艾塞那肽治疗肥胖症的机制研究
2020-10-22叶志伟冼颖欣陈宗兰林倍思徐芬姚斌
叶志伟?冼颖欣?陈宗兰?林倍思?徐芬?姚斌

【摘要】目的 探討艾塞那肽治疗肥胖症的机制及其相互关系。方法 选择15只雄性C57BL/6小鼠随机分为对照组、高脂饮食肥胖模型组(模型组)和高脂饮食肥胖模型艾塞那肽治疗组(治疗组)。在实验第16周记录各组小鼠摄食量、体质量、体脂量,测量空腹血糖,进行腹腔内葡萄糖耐量试验(GTT)和胰岛素耐量试验(ITT)。测量后处死小鼠,收集小鼠血清、脂肪组织检测胰岛素信号通路分子、炎症和氧化应激指标。结果 肥胖指标方面,模型组小鼠的进食量、体脂量、体脂百分比均高于对照组(P均< 0.017),治疗组进食量少于模型组(P < 0.017)。糖代谢方面,模型组小鼠的空腹血糖、GTT及ITT血糖曲线下面积均高于对照组(P < 0.017),治疗组小鼠的空腹血糖、GTT及ITT血糖曲线下面积均较模型组有所下降但与模型组比较差异无统计学意义(P均> 0.017)。胰岛素信号通路分子检测中,模型组IRS1磷酸化比例(p-IRS1/IRS1)低于对照组(P < 0.017),治疗组p-IRS1/IRS1较模型组回升(P < 0.017)。在炎症和氧化应激指标方面,模型组的血清IL-6水平高于对照组(P < 0.017),治疗组的HIF-1α mRNA相对表达量较模型组下降(P < 0.017)。3组动物模型合并计算,血清IL-6水平(r = 0.702,P = 0.011)和脂肪组织IL-6 mRNA相对表达量(r = 0.590,P = 0.043)均与体脂百分比呈正相关。血清IL-6水平还与进食量(r = 0.670,P = 0.017)、脂肪量(r = 0.680,P = 0.015)和空腹血糖(r = 0.780,P = 0.003)呈正相关。脂肪组织IL-6与eNOS mRNA相对表达量呈正相关(r = 0.627,P = 0.029)。脂肪组织的HIF-1α相对表达量与ITT血糖曲线下面积(r = 0.643,P = 0.024)呈正相关,与p-IRS1(r = -0.820,P = 0.046)和p-IRS1/IRS1(r = -0.846,P = 0.034)呈负相关。结论 艾塞那肽可改善肥胖小鼠模型的肥胖指标和相关的糖代谢异常,这种疗效可能是通过抗炎和抗氧化应激实现的。
【关键词】艾塞那肽;肥胖;炎症;氧化应激
Study of mechanism of exenatide in the treatment of obesity Ye Zhiwei, Xian Yingxin, Chen Zonglan, Lin Beisi, Xu Fen, Yao Bin. Department of Endocrinology and Metabolism, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, China
Corresponding author, Yao Bin, E-mail: yaobin1910@ 126. com
【Abstract】Objective To explore the specific mechanism of exenatide in treating obesity and investigate its relationship. Methods Fifteen male C57BL/6 mice were randomly divided into the normal control group, obesity model group (induced by high-fat diet) and exenatide treatment group (treated by exenatide after high-fat diet). At the 16th week of the experiment, the food intake, body weight, weight of body fat, and fasting blood glucose of the mice in each group were recorded, and intraperitoneal glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed. Subsequently, the mice were sacrificed, and the adipose tissue and serum of the mice were collected to detect the insulin signaling pahway molecules and measure the indexes of inflammation and oxidative stress. Results In terms of the obsesity indexes, the food intake, weight and percentage of body fat in the obesity model group were significantly higher compared with those in the control group (all P < 0.017), and the food intake in the treatment group was considerably less than that in the model group (P < 0.017). In the model group, the area under the ROC curve of fasting blood glucose, GTT and ITT was remarkably larger than that in the control group (all P < 0.017). Regarding the glucose metabolism, the area under the ROC curve of fasting blood glucose, GTT and ITT in the treatment group was slightly smaller than that in the model group with no statistical significance (all P > 0.017). In the model group, the percentage of IRS1 phosphorylation was significantly lower than that in the control group (P < 0.017). In the treatment group, the percentage of IRS1 phosphorylation was significantly higher than that in the model group (P < 0.017). The IL-6 level in the model group was significantly higher than that in the control group (P < 0.017). In the treatment group, the relative expression level of HIF-1α mRNA was significantly down-regulated compared with that in the model group (P < 0.017). For all the animals in three groups, the serum IL-6 levels (r = 0.702, P = 0.011) and the relative expression levels of IL-6 mRNA in the fat tissues (r = 0.590, P = 0.043) were positively correlated with the percentage of body fat. The serum IL-6 level was positively associated with the food intake (r = 0.670, P = 0.017), weight of body fat (r = 0.680, P = 0.015) and fasting blood glucose (r = 0.780, P = 0.003). The IL-6 level in the fat tissues was positively correlated with the relative expression level of eNOS mRNA (r = 0.627, P = 0.029). The relative expression level of HIF-1α in the fat tissues was positively associated with the area under ROC curve of ITT (r = 0.643, P = 0.024), whereas negatively correlated with p-IRS1 (r = -0.820, P = 0.046) and the percentage of IRS1 phosphorylation (r = -0.846, P = 0.034). Conclusion Exenatide can improve the obesity indexes and glucose metabolism abnormality probably via the mechanism of anti-inflammation and anti-oxidation.
【Key words】Exenatide;Obesity;Inflammation;Oxidative stress
肥胖是指由于体内脂肪的体积和(或)脂肪细胞数量的增加导致的体质量增加,或体脂占体质量的百分比异常增高,并在某些局部过多沉积脂肪[1]。肥胖是糖尿病、心血管疾病及其他代谢性疾病和肿瘤的潜在危险因素[2]。同时,近二十年,我国肥胖的患病率逐渐增长,肥胖发病出现低龄化表现,严重危害我国人民的健康。肥胖的发病机制复杂,越来越多的临床和基础研究证据提示肥胖是一种慢性炎症,并与氧化应激相关[3-4]。艾塞那肽是一种胰高血糖素样肽1 (GLP-1)受体激动剂。在2型糖尿病患者的治疗中,GLP-1受体激动剂在控制血糖的同时可以降低患者体质量,而抗炎和抗氧化是GLP-1受体激动剂发挥疗效的重要机
制[5-6]。与其他GLP-1受体激动剂类似,在心血管系统、神经系统等领域的多个研究中已初步发现艾塞那肽可能有抗炎和抗氧化的作用[7-8]。由此可见,肥胖发病过程涉及炎症和氧化应激机制,而艾塞那肽具有减重作用和抗炎、抗氧化功能。但目前对艾塞那肽治疗肥胖的研究有限,对其疗效中的抗炎和抗氧化机制尚未阐明。为此,本研究拟探讨艾塞那肽通过抗炎和抗氧化治疗肥胖症的具体机制及这些机制间的相互关系,现报告如下。
材料与方法
一、动物饲养和分组
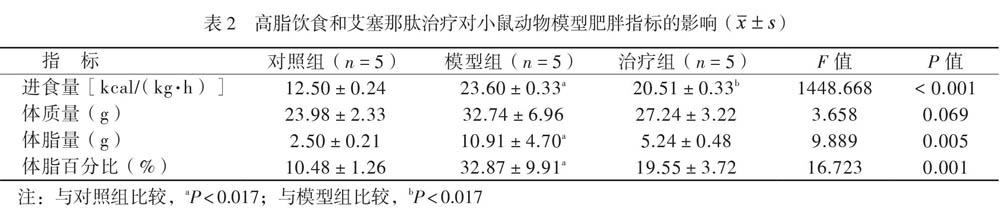
本研究的动物实验研究方案得到中山大学实验动物管理与使用委员会的批准。研究采用7周龄的雄性C57BL/6小鼠(购自南京大学模式动物研究所),体质量为19 ~ 22 g。饲养于屏障环境内,自由进食和饮水。15只雄性C57BL/6小鼠随机分为3组:①对照组,以标准食物(脂肪含量4.0%)饲养16周;②模型组,以高脂食物(脂肪含量34.9%)饲养12周后,以生理盐水腹腔内注射4周;③治疗组,以高脂食物(脂肪含量34.9%)饲养12周后,以艾塞那肽生理盐水腹腔内注射(每日24 nmol/kg)治疗4周。
二、动物模型评估
在实验第16周记录各组小鼠摄食量、体质量、体脂量,测量空腹血糖,并进行腹腔内糖耐量试验(GTT)和腹腔内胰岛素耐量试验(ITT)。其中摄食量的计算方法如下:放入饲料时称重,每次更换饲料时再称量剩余量,两者之差为摄入饲料质量,换算为摄入能量值。并测量小鼠体质量、计算摄入饲料所花费的时间。得到小鼠单位体质量(kg)在单位时间(h)内摄入的能量值(kcal)。体脂量测量按照动物体脂定量分析仪的操作说明书对活体清醒的小鼠进行全身脂肪、瘦肉组织、游离水及全身含水量的分析。体脂百分比(%)即全身脂肪重量与体质量的比值。GTT:小鼠禁食过夜后经腹腔内注射葡萄糖(2 g/kg),并检测0、30、60、90、120 min的尾靜脉血糖水平。ITT试验:小鼠禁食6 h后经腹腔注射重组人胰岛素(0.65 U/kg),并测量0、30、60、90、120 min的尾静脉血糖水平。记录各时间点血糖值,连线得到血糖曲线,曲线下面积= (血糖0 min+血糖120 min+ 血糖30 min×2+血糖60 min×2+血糖90 min×2)×15。血糖检测完成后予异氟烷充分麻醉小鼠,以皮肤捏夹反应及脚趾刺激反应等确保小鼠已经到达深度麻醉,摘除小鼠眼球取血后立即处死小鼠并收集小鼠附睾旁脂肪组织。
三、血清学检测
采用MENDO-75K kit (Millipore)检测血清IL-6水平,所有步骤均严格按照试剂盒说明书进行操作。
四、实时定量PCR
通过实时定量PCR检测模板DNA的浓度,以了解组织内mRNA表达情况:首先应用Trizol总RNA抽提试剂(Invitrogen)收集脂肪组织的mRNA,随后使用Prime Script RT Reagent Kit(Takara)将mRNA逆转录为模板DNA,最后应用Light Cycler? 480 SYBR Green Master (Roche)进行实时定量PCR实验。所有步骤按照试剂盒说明书进行操作。实验中使用的IL-6、缺氧诱导因子-1α(HIF-1α)和内皮型一氧化氮合酶(eNOS)的引物序列见表1。所有实时定量PCR的产物使用鼠源性β-actin作为内参计算标准值(对照组数值等于1)。mRNA表达的倍数变化使用2-ΔΔCT方法计算。
五、蛋白免疫印迹
从脂肪组织(约50 mg)和细胞裂解物中提取总蛋白,然后通过10% SDS-PAGE分离。用特异性一抗进行免疫印迹。所有免疫印迹信号强度使用β-actin作为内参计算标准值(对照组数值等于1)。本研究使用的特异性一抗包括:胰岛素受体-β(IR-β, Cell Signaling Technology)、胰岛素受体底物-1(IRS1,Santa Cruz)、磷酸化IRS1(p-IRS1,Cell Signaling Technology)、磷酸肌醇-3-激酶(PI3K,Cell Signaling)和β-actin(Cell Signaling Technology)。计算IRS1磷酸化比例(p-IRS1/IRS1)。
六、统计学处理
所有统计分析采用SPSS 22.0进行。正态分布的计量资料采用描述,多组定量资料比较用单因素方差分析,两两比较采用t检验并用Bonferroni 法校正检验水准(α' = α/3)。各种指标的相关性采用Pearson相关分析。α= 0.05。
结果
一、对照组、模型组和治疗组小鼠的肥胖指标比较
3组小鼠的进食量、体脂量、体脂百分比比较差异均有统计学意义(P均< 0.05),其中模型组的进食量、体脂量、体脂百分比均高于对照组(P均< 0.017),治疗组进食量少于模型组(P < 0.017)。3组小鼠体质量比较差异无统计学意义(P > 0.05),见表2。
二、对照组、模型组和治疗组小鼠的空腹血糖、GTT和ITT血糖曲线下面积比较
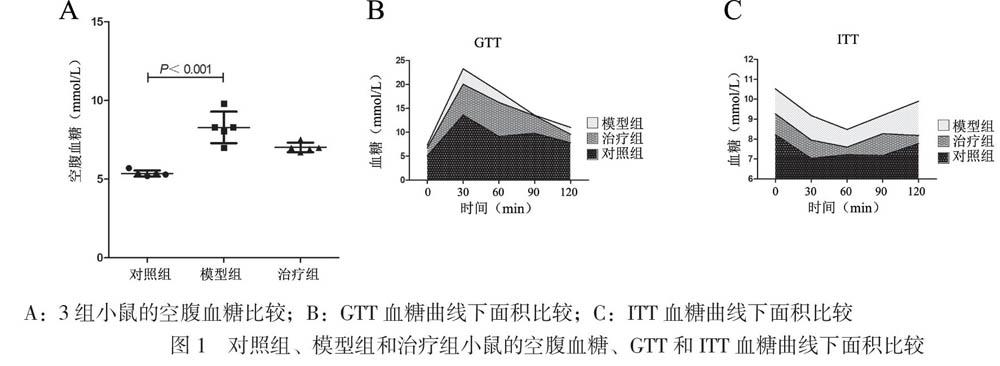
3组小鼠的空腹血糖水平比较差异有统计学意义(F = 28.626,P < 0.001),模型组小鼠的空腹血糖高于对照组(P < 0.017),治疗组小鼠的空腹血糖水平较模型组有所下降但与模型组比较差异无统计学意义(P > 0.017),见图1A。
3组小鼠的GTT血糖曲线下面积比较差异有统计学意义(F = 11.445,P = 0.002),模型组的GTT血糖曲线下面积高于对照组(P < 0.017),治疗组GTT血糖曲线下面积比模型组有所下降但组间比较差异无统计学意义(P > 0.017),见图1B。
3组小鼠的ITT血糖曲线下面积比较差异有统计学意义(F = 9.695,P = 0.003),模型组的ITT血糖曲线下面积高于对照组(P < 0.017),治疗组ITT血糖曲线下面积比模型组下降但组间比较差异无统计学意义(P > 0.017),见图1C。
三、对照组、模型组和治疗组小鼠的胰岛素信号通路相关分子水平及其磷酸化程度比较
3组小鼠的p-IRS1/IRS1比较差异有统计学意义(F = 10.867,P = 0.010),模型组p-IRS1/IRS1低于对照组(P < 0.017),治疗组p-IRS1/IRS1较模型组回升(P < 0.017)。3组小鼠的IR-β、p-IRS1、IRS1和PI3K水平比较差异均无统计学意义(P均> 0.05),见图2。
四、对照组、模型组和治疗组小鼠的炎症和氧化应激指标比较
3组小鼠的血清IL-6水平比较差异有统计学意义(F = 10.988,P = 0.004),模型组的血清IL-6水平高于对照组(P < 0.017),治疗组的血清IL-6水平略低于模型组但组间比较差异无统计学意义(P > 0.017)。各组脂肪组织内IL-6 mRNA相对表达量也呈类似趋势,但组间比较差异无统计学意义(P > 0.05)。3组小鼠脂肪组织HIF-1α mRNA相对表达量比较差异有统计学意义(F = 5.405,P = 0.029),模型组HIF-1α mRNA相对表达量升高但与对照组比较差异无统计学意义(P > 0.017),治疗组的HIF-1α mRNA相对表达量较模型组下降(P < 0.017)。脂肪组织的eNOS水平在不同组别小鼠中存在与HIF-1α类似趋势,但3组比较差异无统计学意义(P > 0.05),见图3。
五、3组小鼠动物模型肥胖、糖代谢指标和炎症、氧化应激指标的相关性分析
3组动物模型合并计算,血清IL-6水平(r = 0.702,P = 0.011)和脂肪組织IL-6 mRNA相对表达量(r = 0.590,P = 0.043)均与体脂百分比呈正相关。血清IL-6水平还与进食量(r = 0.670,P = 0.017)、脂肪量(r = 0.680,P = 0.015)和空腹血糖(r = 0.780,P = 0.003)呈正相关。脂肪组织IL-6与脂肪组织eNOS mRNA相对表达量呈正相关(r = 0.627,P = 0.029)。脂肪组织的HIF-1α相对表达量与ITT血糖曲线下面积(r = 0.643,P = 0.024)呈正相关,与p-IRS1(r = -0.820,P = 0.046)和p-IRS1/IRS1(r = -0.846,P = 0.034)呈负相关。
讨论
艾塞那肽是一种GLP-1受体激动剂,临床上主要用于2型糖尿病的治疗,除了可调节血糖和胰岛素功能外,还能减轻患者体质量。有基础研究显示,艾塞那肽可能还具有抗炎和抗氧化的功效。因此,从机制上推测,对于发病机制中同样涉及炎症和氧化的肥胖患者,艾塞那肽可能有效。本研究成功建立了饮食诱导的小鼠肥胖模型,该模型出现了肥胖、糖代谢异常、胰岛素作用异常,并伴有炎症和氧化应激活化。与模型组小鼠相比,经艾塞那肽干预的治疗组小鼠摄食量减少、IRS1磷酸化比例回升,其氧化应激标志物HIF-1α mRNA相对表达量下降,结果提示艾塞那肽通过抗炎和抗氧化发挥减重和调节糖代谢的作用,从而治疗肥胖症。
IL-6是一种与肥胖相关的重要促炎症细胞因子,研究显示肥胖患者的血清IL-6升高,并且可以预测肥胖所致的2型糖尿病的发生。IL-6在肥胖患者中对炎症的调节是全身性的,肥胖患者唾液中的IL-6比非肥胖者明显增加,其牙龈炎严重程度加重[9]。此外,与IL-6相关的单核苷酸多态性基因 rs2069845致病性变异增加肥胖发生的风险[10]。基础研究证实,IL-6在肥胖的发病机制中具有刺激M2极化和局部脂肪组织巨噬细胞增殖的效应[11]。本研究中,模型组小鼠血清IL-6水平高于对照组,治疗组较模型组有所下降,并且IL-6与肥胖、糖代谢指标相关。再次证实肥胖可以活化炎症应答,并提示艾塞那肽有助于缓解肥胖导致的炎症活化。
氧化应激与炎症密切相关,同时也参与肥胖的发病机制。HIF-1α和eNOS是氧化应激的重要生物学标志物。HIF-1α可通过抑制棕色脂肪组织生热作用、调节胰岛素抵抗等多种途径导致肥胖和糖尿病[12]。eNOS也可通过氧化应激、内皮功能紊乱等诱导肥胖和胰岛素抵抗[13]。本研究中,治疗组HIF-1α mRNA相对表达量低于模型组,并且HIF-1α与ITT血糖曲线下面积相关,表明肥胖动物模型存在氧化应激异常,这种氧化应激可以被艾塞那肽部分缓解。
值得关注的是,本研究中的炎症和氧化应激指标也存在一定相关性。有研究显示,炎症和氧化应激在脂肪组织中是相互联系的。如同样与胰岛素抵抗相关的阻塞性睡眠呼吸暂停综合征,其患者的间歇性缺氧和氧化应激可以引起脂肪组织炎症,并进一步诱导胰岛素抵抗,而纠正这种缺氧和氧化应激可以减轻患者的炎症状况[14-17]。本研究显示,脂肪组织IL-6(炎症指标)与eNOS(氧化应激指标)mRNA相对表达量呈正相关,而这两种效应也可能在艾塞那肽的治疗机制中相互作用,其具体机制值得进一步深入探讨。
綜上所述,艾塞那肽可改善肥胖小鼠模型的肥胖指标和相关的糖代谢异常,这种疗效可能是通过抗炎和抗氧化应激实现的。
参 考 文 献
[1] 中国超重肥胖医学营养治疗专家共识编写委员会. 中国超重/肥胖医学营养治疗专家共识(2016年版). 中华糖尿病杂志, 2016, 8(9):525-540.
[2] Malone JI, Hansen BC. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr Diabetes, 2019, 20(1):5-9.
[3] Stolarczyk E. Adipose tissue inflammation in obesity: a metabolic or immune response? Curr Opin Pharmacol, 2017, 37:35-40.
[4] Gaspar JM, Velloso LA. Hypoxia inducible factor as a central regulator of metabolism-implications for the development of obesity. Front Neurosci, 2018, 12:813.
[5] Guo C, Huang T, Chen A, Chen X, Wang L, Shen F, Gu X. Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages. Braz J Med Biol Res, 2016, 49(12):e5826.
[6] Bu?dak ?, Machnik G, Bu?dak RJ, ?abuzek K, Bo?dys A, Belowski D, Basiak M, Okopień B. Exenatide (a GLP-1 agonist) expresses anti-inflammatory properties in cultured human monocytes/macrophages in a protein kinase A and B/Akt manner. Pharmacol Rep, 2016, 68(2):329-337.
[7] Fang J, Tang Y, Cheng X, Wang L, Cai C, Zhang X, Liu S, Li P. Exenatide alleviates adriamycin-induced heart dysfunction in mice: modulation of oxidative stress, apoptosis and inflam-mation. Chem Biol Interact, 2019, 304:186-193.
[8] Bu?dak ?, Machnik G, Skudrzyk E, Bo?dys A, Okopień B. The impact of exenatide (a GLP-1 agonist) on markers of inflammation and oxidative stress in normal human astrocytes subjected to various glycemic conditions. Exp Ther Med, 2019, 17(4):2861-2869.
[9] Do?an GE, Toraman A, ?ebin S?, Do?an ?, Güng?r A, Aksoy H, Asutay H. Salivary IL-6 and IL-10 levels in subjects with obesity and gingivitis. Am J Dent, 2016, 29(5):261-265.
[10] Todendi PF, Klinger EI, Ferreira MB, Reuter CP, Burgos MS, Possuelo LG, Valim AR. Association of IL-6 and CRP gene polymorphisms with obesity and metabolic disorders in children and adolescents. An Acad Bras Cienc, 2015, 87(2):915-924.
[11] Braune J, Weyer U, Hobusch C, Mauer J, Brüning JC, Bechmann I, Gericke M. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J Immunol, 2017, 198(7):2927-2934.
[12] Jun JC, Devera R, Unnikrishnan D, Shin MK, Bevans-Fonti S, Yao Q, Rathore A, Younas H, Halberg N, Scherer PE, Polotsky VY. Adipose HIF-1α causes obesity by suppressing brown adipose tissue thermogenesis. J Mol Med (Berl), 2017, 95(3):287-297.
[13] Sansbury BE, Hill BG. Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med, 2014,73:383-399.
[14] 冯文雯,潘幸,周昭远.男性肥胖型与非肥胖型OSAHS和胰岛素抵抗的相关分析.新医学, 2015, 46(10):677-681.
[15] Ryan S. Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol, 2017, 595(8):2423-2430.
[16] Murphy AM, Thomas A, Crinion SJ, Kent BD, Tambuwala MM, Fabre A, Pepin JL, Roche HM, Arnaud C, Ryan S. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J, 2017, 49(4):1601731.
[17] Perrini S, Cignarelli A, Quaranta VN, Falcone VA, Kounaki S, Porro S, Ciavarella A, Ficarella R, Barbaro M, Genchi VA, Nigro P, Carratù P, Natalicchio A, Laviola L, Resta O, Giorgino F. Correction of intermittent hypoxia reduces inflam-mation in obese subjects with obstructive sleep apnea. JCI Insight, 2017, 2(17):e94379.
(收稿日期:2020-02-29)
(本文編辑:林燕薇)
