AC mainly include carboxyl groups, phenolic hydroxyl groups, ester groups, ketone groups, and ethyl ether[18-19]. To determine the functional groups, FTIR was used to analyze the changes in the functional groups on the surface of the carbon materials before and after modifications.


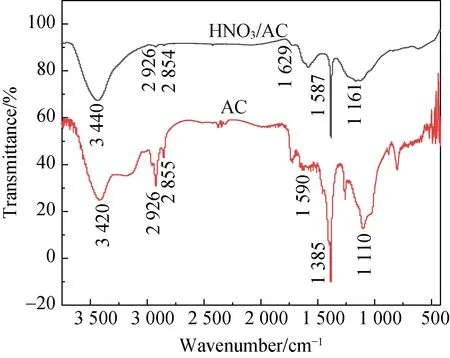
(a)
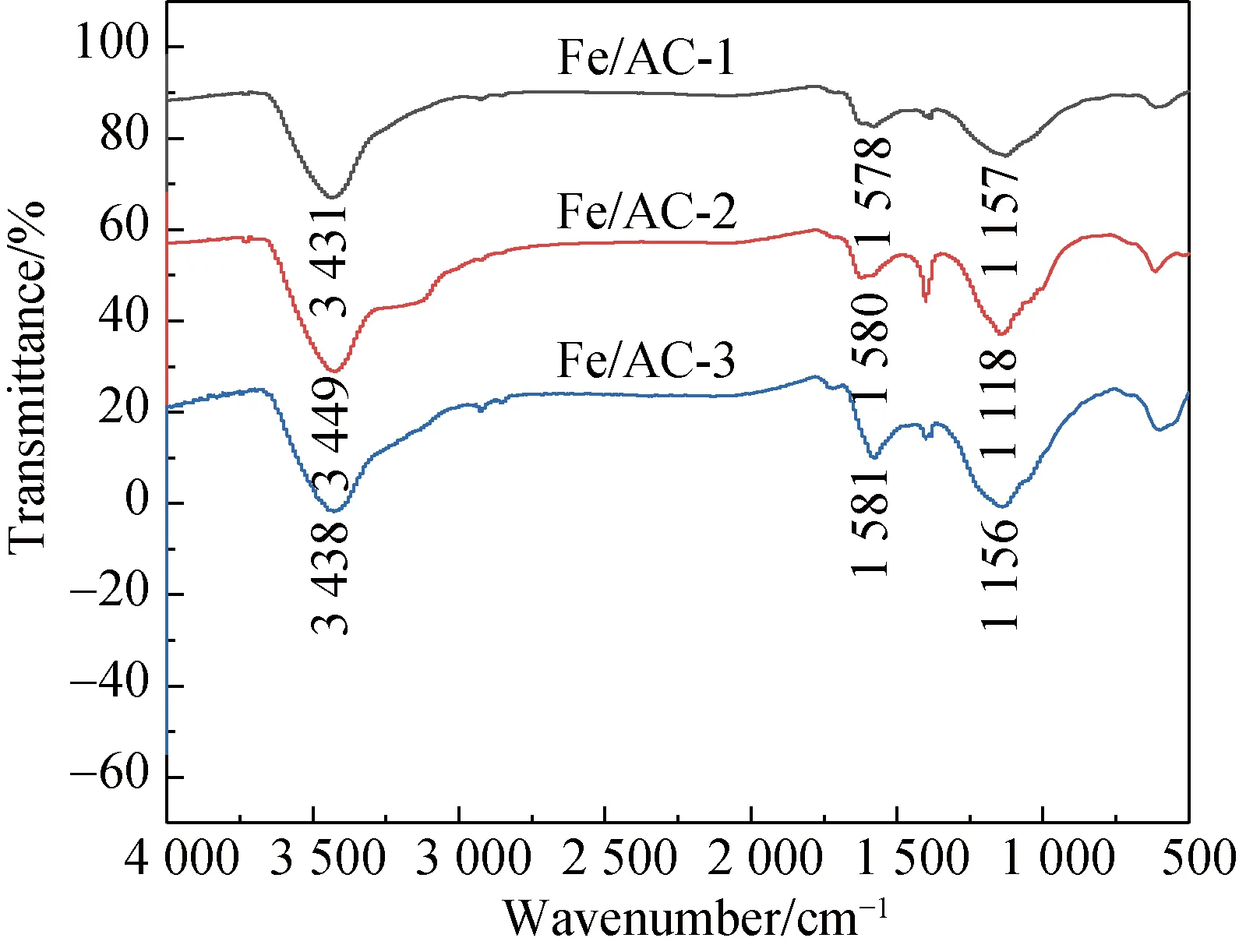
(b)
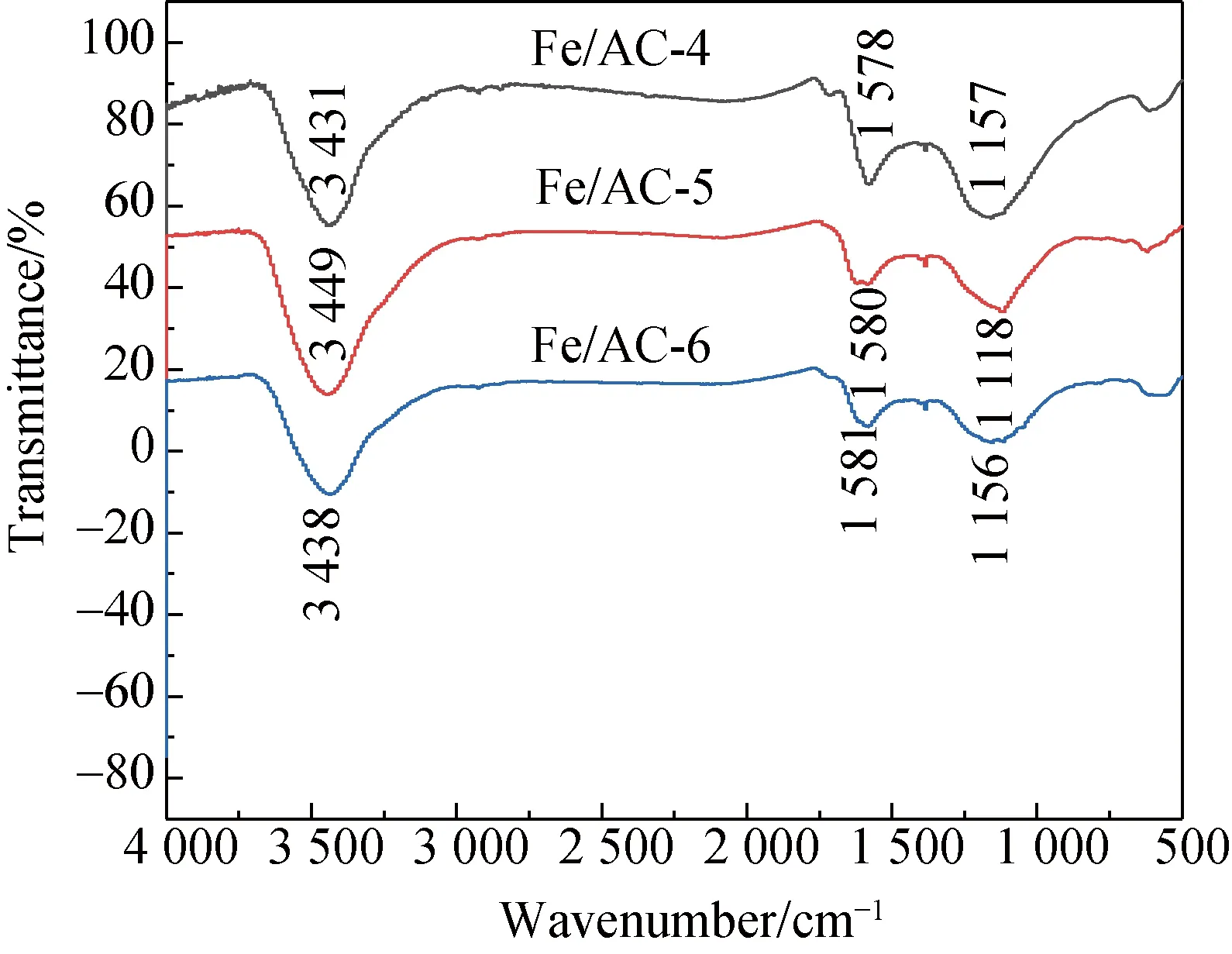
(c)

3 Conclusions
(1) The surface micro topographies of HNO3/AC became unsystematic and disordered. Upon FeSO4modification, the ferriferous oxide was mainly present as a near-spherical crystal. The ferriferous oxide of Fe/AC modified with Fe(NO3)3was mainly present as a plate shape.
(2) Modification with nitric acid enlarged the pores but decreased the specific surface area of AC. FeSO4modification formed a new net post structure in the pore canals of AC. Fe(NO3)3modification caused pore space structures to develop in the interior, and a higher calcination temperature was favorable for ablation.
(3) The ash content of AC substantially decreased upon modification with HNO3. For FeSO4modification,α-FeOOH,α-Fe2O3andγ-Fe2O3coexisted under the conditions of a lower concentration and a lower calcination temperature, and a higher FeSO4concentration and calcination temperature generated moreα-Fe2O3. Fe/AC-2 resulted in moreγ-Fe2O3active material. The calcination temperature during Fe(NO3)3modification determined the Fe graphitization degree, and a higher loading amount resulted in a lower graphitization degree.

