Artificial intelligence in gastric cancer: Application and future perspectives
2020-10-22PengHuiNiuLuLuZhaoHongLiangWuDongBingZhaoYingTaiChen
Peng-Hui Niu, Lu-Lu Zhao, Hong-Liang Wu, Dong-Bing Zhao, Ying-Tai Chen
Abstract Gastric cancer is the fourth leading cause of cancer-related mortality across the globe, with a 5-year survival rate of less than 40%. In recent years, several applications of artificial intelligence (AI) have emerged in the gastric cancer field based on its efficient computational power and learning capacities, such as imagebased diagnosis and prognosis prediction. AI-assisted diagnosis includes pathology, endoscopy, and computerized tomography, while researchers in the prognosis circle focus on recurrence, metastasis, and survival prediction. In this review, a comprehensive literature search was performed on articles published up to April 2020 from the databases of PubMed, Embase, Web of Science, and the Cochrane Library. Thereby the current status of AI-applications was systematically summarized in gastric cancer. Moreover, future directions that target this field were also analyzed to overcome the risk of overfitting AI models and enhance their accuracy as well as the applicability in clinical practice.
Key Words: Gastric cancer; Image-based diagnosis; Prognosis prediction; Artificial intelligence; Machine learning; Deep learning
INTRODUCTION
Gastric cancer has long been believed to be an aggressive malignancy with a 5-year survival rate of less than 40%[1]. Despite the decrease in incidence and mortality over the past few decades in some countries, gastric cancer is still the sixth most common malignancy and remains the fourth leading cause of cancer-related deaths across the globe[2-4]. Due to the early atypical symptoms of gastric cancer and its advanced aggressive behaviors, reducing recurrence and prolonging survival are increasingly dependent on advanced screening, diagnosis, treatment, prognosis prediction, and other new technologies. Artificial intelligence (AI), with its efficient computational power and learning capacity, has caught considerable attention in the field of gastric cancer.
Contrary to human intelligence, AI is the intelligence displayed by machines, which first emerged in 1956. The term “artificial intelligence” was commonly used to describe machines (or computers) that imitate human “cognitive” functions (e.g., learning and problem solving) related to human thinking[5]. As a subset of AI, machine learning (ML) can be defined as the computer algorithms that can automatically improve through experience[6]. Based on training data, the learner utilizes ML algorithms to build models with which the predictions or decisions can be made without explicit programming. In the last few years, ML algorithms, including random forest and support vector machines (SVM), were applied in various domains, especially in medicine. As of 2020, in the field of ML, deep learning (DL) had become the primary approach adopted in much ongoing work. DL is a type of ML algorithm that uses multiple layers to extract higher-level features from the original input gradually. Briefly, ML is a significant branch of AI, and DL is performed to implement ML. Recent developments of efficient hardware and computational power led to several AI models emerging in the field of gastric cancer[7-43]. AI-assisted diagnosis mainly included pathology, endoscopy, and computed tomography (CT)[7-35], while related researchers in prognosis focused on recurrence, metastasis, and survival prediction[36-43].
In this review, we searched the relevant works published up to April 2020 from the databases of PubMed, Embase, Web of Science, and the Cochrane Library, thus comprehensively summarizing the current status of AI-applications in gastric cancer. In addition, challenges and future directions that target the field were also discussed to improve the accuracy and applicability of AI-models in clinical practice.
AI IN THE DIAGNOSIS OF GASTRIC CANCER
Gastric cancer was mostly diagnosed at advanced stages because of their latent and nonspecific symptoms, which led to poor prognosis. It was reported that early accurate detection of gastric cancer could increase the overwhelming 5-year survival rate by approximately 90%[44,45]. However, an early gastric cancer diagnosis was mainly limited to the number of experienced imaging experts. Furthermore, the diagnostic accuracy largely depended on the clinical experience of experts and was vulnerable to multiple factors. It is impossible for qualified experts to avoid all misdiagnoses and missed diagnoses. AI methods, which imitated human cognitive functionviaa computer, were adept at processing and analyzing large amounts of data and thus could assist gastroenterologists in clinical diagnosis and decision making. To date, AI has been applied in many medical imaging fields, such as endoscopy, pathology as well as CT imaging. AI-assisted endoscopic diagnosis included the extraction of image features[7,8], the detection of early gastric cancer[9-14], the detection of precancerous conditions[15], the optimization of magnifying endoscopy with narrow-band imaging (M-NBI)[16-19]and the application of Raman endoscopy[20,21]. AI-assisted pathologic diagnosis involved the automatic identification of gastric cancer[22], the detection of gastric cancer based on the whole slide imaging (WSI)[23-26], the automatic detection of tumor-infiltrating lymphocytes (TILs)[27]and the segmentation of lesion regions[28-31], while AI-assisted CT diagnosis focused on the identification of preoperative peritoneal metastasis[32], the detection of perigastric metastatic lymph nodes[33]and two other new imaging techniques[34,35]. Under certain conditions, the diagnostic performance of these AI models was not inferior to human experts.
AI-assisted diagnosis in endoscopy
Endoscopy has played an essential role in the detection of gastric cancer because it enables endoscopists to observe cancerous sites directly. Accurate diagnosis of early gastric cancer by using endoscopic images is an urgent need to improve the patient’s poor prognosis. However, recent studies showed that the detection accuracy of conventional endoscopy only ranged from 69% to 79%[46]. Due to the heavy workload of medical image analysis, it was inevitable for experienced endoscopists to run into misdiagnosis and missed diagnosis. Recent efforts in endoscopy focused on adopting AI techniques to enhance the inspection and diagnosis of gastric cancer (summarized in Table 1).
The key to obtaining high detection accuracy lies in the extraction of discriminative features, which can significantly distinguish the lesion images from standard images. Liuet al[7]designed an algorithm of ML, which was called joint diagonalization principal component analysis for the dimension reduction of endoscopic images. Then, a novel AI-assisted method was presented to detect early images of gastric cancer by combining joint diagonalization principal component analysis and conventional algorithms without learning, which revealed a better performance than traditional related methods. Aliet al[8]presented a novel texture extraction method named Gaborbased gray level co-occurrence matrix to detect the abnormal frames from the whole chromoendoscopy sequence. Then, the authors combined the SVM classifier and Gabor-based gray level co-occurrence matrix texture features to screen for early gastric cancer. The detection accuracy, specificity, sensitivity and the area under the curve (AUC) value were 87%, 82%, 91% and 0.91, respectively, which were higher than those results obtained by the SVM classifier combined with other texture extraction methods.
A study conducted by Luoet al[9]constructed the Gastrointestinal Artificial Intelligence Diagnostic System to detect upper gastrointestinal cancer in real time automatically. They used 1036496 endoscopy images with standard white light from 84424 cases across China for training and testing. In the different large-scale validation and prospective sets, the diagnostic accuracy was satisfactory as it ranged from 0.915 to 0.977. Moreover, the experimental results demonstrated that the Gastrointestinal Artificial Intelligence Diagnostic System attained sensitivity comparable to that of the human experts (0.942vs0.945). Sakaiet al[10]introduced a convolutional neural network (CNN)-based automatic detection model with high accuracy and believed that the model could enhance the diagnostic capabilities of endoscopists. Yoonet al[11]adopted a lesion-based model for the accurate detection and depth prediction of early gastric cancer and evaluated the significant factors associated with AI-assisted diagnosis.
Nakahiraet al[12]described that the analysis system of AI-assisted endoscopic images could effectively stratify the risk of gastric cancer and further evaluated the consistency of the AI model with the consensus diagnoses of three endoscopists. Zhuet al[13]also constructed a CNN computer-aided detection system to determine the invasion depth of early gastric cancer. Their proposed model achieved substantially higher specificity and accuracy compared to endoscopists with an AUC of 0.94. Another study suggested a multicolumn CNN to improve gastric cancer screening[14]. The novelty of their method lies in combining electronic gastroscopy with the analysis tools of cloud-based endoscopic images. Experimental results revealed that the proposed multicolumn CNN method dramatically outperformed the other CNN models (including AlexNet[47], GoogLeNet[48]and VGGNet[49]) and non-DL methods (including kNN[50]and SVM-based[51]classifiers).
Chronic atrophic gastritis is a common precancerous gastric condition that might lead to the appearance and development of gastric cancer[52]. Conventional endoscopy had high variability among different endoscopists for distinguishing of precancerousconditions. Therefore, Guimarãeset al[15]developed and trained a DL approach by using 200 real-world endoscopic images to diagnose atrophic gastritis. The DL model achieved a diagnostic accuracy of 93% and an AUC of 0.98, outperforming the combined results of expert endoscopists.
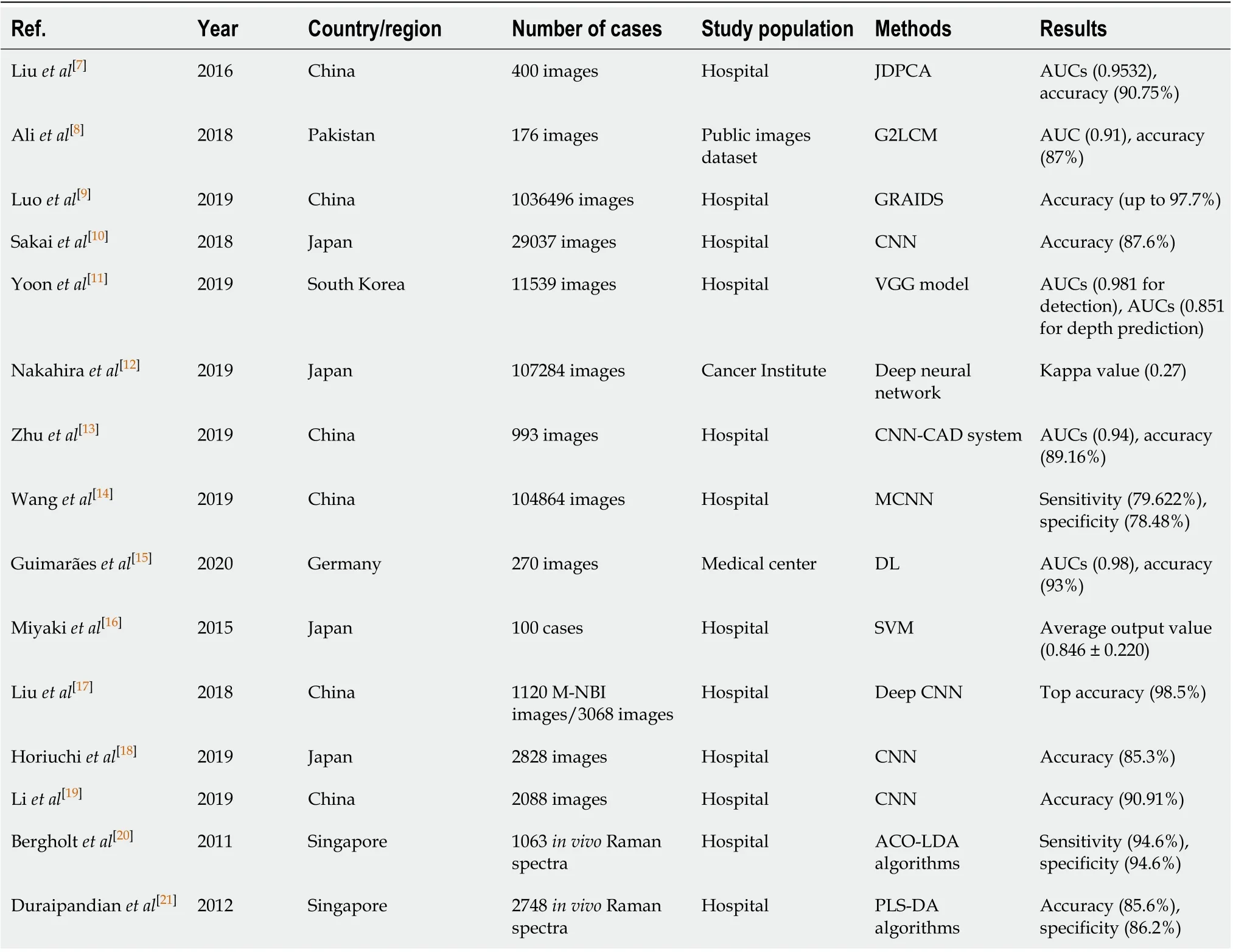
Table 1 Applications of artificial intelligence in endoscopy based on different study population
Given the rapid advances of narrow-band imaging, magnifying endoscopy with MNBI had been given great attention to the diagnosis in early gastric cancer, which showed a more conspicuous accuracy than that by general white light imaging[53]. However, interobserver variability was a limitation for the diagnosis of the lesions using M-NBI, and it was difficult for endoscopists to master the diagnostic technology in a short time[54]. Given that the advance of AI may offer such a solution, several studies related to AI-assisted image diagnosis have emerged in recent years. Miyakiet al[16]developed the SVM system to quantitatively identify gastric cancer based on magnifying endoscopy with blue-laser imagery. Liuet al[17]first applied transfer learning of fine-tuning deep CNN features to classify the gastric mucosal lesions of MNBI images. Horiuchiet al[18]and Liet al[19]adopted a CNN system to boost the capability to distinguish early gastric cancer from noncancerous lesions efficiently and obtained excellent diagnostic performances. Their results suggested that the diagnostic sensitivity of the CNN model with M-NBI was superior compared to that of endoscopists.
Furthermore, a previous report suggested that M-NBI was still inadequate to effectively and accurately diagnose grossly invisible lesions due to the lack of sufficient biochemical information[55]. Raman spectroscopy, as a novel point-wise spectroscopic technique, could comprehensively display the surface and subsurface cellular structures from diagnostic tissue. It was suggested that Raman endoscopy had the promising potential for the diagnosis of early gastric cancer. Bergholtet al[20]first combined the real-time Raman endoscopy with AI-based algorithms to distinguish neoplastic and normal gastric tissues. Later, another study devised an automated Raman spectroscopy diagnostic framework named PLS-DA algorithms to detect gastric cancer with a diagnostic accuracy of 85.6%[21].
AI-assisted diagnosis in pathology
The traditional diagnosis of gastric cancers was to identify morphological features of the malignant cells by using histopathological biopsy specimens, and manual pathological inspection of gastric slices was time-consuming and laborious. The need for automatic image analysis and histological classifications of gastric cancer has been increasing. Liet al[22]proposed a novel DL-based framework, called GastricNet, to automatically identify gastric cancers. The classification accuracy of the proposed framework was 100% on gastric pathological slices, which was substantially higher than other existing networks, including DenseNet[56]and ResNet[57].
In addition, the WSI, as a virtual counterpart of glass slides[58], was considered to be comparable to optical microscopy for the diagnosis of gastric cancers. The advances of WSI led to the emergence of several AI applications in pathological diagnosis. Sharmaet al[23]described that the CNN architecture could efficiently analyze pathological images with an accuracy of 0.6990 for cancer classification and 0.8144 for necrosis detection. Leonet al[24]assessed the application of deep CNN in the automatic detection of gastric cancer pathological images. Two approaches based on deep CNN were presented: One was performed to analyze the morphological features from the whole images, while the other independently investigated the local characteristic properties. Experiment results showed an average accuracy of up to 89.72%, which demonstrated the excellent performance of the proposed model in the detection of gastric cancers. Iizukaet al[25]trained CNNs and recurrent neural networks to distinguish stomach adenocarcinoma, adenoma and non-neoplastic. On three independent test sets of biopsy histopathology WSI, DL applications achieved AUCs up to 0.97 for the classification of gastric adenocarcinoma. Yoshidaet al[26]compared the classification results of experienced pathologists with that of the e-Pathologist constructed by NEC Corporation. Although the overall concordance rate between the two methods was only 55.6% (1702/3062), the concordance rate for the negative biopsy specimens was as high as 90.6% (1033/1140). Furthermore, current evidence revealed that TILs were associated with the prognosis of gastric cancer[59]. A related study presented the CNN model to automatically detect TILs on histopathological WSI with an acceptable accuracy of 96.88%[27].
The automatic segmentation of lesion regions was a challenge in the AI-assisted pathological diagnosis of gastric cancer. To alleviate the shortage of well-annotated pathology image data, Lianget al[28]firstly applied the DL method to segment the pathological images. They presented a new neural network architecture and algorithm named as an overlapped region forecast for the detection of gastric cancers. An intersection over union coefficient (IoU) of 88.3% and 91.1% accuracy indicated that the model had reached the standard of supervised learning. Quet al[29]presented a novel type of intermediate dataset and developed a stepwise fine-tuning-based scheme to improve the classification performance of deep neural networks. Sunet al[30]also demonstrated that the proposed DL model was a powerful image segmentation tool with 91.60% for the mean accuracy and 82.65% for the mean IoU. Another study also demonstrated that the Mask R-CNN model was an effective method to target the field of medical image segmentation[31].
Hence, these satisfactory results of AI-assisted applications highlighted the enormous potential benefit to help the pathologists and support the pathological detection of gastric cancer, especially for improving the efficiency of image segmentation and reducing the diagnostic time (summarized in Table 2).
AI-assisted diagnosis in CT imaging
Attributable to the noninvasiveness and convenience, CT was widely used for the clinical diagnosis of gastric cancer[60,61]. However, the diagnostic accuracy was mainly dependent on the clinical experience of radiologists. When interpreting large amounts of CT images, the radiologist’s diagnostic accuracy would inevitably decrease, and errors were more prone to occur. Several approaches based on ML and DL reported that they could effectively extract valuable information on CT images (summarized in Table 3). For example, Huanget al[32]applied the DL method on diagnostic analysis and created a deep CNN model to identify the preoperative peritoneal metastasis in advanced gastric cancer.

Table 2 Applications of artificial intelligence in pathology and computerized tomography based on different study population

Table 3 Applications of artificial intelligence in computerized tomography based on different study population
Compared to the current status of poor CT depiction in lymph node metastasis and low detection sensitivity, the novel DL-based model was expected to obtain an excellent performance of CT imaging. Gaoet al[33]developed and validated faster region-based CNN based on CT images. The experimental results showed that faster region-based CNN obtained a high accuracy for the diagnosis of perigastric metastatic lymph nodes with the mean average precision value of 0.7801 and AUC value of 0.9541.
Moreover, dual-energy spectral CT (DEsCT), as a new imaging technique, could easily switch between high-energy and low-energy datasets, which enabled the precise creation of virtual images based on monochromatic spectra. The recent improvement of DEsCT made it available for routine clinical practice. However, it was difficult for radiologists to take full advantage of more quantitative data obtained by the DEsCT system. A recent study introduced the AI-assisted utility of DEsCT imaging for the stages and characteristics of gastric cancer[34]. The authors used a new multiple instance learning method to determine the invasion depth of gastric cancer and achieved a gross accuracy of 0.7692 after optimization. Also, it was reported that gemstone spectral imaging could provide more valuable image information to radiologists than conventional CT[62]. Liet al[35]proposed the ML-based gemstone spectral imaging analysis for lymph node metastasis in gastric cancer, which achieved a higher accuracy of detection. The feasibility and the effectiveness of gemstone spectral imaging-CT to diagnose lymph node metastasis outperformed traditional detection methods, such as endoscopic ultrasound and the multidetector-row CT.
AI IN PROGNOSIS PREDICTION OF GASTRIC CANCER
Accurate prognosis prediction of gastric cancer was of significance for both clinicians and patients. Such information could assist clinicians in decision-making and improve management over patients. It was appreciated that the demographics, pathological indicators, physiological states and even social contacts had an impact on the prognosis of gastric cancer patients. However, conventional statistical methods, such as the tumor–node–metastasis staging system and nomogram, could hardly analyze the complicated internal connections among these characteristics. Based on its excellent computational power and integration capability, AI models had been applied to improve the survival rates of gastric cancer patients.
In the last few years, the applications of AI in prognosis involved the predictions of survival time[36-38], recurrence risk[39,40]and metastasis[41-43](summarized in Table 4). Jianget al[36]applied SVM to survival analysis and developed a prognostic classifier. The results showed a higher predictive accuracy of overall survival and disease-free survival than the tumor–node–metastasis staging system defined by the American Joint Committee on Cancer. Besides, the proposed gastric cancer-SVM classifier was also used to predict adjuvant chemotherapeutic benefit, which was able to facilitate the individualized treatment for gastric cancer. Combining demographics, pathological indicators and physiological characteristics of 939 cases, Luet al[37]created a novel multimodal hypergraph learning framework to improve the accuracy of survival prediction. The result showed that the proposed approach outperformed random forest and SVM in overall survival prediction. Another study compared the value of artificial neural network and Bayesian neural networks (BNN) in survival prediction of gastric cancer patients, and the findings indicated BNN was superior to the artificial neural network method[38].
Recurrence was one of the leading causes of death for gastric cancer patients[63], thereby the accurate evaluation of recurrence risk was relevant in routine clinical work. Recent reports indicated that the AI-assisted recurrence prediction system achieved better performances than traditional statistical methods. Zhanget al[39]used ML methods to extract radiomic signatures from CT images of 669 consecutive patients diagnosed with advanced gastric cancer. Then they constructed a CT-based radiomic model to predict the recurrent risk of advanced gastric cancer. Liuet al[40]trained the SVM classifier to predict the recurrence in patients with gastric cancer. Using the gene expression profiling dataset GSE26253[64], they discovered that a set of feature genes (includingPLCG1, PRKACAandTGFBR1) potentially correlated with gastric cancer recurrence.
Lymph node metastasis was a significant prognostic indicator for gastric cancer[65]. The lack of accurate methods to predict gastric cancer metastasis has led to the application of AI-assisted prediction techniques to evaluate the metastasis risk better. Bollschweileret al[41]demonstrated that artificial neural networks could broadly enhance the predictive accuracy of lymph node metastasis. Hensleret al[42]proposed a novel artificial neural network approach for the preoperative prediction of lymph node metastasis. Compared with the Maruyama Diagnostic System developed at the National Cancer Center in Tokyo, the proposed model showed higher accuracy and better reliability. Also, it was shown that liver metastases could severely diminish the long-term survival of gastric cancer patients. Jagricet al[43]presented a learning vector quantization networks to predict postoperative liver metastasis in patients suffering from gastric cancer and obtained a reasonably high predictive value.
CHALLENGES AND FUTURE PERSPECTIVES
Despite the reported great success of AI in medical image-based diagnosis and prognosis prediction, several barriers must be removed before widespread clinical practice occurs.
A flexible AI model requires a large amount of well-annotated data for training, validating and testing, while the related research with small sample sizes is prone to have measurement errors[66]. With advances in medical-based imaging, such as endoscopy and pathology, numerous data is generated continuously to help physicians in clinical diagnosis and decision making. However, such data are rarelylabeled or annotated, which are not suitable for algorithm training. Hence, the availability of high-quality data is a significant challenge for the development and optimization of AI. A meaningful way to access these qualified data sets is to establish large-scale open-access databases. Moreover, existing data resources should also be utilized effectively. Single hospitals and institutions are encouraged to share validated data to improve the applicability of AI in gastric cancer, which is similar to previous research related to Alzheimer’s disease[67].
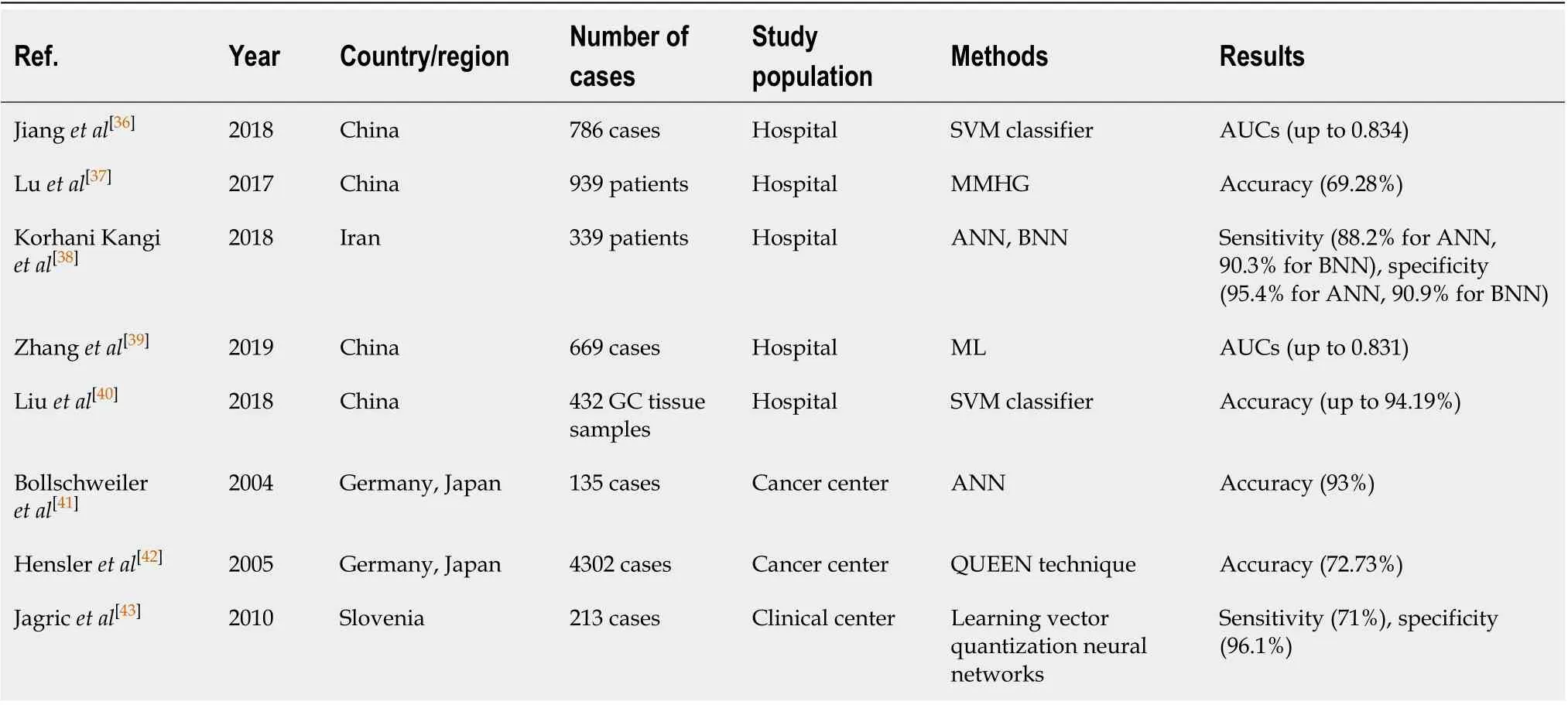
Table 4 Applications of artificial intelligence in gastric cancer prognosis based on different study population
An additional hurdle to the improvement of robust algorithms for gastric cancer is the interpretability of AI. In some studies, the applications of ML and DL revealed higher sensitivity and fewer false positives than radiologists[68,69]. However, they also inevitably ran into the risk of overfitting, leading to a tradeoff between accuracy and interpretability. In addition, the “black box” feature of algorithms may cause clinician’s suspicion of ML applications. Cabitzaet al[70]offered that the “black box” of ML may bring the unintended negative consequences in clinical practice. Fortunately, recent advances in data visualization tools deepened the visual understanding of algorithm decision making[71]thus contributing to the promotion of the optimization algorithms and widespread clinical acceptance.
Given its advantages of computational power and learning capacity, AI will appear in various gastric cancer fields. Increasingly, it is appreciated that the characteristics of diseases, the physiological, psychological states of patients and even social communication have an impact on the prognosis of gastric cancer patients. It is difficult for physicians to integrate complex data manually. An AI model is adept at integrating much information from the vast majority of data, which has the potential to reduce the workload of clinicians substantially. However, due to some ethical and safety issues, the predictions that are generated by AI require further evaluation and interpretation by professional physicians. Thereby, AI techniques will not wholly replace physicians in future clinical practice and combining human beings with AI can achieve the ideal state of higher efficiency.
CONCLUSION
AI techniques, especially ML and DL, are making remarkable progress in the field of gastric cancer. The current status and future perspectives of AI-assisted diagnosis and prognosis were comprehensively introduced in this review. Numerous related researchers reported the impressive performance of AI, which was superior to the standard statistical methods. Despite several limitations and hurdles that exist in AI, such as the lack of well-annotated data and the interpretability of models, based on its efficient computational power and learning competence, AI will revolutionize the diagnosis and prognosis of gastric cancer in the foreseeable future.
杂志排行
World Journal of Gastroenterology的其它文章
- Pediatric non-alcoholic fatty liver disease and kidney function: Effect of HSD17B13 variant
- Major gastrointestinal bleeding and antithrombotics: Characteristics and management
- Treatment of eosinophlic esophagitis with swallowed topical corticosteroids
- Granulocyte-macrophage colony-stimulating factor protects mice against hepatocellular carcinoma by ameliorating intestinal dysbiosis and attenuating inflammation
- SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma mimicking solid pseudopapillary neoplasm: A case report and review of the literature
- Solitary peritoneal metastasis of gastrointestinal stromal tumor: A case report
