Influence of sulfur on inclusion and pitting resistance of 316L stainless steel
2020-10-13
1)New Material Industry Innovation Center,China Baowu Steel Group Co.,Ltd.,Shanghai 201999,China;2)Research Institute,Baoshan Iron & Steel Co.,Ltd.,Shanghai 201999,China
Abstract: The effect of the sulfur content on the microstructure and inclusions of 316L stainless steel was studied by optical microscopy,scanning electron microscopy,and image analysis,and the effect of sulfur on the pitting corrosion resistance of 316L stainless steel was studied by conducting ferric chloride immersion test and plotting the electrochemical polarization curves.The results show that the added sulfur is mainly in the form of manganese sulfide inclusions in 316L stainless steel.With increases in the sulfur content,the grade and percentage of the sulfide in the steel gradually increased,and its distribution became increasingly dense.When the sulfur content exceeded 0.1%,the number of sulfide inclusions in the sample increased sharply.When the sulfur content reached 0.199%,the sulfides in the steel were primarily in spindle form,and a large number of spindles were found to refine the grain size of 316L stainless steel.The pitting corrosion weight loss rate of 316L stainless steel increased with increases in the sulfur content,while the pitting potential gradually decreased.However,the pitting potential of 316L stainless steel rebounded when the sulfur content reached 0.199%,which may be related to the grain refinement of the test steel and requires further study.
Key words: stainless steel; manganese sulfide; pitting corrosion resistance; pitting potential
1 Introduction
With the ongoing demand for social and economic advances,the mass production of free-cutting stainless steel has rapidly developed and is widely used.Adding appropriate amounts of sulfur to stainless steel can improve its cutting performance,reduce the surface roughness of a workpiece,increase wear resistance,extend the tool service life,and improve production efficiency.However,the addition of sulfur also brings some adverse effects.Sulfide inclusions easily stretch into strips during the hot-working process,which results in anisotropic plasticity and toughness and deterioration of the mechanical properties of the steel.Sulfide inclusions also reduce the corrosion resistance,especially the pitting corrosion resistance[1-2].In this paper,austenitic stainless steel 316LB with different sulfur contents was designed to study the effect of the sulfur content on the microstructure,inclusions,and pitting corrosion resistance of the stainless steel.
2 Materials and experiments
2.1 Materials
The materials used in this test included a 316L hot-rolled sheet and four 316LB austenitic stainless steel samples with different sulfur contents,which were recorded as samples 0#,1#,2#,3#,and 4#,respectively.The chemical composition of the steel samples is listed in Table 1.With the exception of sample 0#,the test materials were smelted in a 150-kg vacuum induction furnace protected by argon gas.They were cast into round ingots,hot forged into 40-mm-thick intermediate billets,and then rolled into 4-mm-thick hot-rolled plates by a four-roll mill.All the hot-rolled plates were subjected to solution treatment at 1 050 ℃ for 8 min and then water-cooled to room temperature.

Table 1 Chemical composition of the test steels %
2.2 Test methods
2.2.1 Metallographic examination
After grinding and polishing,the inclusion grade and percentage content of the inclusion area were determined according to GB/T 10561-2005 and GB/T 18876.1-2002 for longitudinal specimens.The microstructures of the samples were displayed by 10% oxalic-acid electrolysis,and were then observed and photographed using an optical microscope.The austenite grains were displayed by electrolysis in a 60% nitric acid aqueous solution.The grain sizes of the samples were measured using the three-circle cut-off point method described in GB/T 6394-2017 and then examined using an inverted metallographic microscope OLYMPUS GX71.
2.2.2 Scanning electron microscopy analysis
The composition of the sulfide inclusions in the test steel was qualitatively analyzed using an FEI Quanta 600 FEG scanning electron microscope and an Oxford energy spectrometer.
2.2.3 Corrosion test
In the corrosion test,a 0.16%HCl+6%FeCl3solu-tion was used and the test temperature was (35±1)℃.According to the method described in GB/T 17897-2016,the corrosion weight loss rate was measured after continuous immersion for 24 h.The electro-chemical test solution was 3.5% NaCl,the tempera-ture was 30 ℃,and the scanning rate was 20 mV/min,as per GB/T 17899-1999.The test equipment was a VMP3 multi-channel potentiostat produced by the Princeton company.
3 Results and discussion
3.1 Analysis of inclusions
According to the method described in GB/T 10561-2005,nonmetallic inclusions were graded using the method A (worst view method),the results are shown in Table 2.
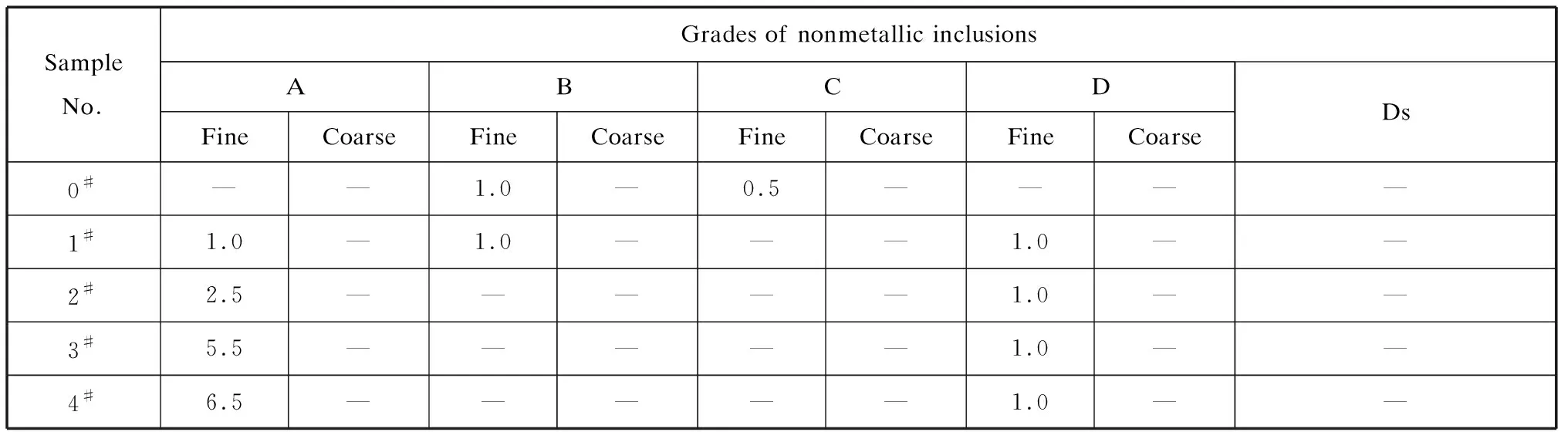
Table 2 Nonmetallic inclusion ratings of the test materials
The inclusions in sample 0#included few B(alumina) and C types(silicate),and fewer A types(sulfide).There were a small number of B-type and D-type(spherical oxide) inclusions in addition to A type in sample 1#.Samples 2#- 4#had A-type inclusions and very few D-type inclusions.With increases in the sulfur content,the grade of the sulfide in the steel gradually increased,and the sulfide distribution increased in density.Fig.1 shows the morphology of the sulfides in the samples.
In Fig.1,it can be seen that some sulfide inclusions in the samples are long strips and some are spindles.There were more long-strip sulfides than spindles in samples 1#-3#.In sample 4#,however,the length-width ratio of the sulfide inclusions decreased,with more spindle sulfide inclusions than long-strip inclusions.The results of scanning electron microscopy (SEM) and energy dispersive spectrometry (EDS) revealed that the sulfides were mainly MnS (Fig.2).
To further analyze the effect of sulfur content on the inclusions in the test samples,the area percen-tages of the inclusions were measured with an image analyzer.The results showed that the inclusions in samples 0#- 4#accounted for 0.13%,0.33%,0.51%,0.89%,and 2.69%,respectively.Thus,with increases in the sulfur content,the inclusion content gradually increased.When the sulfur content exceeded 0.1%,the sulfide inclusions in the sample increased sharply,as shown in Fig.3.
3.2 Microstructure and grain size
The microstructures of the all test materials are austenitic.There are a few deformation bands in sample 0#and some short black rods or long-strip sub-stances in samples 1#- 4#.With increases in the sul-fur content,the amount of these substances increased.The black strips show the sulfide morphology in steel after electrolysis.Fig.4 shows the metallo-graphic morphology of the test materials.
To reveal the grain sizes of the samples and reduce the appearance of twins,60% nitric acid electrolysis was used,for which the voltage was 2 V and the electrolysis time was 15 s.The grades of the grain size for samples 0#-4#were 7.4,6.5,7.3,7.4,and 8.9,respectively.The reason for the finer grain size of sample 4#is the greater number of spindle-shaped sulfide inclusions.Research has shown that spindle-shaped sulfide inclusions play an important role in grain refinement[3].Fig.5 shows the grain morphology of the samples.
3.3 Evaluation of pitting corrosion resistance of steels
3.3.1 Pitting corrosion immersion results
The pitting corrosion resistance was evaluated by an accelerated corrosion test with ferric chloride.After soaking,the corrosion products were removed using a brush and ultrasonic wave.After drying,the macroscopic corrosion morphology of the steel was examined.Fig.6 shows the corrosion morphology of the surfaces and sides of the samples with different S contents after the immersion test.
In Fig.6,no large corrosion pits can be seen on the surface of the samples after immersion,although there are pagoda-like corrosion pits on the surfaces of samples 0#and 2#.Unlike samples 2#- 4#,there are no obvious fine pits on the surfaces of the samples 0#and 1#.From the side profiles of the samples,it is evident that the corrosion severity increased with increases in the sulfur content.
Some studies[4-5]have shown that nonmetallic inclu-sions in steel were the main source of pitting cor-rosion.Sulfide inclusions induce pitting corrosion ear-lier than other inclusions,and the pitting corrosion is more severe.Manganese sulfide inclusions are good conductors of electricity and their electrode potential is much more positive than that of the steel matrix.As such,micro-battery corrosion readily occurs between the long-strip manganese sulfide and the steel matrix.The corrosion begins in the matrix around the strip manganese sulfide.Deep corrosion grooves then form on both sides of the manganese sulfide inclusion and the inclusion itself dissolves to a significant degree.In this study,the number of manganese sulfide inclusions increased with increases in the sulfur content,which increased the corrosion rate.The results are shown in Fig.7.
In Fig.7,it can be seen that with increases in the sulfur content,the corrosion rate of the test materials gradually increased.When the sulfur content reached 0.1%,the corrosion rate increased sharply,which is consistent with the content of sulfide inclusions in the samples.
3.3.2 Measurement results of pitting potential
The anodic polarization curves were plotted from the results of the electrochemical experiments,and the pitting potentialsEbof the materials were determined by the corresponding current densities of 100 μA/cm2in the polarization curves.Fig.8 shows the anodic polarization curves of the test materials in 3.5%NaCl (30 ℃).It can be seen that the polarization curves of all five samples have wide passivation zones.The difference between them is that the polarization curves of sample 0#in-creases linearly when the breakdown potential is reached,whereas the polarization curves of samples 1#- 4#fold upward prior to reaching the breakdown potentials,which is likely related to the large amount of sulfide inclusions in samples 1#-4#.
Fig.9 shows the changes in the pitting potentials and grain sizes of the steel samples in the test.With increases in the sulfur content,the pitting potentials of the samples decreased gradually,but that of sample 4#recovered somewhat,which may be related to its small grain size.
The impact on the pitting corrosion resistance of austenitic stainless steel with respect to grain size is rarely reported.Some researchers have studied the relationship between the grain size of 304 austenitic stainless steel and its corrosion resistance,and proposed that the corrosion resistance of a material increased with decreases in the grain size[6].Based on test results obtained using the electrochemical method,Zheng et al.also indicated that grain refinement improved the tightness and stability of the surface passivation membrane of 304 stainless steel[7]and increased the corrosion resistance.Toppo et al.studied the pitting behavior in a solid solution state and with surface thermo-mechanical treatment on the surface of 304 stainless steel[8].The results showed that the pitting resistance of 304 stainless steel after surface heating and shot blast treatment was significantly improved,due to the grain refinement after surface shot blast,and the stability of the surface passive film was also improved.
The results of the pitting corrosion resistance test of the steel samples differed,as determined by an immersion corrosion test and electrochemical test,mainly in the evaluation of sample 4#.This is because the pitting corrosion behaviors of the materials were macroscopically characterized via an immersion corrosion test and the results were greatly influenced by number of inclusions in the materials,the test temperature,and the surface roughness.Corrosion dissolution of stainless steel occurs preferentially at inclusions,which accelerates the rate of pitting corrosion.There were many spindle manganese sulfide inclusions in sample 4#,which means there were more pitting nucleation sites,which led to a higher weight loss rate in the immersion corrosion test.The pitting corrosion potential obtained in electrochemical tests indicates the stability of the passive film on a material surface.The higher the pitting corrosion potential,the greater the stability of the passive film and the pitting corrosion resistance.
4 Conclusions
(1) When added into 316L stainless steel,sulfur exists mainly in the form of manganese sulfide inclusions.With the increases in the sulfur content,the grades and contents of sulfide gradually increase,and the distribution of sulfide becomes increasingly concentrated.When the sulfur content exceeds 0.1%,the number of sulfide inclusions increases sharply.
(2) The addition of sulfur has an impact on the grain size of 316L stainless steel.When the sulfur content reaches 0.199%,the sulfur compounds are mostly spindle-shaped,and a large number of spindle-shaped sulfur compounds facilitate grain refinement.
(3) The sulfur content has a significant impact on the pitting resistance of 316L stainless steel.With increases in the sulfur content,the point corrosion weight loss rate of steel increases,and the pitting potential gradually decreases.However,when the sulfur content reaches 0.199%,the pitting potential of 316L stainless steel increases somewhat due to grain refinement,which improves the pitting resistance.Further research is needed to determine whether grain refinement is the main reason for the increased pitting potential of sample 4#.
杂志排行
Baosteel Technical Research的其它文章
- Mechanical and corrosion properties of N08825 CRA clad pipeline steel
- Research on material design and high-temperature friction and wear properties of new graphitic steel
- Bottom dross defect and its transformation behavior during the galvannealing process
- Theoretical study on the torque values of premium connections
- Causes of the formation of pit defects on the surface of the enamel layer
