Histopathological validation of magnifying endoscopy for diagnosis of mixed-histological-type early gastric cancer
2020-10-22YuichiroOzekiKingoHirasawaRyosukeKobayashiChikoSatoYokoTateishiAtsushiSawadaRyosukeIkedaMasafumiNishioTakehideFukuchiMakomoMakazuMasatakaTaguriShinMaeda
Yuichiro Ozeki, Kingo Hirasawa, Ryosuke Kobayashi, Chiko Sato, Yoko Tateishi, Atsushi Sawada, Ryosuke Ikeda, Masafumi Nishio, Takehide Fukuchi, Makomo Makazu, Masataka Taguri, Shin Maeda
Abstract
Key Words: Early gastric cancer; Endoscopic submucosal dissection; Mixed-histologicaltype; Undifferentiated-type; Narrow-band imaging
INTRODUCTION
Endoscopic submucosal dissection (ESD) has made it possible to treat early gastric cancers less invasively[1,2]. ESD is currently performed according to the guidelines issued by the Japanese Gastric Cancer Association[3]. Principal indications for endoscopic treatment are tumors with a very low possibility of lymph node metastasis, established by estimation with a large number of surgical cases[4-6]. The Japanese Classification of Gastric Carcinoma[7]classifies gastric cancers into two major histological types, differentiated-type (DT) and undifferentiated-type (UDT). Gastric cancers that contain a mixture of DT and UDT components are called mixedhistological-type (MT) and are classified according to the predominant histological type in the above classification and guidelines[3,7]. For UDT early gastric cancers, the resection is classified as curative withen blocresection, tumor size ≤ 2 cm, pT1a, without ulceration, negative horizontal margin, negative vertical margin, and no lymphovascular infiltration. Since UDT-predominant MT gastric cancers are regarded as UDT gastric cancers and are, hence, subject to stricter criteria for endoscopic curative resection[8], it is important to accurately diagnose the UDT component before making treatment decisions. Moreover, the presence of the UDT component is an independent risk factor for non-curative endoscopic resection, even in DTpredominant MT gastric cancers[9-11]. However, few studies have investigated whether the UDT component within MT early gastric cancers can be recognized preoperatively. Thus, the present study investigated the feasibility of preoperative diagnosis of the UDT component within MT early gastric cancers. First, we attempted to clarify the histopathological characteristics of the endoscopically-resected MT early gastric cancers with emphasis on the UDT component. Then, we reviewed preoperative endoscopic images, including magnifying endoscopy with narrow-band imaging (MENBI), emphasizing the area where the UDT component was present in the histopathological examination. Finally, we analyzed the comparative relationships between the preoperative endoscopic findings and the histopathological findings to investigate whether the UDT component could be recognized preoperatively.
MATERIALS AND METHODS
Patient selection
This was a single-center retrospective study. Figure 1 shows the flowchart of patient recruitment. We treated 2011 consecutive early gastric cancers in 1558 patients with ESD between January 2005 and June 2016 at the Yokohama City University Medical Center. Of these, 92 lesions in 87 patients were histopathologically diagnosed as MT gastric cancers after ESD. After excluding lesions that did not precisely undergo MENBI of the whole lesion before treatment, we analyzed 49 MT gastric cancers in 44 patients. Preoperative endoscopic images and postoperative histopathological slides were reviewable for all lesions. All patients were fully informed about the treatment and histopathological examination and provided consent. Our institutional review board approved the study, which was performed in accordance with the 1964 Declaration of Helsinki and later revisions.
Histopathological examination
All specimens resected with ESD were “pinned out” onto polystyrene receivers to facilitate subsequent histopathological sectioning before immediate fixation in 10% buffered formalin solution, then cut into 2-mm-thick slices. The slices were embedded in paraffin, cut into 3 µm sections, stained with hematoxylin and eosin, and microscopically examined for histopathological findings by two or more expert pathologists. In addition to the items that are stipulated in guidelines issued by the Japanese Gastric Cancer Association[3,7], we investigated the UDT component within a lesion with regard to the presence or absence of the following three factors that we hypothesized could potentially be associated with recognition using ME-NBI: (1) The whole mucosal layer occupation of the UDT component; (2) Exposure of the UDT component to the surface of the mucosa; and (3) Existence of a clear border between the DT and UDT components (Figure 2). Cases that met category (1) criteria included cases that met category (2) criteria. However, we distinguished category (1) from category (2) by the UDT cancer status, since category (1) had more progressed cancer compared to category (2). UDT early gastric cancers are thought to progress from the intermediate-layer type to the superficial type and finally to the whole-layer type[12].
Endoscopic examination

Figure 1 Patient recruitment. ESD: Endoscopic submucosal dissection; ME-NBI: Magnifying endoscopy with narrow-band imaging.
We used the Evis Lucera Spectrum endoscopy system from January 2005 to October 2012 and the Evis Lucera Elite system from November 2012 onwards (Olympus Medical Systems, Tokyo, Japan), along with high-resolution optical magnifying endoscopies (GIF-H260Z; Olympus Optical, Tokyo, Japan) for NBI. We used a black soft hood attachment (MB46; Olympus) on the tip of the scope in all cases. All patients were examined under conscious sedation, as described in our previous report[13]. Three specialists certified by the Japan Gastroenterological Endoscopy Society retrospectively reviewed the preoperative endoscopic images of all lesions. In the review, we first mapped the histological type diagnosed in the histopathological examination onto the corresponding endoscopic images (Figure 3A and B). Then, we focused on the area where the UDT component was histopathologically present and investigated whether the endoscopic finding of the UDT component could be identified (Figure 3C and D). In this study, the endoscopic finding of the UDT component was evaluated by the ME-NBI finding because we aimed to focus on the exact area where the UDT component existed histopathologically, even if the area was limited in size. The ME-NBI finding of the UDT component was defined by two elements: a microvascular pattern, which has an isolated and disordered quality, and a microstructure pattern, which lacks visibility or even disappears (Figure 4). This complies with the ME-NBI finding described as a corkscrew pattern in the study by Nakayoshiet al[14], who reported that 85.7% of UDT early gastric cancers showed a corkscrew pattern in ME-NBI. Kishinoet al[15]recently reported that the positive predictive value of ME-NBI for UDT early gastric cancer was 88.9% when a lesion showed both an unclear surface pattern and severe vascular irregularity in accordance with the corkscrew pattern. The results of the preoperative biopsies were also investigated.
Statistical analysis
Statistical analyses were performed using JMP Pro 12 (SAS Institute, Cary, NC, United States). Continuous variables were expressed as the mean ± SD, and categorical parameters were expressed as numbers and frequencies. We used the Chi-square test or Fisher’s exact test for the categorical parameters, andt-test to compare the mean values of the continuous variables for two-group comparisons.P< 0.05 was considered statistically significant. The statistical methods and analyses of this study were reviewed by Professor Masataka Taguri of the Yokohama City University School of Data Science.
RESULTS
Among 2011 early gastric cancers treated at our hospital with ESD between January 2005 and June 2016, 92 lesions (4.6%) were histopathologically diagnosed as MT gastric cancers after ESD (Figure 1). Table 1 shows the clinicopathological characteristics and treatment outcomes of MT gastric cancers compared to pure DT gastric cancers treated with ESD during the same period. Outcomes of pure UDT gastric cancers are alsoshown in Table 1 for reference. Treatment outcome revealed that 45.7% (42/92) of MT early gastric cancers were histopathologically identified as submucosal cancers. Lymphovascular invasion was present in 22.8% (21/92) of the MT lesions. Thus, the overall endoscopic curative resection rate of MT gastric cancers was only 38.0% (35/92), which was significantly lower than that of pure DT gastric cancers. After excluding 43 lesions that did not precisely undergo preoperative ME-NBI of the whole lesion, we included 49 MT gastric cancers in 44 patients for additional analysis. Table 2 shows the clinicopathological characteristics and treatment outcomes of the 49 lesions. In the histopathological examination, the whole mucosal layer occupation of the UDT component and exposure of the UDT component to the mucosal surface were seen in 67.3% (33/49) and 71.4% (35/49) of the MT gastric cancers, respectively. A clear distinction of the border between the DT and UDT components could not be drawn in 65% (32/49) of MT lesions. In the endoscopic examination, a review of pretreatment ME-NBI images revealed that 24.5% (12/49) of the MT lesions showed an endoscopic finding of the UDT component within the area where it was present in the histopathological examination. Only 26.5% (13/49) of the lesions were diagnosed from the pretreatment biopsy as having a UDT component. Table 3 shows the comparative relationships between the presence or absence of an ME-NBI UDT component finding and histopathological UDT component findings. Tumor diameter did not differ significantly between the lesions with an ME-NBI UDT component finding and those without the ME-NBI UDT component finding. Fourteen of 15 lesions (93%) with submucosal invasion did not show an ME-NBI UDT component finding. The UDT component did not expose to the mucosal surface in 10 lesions histopathologically and none of them showed an ME-NBI UDT component finding. In this analysis, histopathological UDT predominance was the single significant factor associated with an ME-NBI UDT component finding, with 62% (8/13) of UDT-predominant MT gastric cancers showing an ME-NBI UDT component. Of the 36 lesions that were diagnosed
from the pretreatment biopsy as having only a DT component, seven showed an MENBI UDT component finding. Therefore, the addition of ME-NBI improved the pretreatment diagnosis rate of a UDT component by 14%. Consequently, combined results of the pretreatment biopsy and an ME-NBI finding showed the presence of a UDT component within the lesion in 40% (20/49) of MT gastric cancers in this study (Figure 5).

Table 1 Clinicopathological characteristics and treatment outcomes of all the patients and lesions treated with endoscopic submucosal dissection at our facilities between January 2005 and June 2016, n (%)
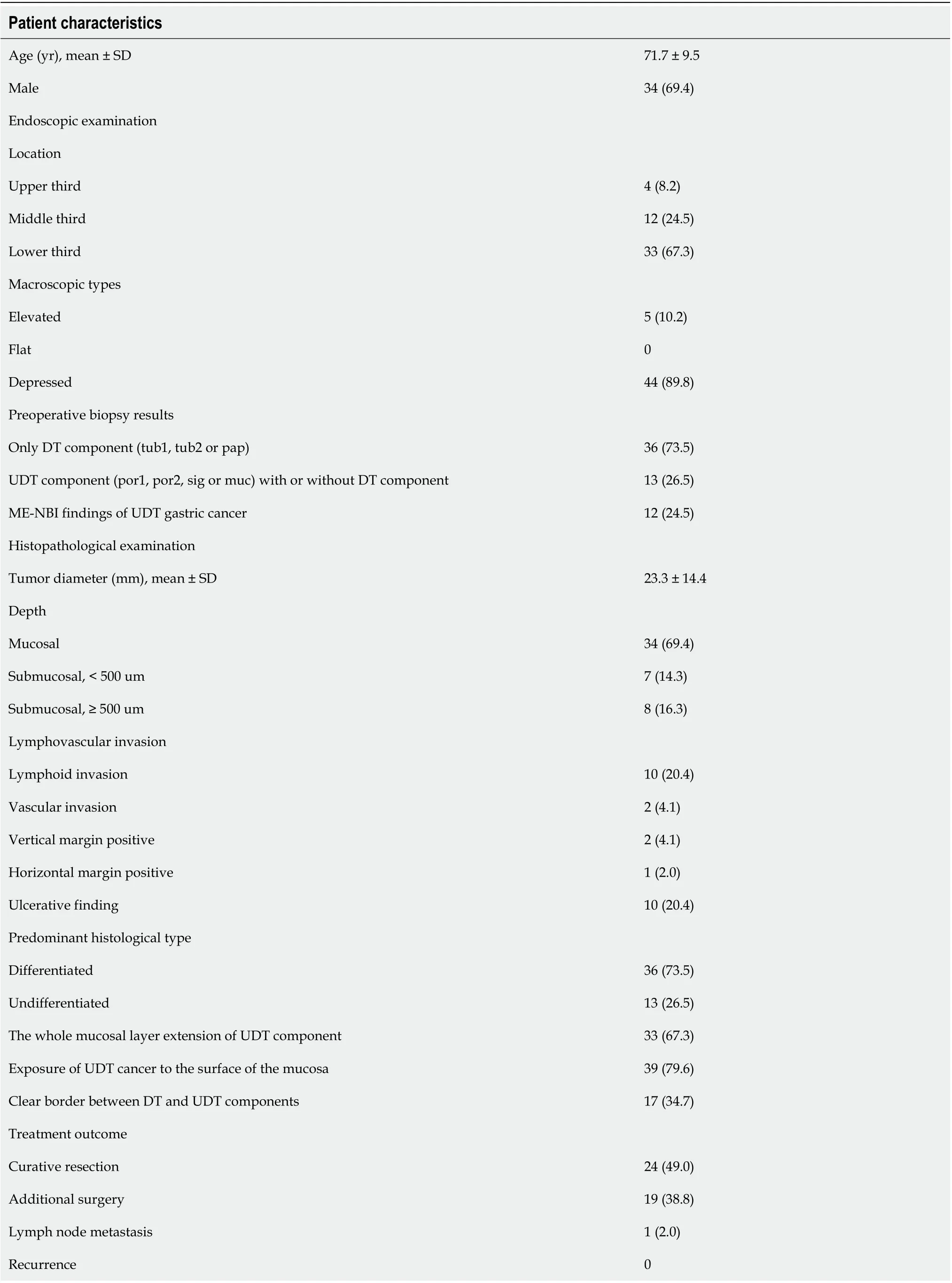
Table 2 Clinicopathological characteristics and treatment outcome of the 49 mixed histological-type early gastric cancers investigated in this study (n = 49), n (%)
DISCUSSION
In this retrospective study, we investigated whether a UDT component within MT early gastric cancers could be recognized preoperatively, since the presence of a UDT component profoundly affects the clinical course and treatment decision of early gastric cancers. Some retrospective studies of surgical cases reported a higher probability of lymph node metastasis[16-20]and even poorer prognosis[21-24]in MT gastric cancers, while other studies have reported that these relationships may not apply to MT gastric cancers that meet the criteria for endoscopic curative resection[25,26]. However, retrospective studies of surgical cases tend to ignore the uncertainty of a preoperative diagnosis. A previous study of endoscopically treated cases reported that the presence of a UDT component was an independent risk factor for non-curative endoscopic resection in early gastric cancers[9-11,27]. The present study included cases that underwent ESD as an initial treatment, and only 38% (35/92) of them turned out to fulfill criteria for endoscopic curative resection. Therefore, preoperative recognition of a UDT component may reduce the incidence of cases that need additional gastrectomy after undergoing ESD.
Histopathological examination of ESD resected specimens showed that in 67.3% (33/49) of MT gastric cancers, the UDT component occupied the whole mucosal layer, and 79.6% (39/49) showed the UDT component exposed to the surface of the mucosa. Therefore, it is reasonable to assume that preoperative ME-NBI may contribute to diagnosing a UDT component within MT gastric cancers. However, a thorough review of preoperative ME-NBI images by three specialists certified by the Japan Gastroenterological Endoscopy Society revealed that only 24.5% (12/49) of MT gastric cancers showed an endoscopic finding of a UDT component within the area where the UDT component existed histopathologically. Taking the results of pretreatment biopsy into account, only 40% (20/49) of MT early gastric cancers in this study showed preoperative findings suggestive of a UDT component. These results indicate that the accurate pretreatment diagnosis of a UDT component is difficult even when the pretreatment biopsy and ME-NBI are combined.
One reason why pretreatment recognition of a UDT component in MT early gastric cancers using ME-NBI is difficult is that histopathologically, a clear distinction between DT and UDT components within a lesion could not be drawn in 65% (32/49) of cases where the DT and UDT components were mixed at a glandular structure level such as tub2+por. Thus, they did not show an endoscopic finding of the UDT component, such as a corkscrew pattern. A second reason is that even though there was an area where the UDT component occupied the whole mucosal layer or was exposed to the mucosal surface histopathologically, it could not be recognized endoscopically using ME-NBI when the area was not large enough. In other words, an area with a certain amount of UDT component may be necessary for the endoscopic finding to appear. This might be why histopathological UDT predominance was the only significant factor associated with an ME-NBI finding of a UDT component in this study (P= 0.0009). Therefore, the possibility of UDT predominance should be suspected when a lesion shows an endoscopic finding of the UDT component. Third, MT gastric cancers with submucosal invasion were less likely to show an ME-NBI finding of a UDT component compared to mucosal cancers, although the difference was not statistically significant. In lesions with submucosal invasion, microstructure and microvascular patterns were occasionally destructed, making the ME-NBI finding hard to interpret. Moreover, some lesions harbor a UDT component only in the submucosa. Previous studies have shown that MT gastric cancers tend to be glandular in the mucosa and then grow diffusely in the deeper layers[28].
Few reports have dealt with the pretreatment diagnosis of MT early gastric cancer. Horiuchiet al[29]reported that accuracy in diagnosing the predominant histological type in MT early gastric cancers was 177/192 (92.2%) when using a combination of biopsy and ME-NBI. A possible explanation for the discrepancy between previous results and our finding is that we focused on the preoperative recognition of the UDT component within MT early gastric cancers regardless of the UDT component amount,while the previous study distinguished the predominant histological type of MT early gastric cancers. It is considered more difficult to preoperatively detect a UDT component when the UDT component amount is small or when the UDT and DT components are mixed at a glandular level. Given that the presence of a UDT component adversely affects the treatment outcome of even DT-predominant MT early gastric cancers[9-11,27], we need to pay attention to even minuscule amounts of UDT component. To the best of our knowledge, the present study is the first to address the feasibility of preoperative recognition of a UDT component within early gastric cancers, regardless of histological predominance. By investigating the comparative relationships between the histopathological and endoscopic findings, the present study demonstrated the difficulty in preoperative recognition of a UDT component in MT early gastric cancers. Our results suggest that endoscopic resection plays a significant role not only in treatment but also in diagnosis.
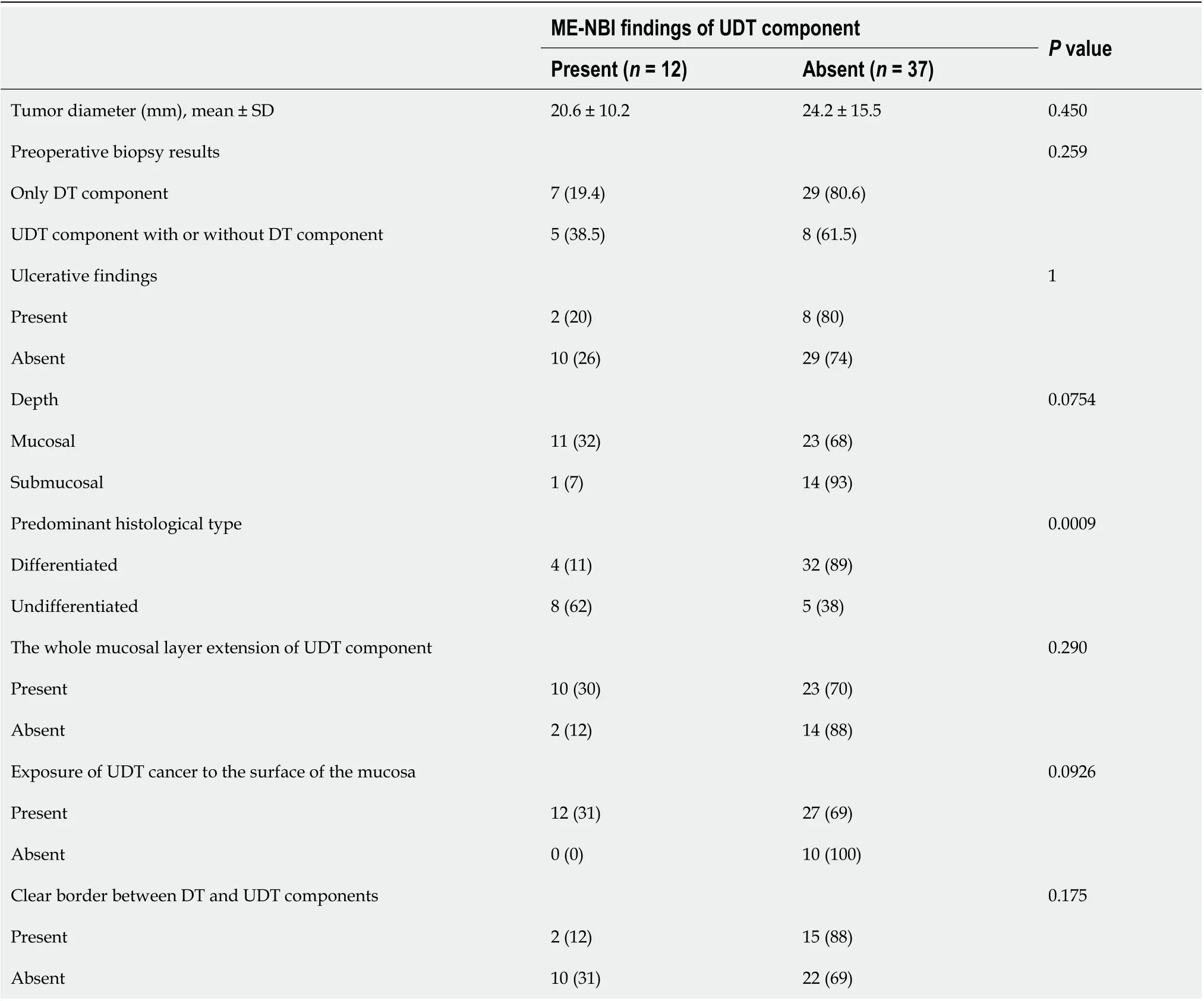
Table 3 Comparison between magnifying endoscopy with narrow-band imaging and histopathological findings
Our study has several limitations. First, there was an inherent selection bias because this was a single-center retrospective study. Notably, we excluded early gastric cancers that had not undergone preoperative ME-NBI of the whole lesion and that had undergone gastrectomy instead of ESD as the first therapy. Thus, this study may have eliminated the MT early gastric cancers that had been easily diagnosed with a predominant UDT component, which, in turn, could have lowered the preoperative diagnosis rate of UDT components. However, we believe that the results of this study accurately reflect actual clinical practice, given that the proportion of UDTpredominant MT gastric cancers in this study was similar to that of previous studies[11,29]. Second, although we investigated 2011 consecutive early gastric cancers in 1558 patients treated with ESD over a decade in a tertiary referral center, the sample size was small and may have been insufficient. This was mainly a result of the rarity of MT early gastric cancer. Multicenter studies with larger samples are needed. Finally, we retrospectively reviewed the stored preoperative endoscopic images knowing that the final diagnoses were MT gastric cancers, which may have influenced the diagnosis rate of the UDT component. Thus, the accurate pretreatment diagnosis rate of a UDT component using ME-NBI in actual clinical practice is likely to be lower than that seen in this study.
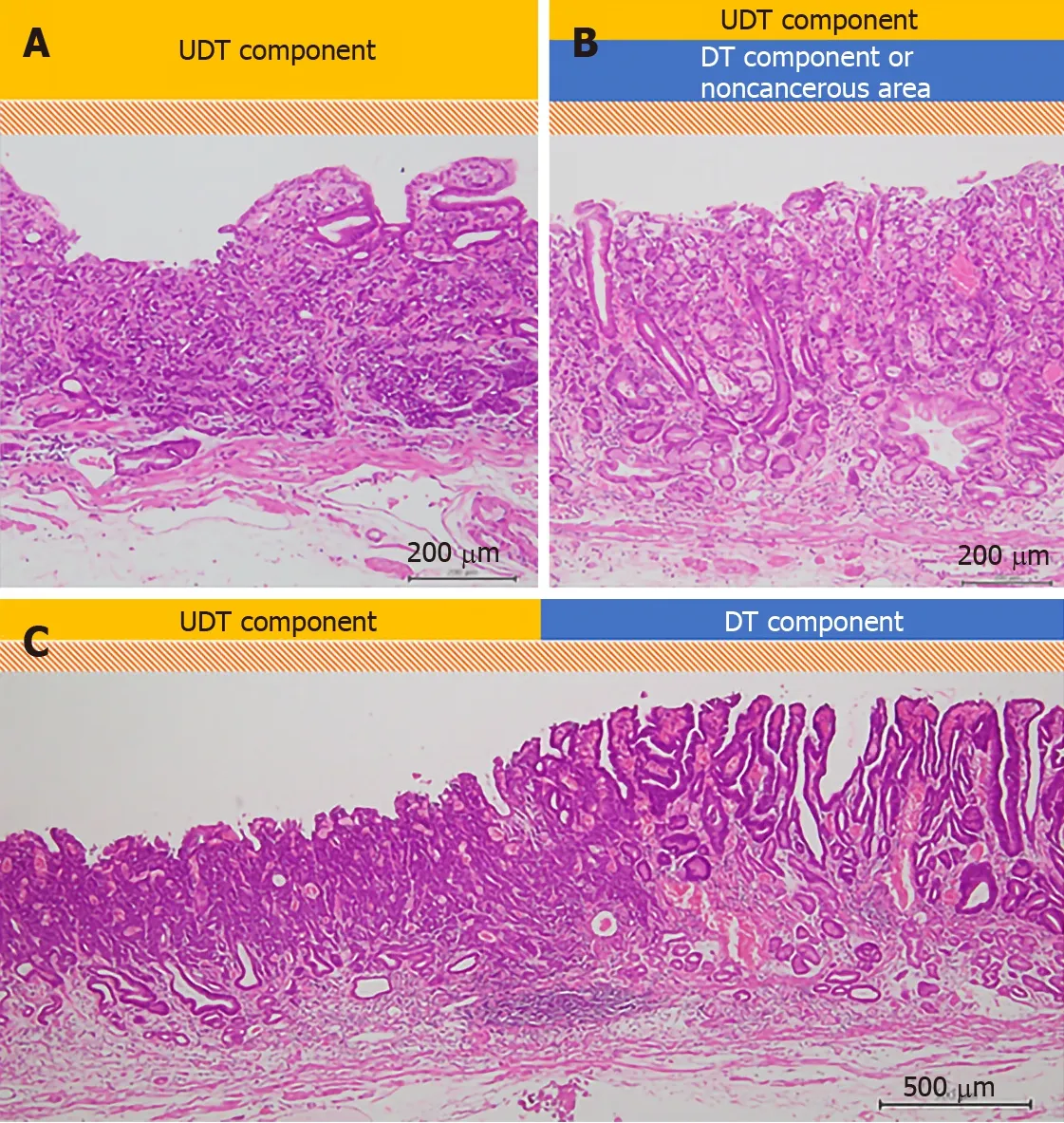
Figure 2 Evaluation of histopathological examination (hematoxylin-eosin staining). A schema of the three histopathological findings investigated in our study. The scale bars show 200 μm in (A) and (B), and 500 μm in (C). A: The whole mucosal layer occupation of undifferentiated-type (UDT) component (100 ×); B: Exposure of UDT component to the mucosal surface (100 ×); C: Clear border between differentiated-type and UDT components (40 ×). DT: Differentiated-type; UDT: Undifferentiated-type.
CONCLUSION
The present study is the first to address the feasibility of preoperative UDT component recognition in early gastric cancers regardless of histological predominance. Accurate pretreatment diagnosis of a UDT component within MT early gastric cancers is difficult even when pretreatment biopsy and ME-NBI are combined. Considering our results, endoscopic resection plays a significant role in both treatment and diagnosis.
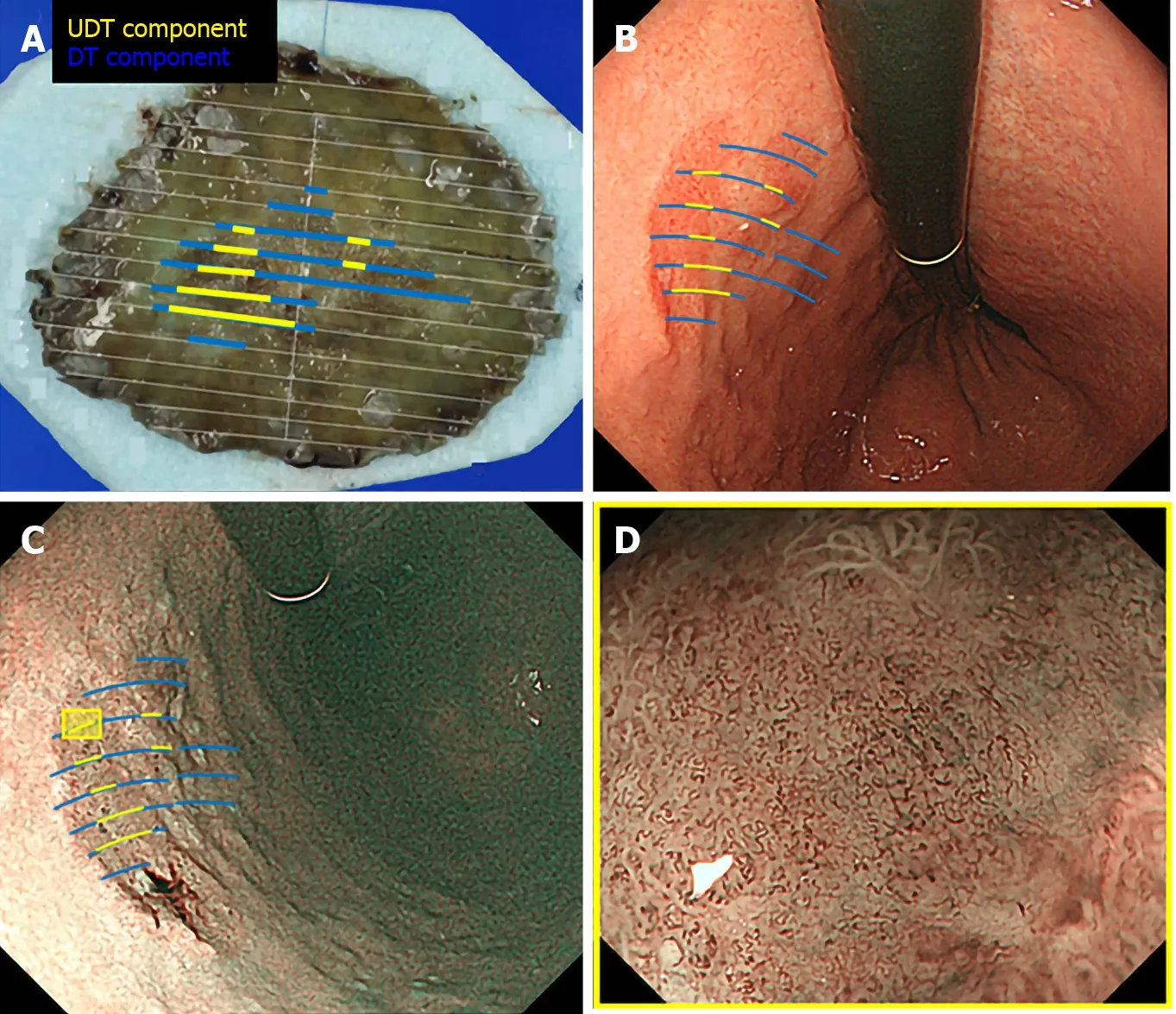
Figure 3 Correlation between pathology and endoscopic findings. Preoperative endoscopic images were reviewed to determine whether the endoscopic finding of undifferentiated-type (UDT) component was recognizable within the area where UDT component was present in the histopathological examination. A: Endoscopically resected specimen cut into 2-mm-thick slices with mapping according to the histological types. Blue lines indicate differentiated-type component, while yellow lines indicate UDT component; B: Endoscopic images upon white light imaging onto which the histological type is mapped; C: Endoscopic images upon narrow-band imaging onto which the histological type is mapped; D: Magnifying endoscopy with narrow-band imaging finding of the designated UDT area with the yellow square in Figure 3C. ME-NBI: Magnifying endoscopy with narrow-band imaging; DT: Differentiated-type; UDT: Undifferentiated-type.
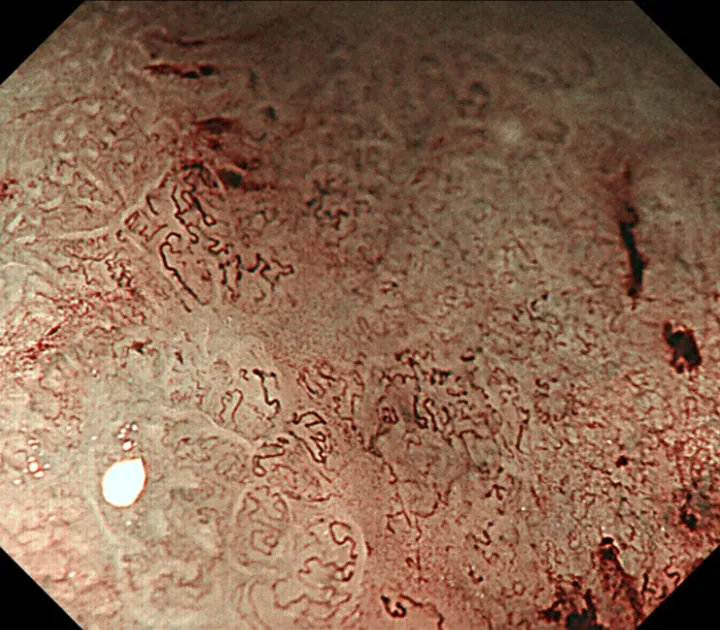
Figure 4 A representative magnifying endoscopy with narrow-band imaging image of the undifferentiated-type component. Magnifying endoscopy with narrow-band imaging finding of undifferentiated-type. Component was defined as satisfying both a microvascular pattern, which has an isolated and disordered quality and a microstructure pattern, which lacks visibility or even disappears. It complied with the finding described as a corkscrew pattern.
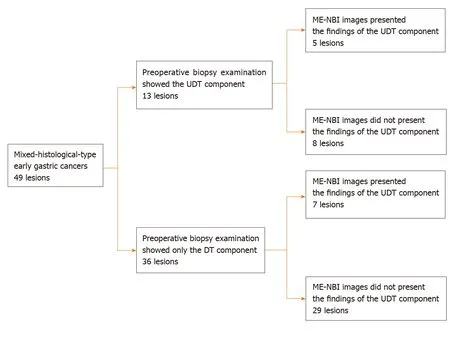
Figure 5 Diagnostic accuracy of pretreatment biopsy examination for undifferentiated-type-component and possible additional effect of magnifying endoscopy with narrow-band imaging. DT: Differentiated-type; UDT: Undifferentiated-type; ME-NBI: Magnifying endoscopy with narrow-band imaging.
ARTICLE HIGHLIGHTS
Research background
Endoscopic resection has become a mainstay therapy for early gastric cancers (EGCs). EGCs with very low risk of lymph node metastasis are indications for endoscopic resection. The risk of lymph node metastasis markedly differs according to the histological types.
Research motivation
Although the undifferentiated-type (UDT) component adversely affects the clinical course of EGCs, whether the UDT component within histological-mixed-type (MT) EGCs can be recognized preoperatively is not known. This is particularly important because accurate pretreatment diagnosis of the UDT component can help make the right treatment decision for EGC.
Research objectives
We investigated the feasibility of the preoperative diagnosis of the UDT component within MT EGCs.
Research methods
This is a retrospective study using clinical data and samples from patients with EGCs treated by endoscopic submucosal dissection. Through histopathological examination, we investigated each lesion’s UDT component: (1) Whole mucosal layer occupation; (2) Exposure to the mucosal surface; and (3) Existence of a clear border between the differentiated-type and UDT components. We examined endoscopic images using magnifying endoscopy with narrow-band imaging to identify whether the endoscopic UDT component finding was recognizable within the area where it was present in the histopathological examination. The preoperative biopsy results and comparative relationships between endoscopic and histopathological findings were also examined.
Research results
Whole mucosal layer occupation of the UDT component and exposure of the UDT component to the mucosal surface were observed in 67.3% and 79.6% of samples, respectively. However, the preoperative endoscopic images showed that only 24.5% of MT EGCs revealed the UDT component within the area where it was present histopathologically. Histopathological UDT predominance was the single significant factor associated with the presence of the endoscopic UDT component finding (61.5%vs11.1%,P= 0.0009). Combined results of the pretreatment biopsy and magnifying endoscopy with narrow-band imaging showed the preoperative presence of the UDT component in 40% of MT EGCs.
Research conclusions
Accurate pretreatment diagnosis of the UDT component was hardly achieved even when pretreatment biopsy and diagnostic endoscopy were combined. However, the possibility of UDT predominance should be suspected when a lesion shows an endoscopic finding of the undifferentiated-type component.
Research perspectives
Currently, endoscopic resection plays a significant role in both the diagnosis and treatment of MT EGCs. Future studies should seek novel UDT findings to distinguish MT EGCs from pure differentiated-type EGCs.
ACKNOWLEDGEMENTS
We thank all the members of the Department of Pathology at Yokohama City University Medical Center for their devotion to the work and excellent evaluation of the samples.
杂志排行
World Journal of Gastroenterology的其它文章
- Pediatric non-alcoholic fatty liver disease and kidney function: Effect of HSD17B13 variant
- Major gastrointestinal bleeding and antithrombotics: Characteristics and management
- Artificial intelligence in gastric cancer: Application and future perspectives
- Treatment of eosinophlic esophagitis with swallowed topical corticosteroids
- Granulocyte-macrophage colony-stimulating factor protects mice against hepatocellular carcinoma by ameliorating intestinal dysbiosis and attenuating inflammation
- SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma mimicking solid pseudopapillary neoplasm: A case report and review of the literature
