爆炸冲击合成纳米碳化钛的研究
2020-10-10于雁武贾康辉魏子辉茹锐锋
于雁武,贾康辉,魏子辉,茹锐锋
(中北大学环境与安全工程学院,山西 太原 030051)
Titanium carbide (TiC) is a typical transition metal carbide characterized by its high elastic modulus, low coefficient of thermal expansion, remarkable high-temperature strength, and superior resistance to abrasion and oxidation[1]. It is widely used in structural materials for cutting tools, abrasion resistance coatings, mechanical parts and other fields. In addition, transparent ceramics TiC can also be used as excellent optical materials. TiC has excellent thermal shock resistance, which is often used as a special refractory in reductive or neutral atmospheres. TiC based cermets possess high hardness, high strength, oxidation resistance, high temperature resistance and chemical stability, as well as good toughness[2]. As an excellent material, TiC has attracted wide attention all over the world and many researchers have used different methods to synthesize it. These methods mainly include carbothermal reduction method[3-5], direct synthesis method[6-7], sol-gel method[8], gas phase method[9-10], mechanical alloy[11], etc. In view of the fact that the research has not been carried out at home and abroad on the synthesis of titanium carbide by precursors directly driven by detonation shock of explosives, and it’s only reported that the synthesis of titanium carbide may require a high temperature and high pressure condition. So the detonation shock method is used to synthesize TiC in this paper.
The detonation shock synthesis is a new field of science and technology rising in recent years, and the instantaneous high temperature and high pressure condition provided by detonation shock process can make the properties of materials change complicatedly. At present, the detonation shock method has been applied to powder processing, synthesis of super-hard materials, sinter and weld of new materials, and other fields. In this paper,nanometer TiC powder was synthesized by detonation shock wave. The structure, morphological characterization and elemental composition of the as-prepared samples were analyzed by X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive spectrometer (EDS), respectively.
1 Experimental
The schematic diagram of the experiment in a closed detonation reactor designed by our team is shown in Fig.1. HMX was selected as the pressure and temperature source. Detonation shock wave was adjusted by polymethylmethacrylate (PMMA, density 1.18 g/cm3). The precursors are TiO2and activated carbon. The HMX and the precursors were respectively pressed into a cylinder with a diameter of 10 mm and a height of 5 mm. The density of HMX cylinder and the precursor cylinder are 1.8 and 1.5 g/cm3, respectively. By the end of detonation shock wave, black powder products were obtained, accompanied by the release of ammonia gas. The black powder turned brown after being soaked in aqua regia for 24 hours, then it was calcined in muffle furnace for 400 min at 400 ℃. Ultimately, the light grey powder was obtained.
Fig.2 shows the SEM diagram of the raw material. In the Fig.2(a), the activated carbon has a large layer and a flat surface. It has a obvious delamination and the edge is defective but not very large. Fig.2(b) shows that the particle size of titanium dioxide is very small.

Fig.2 SEM images of raw material
In order to improve the pressing formability of HMX cylinder, the water suspension granulation method was used to coat HMX with fluoro rubber 2 602 whose mass fraction is 3% as a binder. The shock pressure required for the experiment is theoretically calculated according to the attenuation model of detonation shock wave[12],which considers that the detonation shock wave decays exponentially during the propagation in the medium, and the attenuation relationship can be expressed by the following formula

where p is the output pressure after attenuation, k is the correction factor, which is related to the density of HMX,a is the parameter of organic glass material, and x is the thickness of PMMA plate.
The XRD instrument used in the experiment is the D/MAX-rBX ray diffractometer by Japanese Science. The test was processed by using the standard θ−2θ scanning method. The copper target Kα1 radiation (λ=1.540 56×10−10m) was projected onto the powder. For each measurement, the machine was operated at 80 keV with a current of 100 mA, the scanning range is 20°−100° and the scanning rate is 4 (°)/min. The SEM instrument used in the experiment was Tecnai G2 F20 S-TWIN type of FEI Company of the United States.
2 Result and conclusion
2.1 Analysis of structure
Fig.3 is the XRD curve of the sample produced by the detonation shock wave in the pressure of 25 GPa. The mole ratio of the reaction precursor TiO2and activated carbon is 1∶6. There appeared 13 distinct peaks among which the Numbers 3, 4, 8 and 12 correspond to the crystal surface (111), (200),(220) and (400), respectively. The Numbers 5, 9, 10 are the peaks of TiCx(x<1), and the Numbers 1, 2,6, 7, 11 and 13 are the ones of TiO2.
It is seen in Fig.3 that under the instantaneous high temperature and high pressure provided by detonation shock wave, C in activated carbon was used as a reducing agent to reduce TiO2to obtain crystalline TiC and TiCx(x<1), and a few unreacted TiO2were found in the samples. Sen[13]used thermodynamic methods to determine the possibility of using carbothermic method to obtain TiC by calculating the relationship between the reaction enthalpy and temperature of C and TiO2, and the relationship between the Gibbs free energy of the reaction and the temperature and pressure. In addition, according to the C-Ti binary equilibrium phase diagram, it is known that TiC is non-stoichiometric, and the range of the atomic ratio of C to Ti is from 0.49 to 1.0 with the continuous change of reaction conditions, which explains the existence of TiCx(x<1) in the sample. In Fig.3, the TiC characteristic peak deviates from the standard value,which is mainly due to the formation of lattice defects caused by the instantaneous high temperature and high pressure. Besides, the molar ratio of C and TiO2also has some effect on the deviation of the characteristic peaks,which is consistent with the references[13-14]. There is no peak of C in its elemental form due to the oxidation of partial C into CO gas. The remaining activated carbon is soaked in aqua regia and calcined at high temperature for 31 hours during the purification process, and is released in the form of gaseous CO2. TiO2is not completely removed in the purification process because of its stable physical and chemical properties and remains in the sample ultimately.
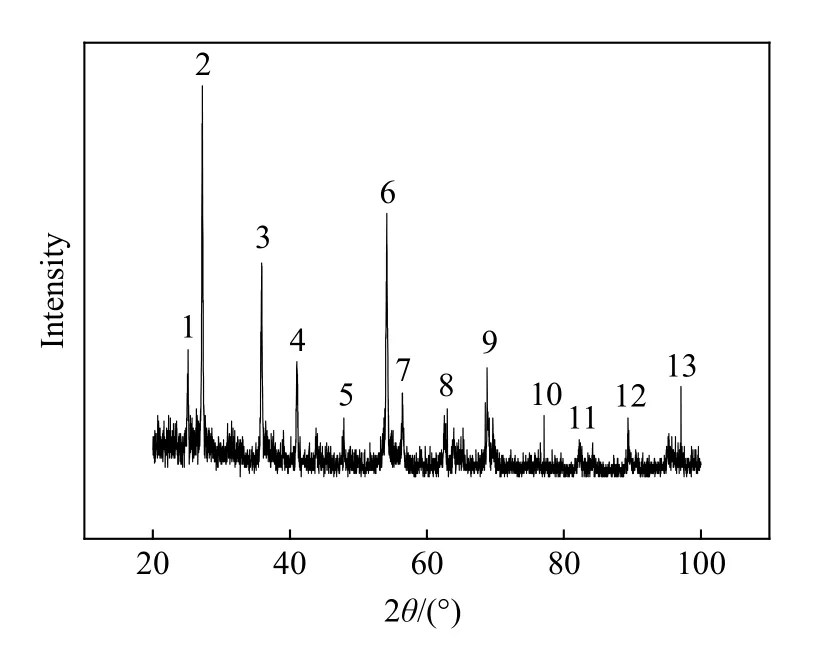
Fig.3 XRD patterns of the prepared titanium carbide
2.2 Characterization of sample
Fig.4 is the SEM images of the samples. In Fig.4(a), there are particles distributed evenly with a size less than 50 nm. It shows that a small number of micron-sized spherical aggregates are attached to the nano-particles and band-shaped substances in Fig.4(b). The band-shaped substances should be residual of anatase TiO2according to the reference [15].
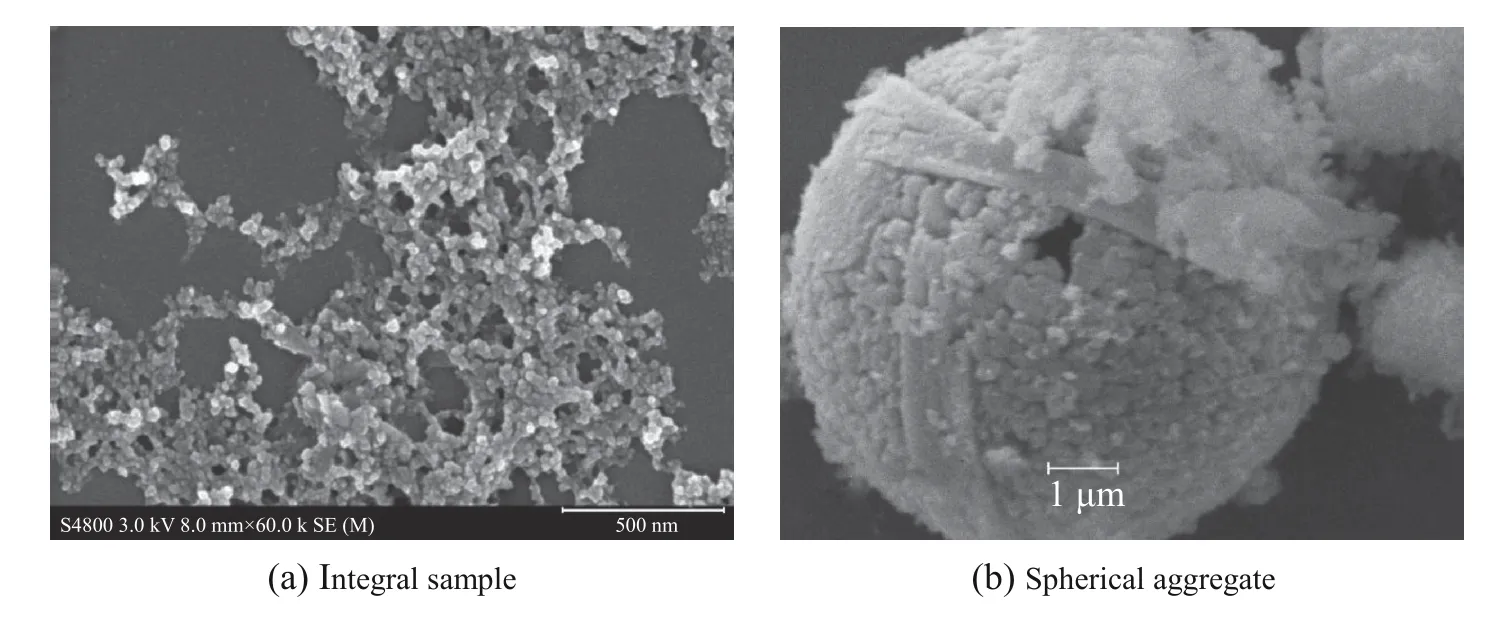
Fig.4 SEM images of the samples
Table 1 and Table 2 show the results of the EDS spectrum of the samples. It is showed that four elements of Si, O, Al and Fe appears, besides C and Ti. According to the XRD results, TiC, TiCx(x<1) and TiO2were found in the samples. The element Si is derived from the organic glass filler, the sheath of detonator’s lead and the adhesive tape during the experiment. Under the instantaneous high temperature and high pressure of the detonation shock, element Si is oxidized rapidly to amorphous SiO2, which is consistent with the reference[16]. A small amount of metallic elements in the sample mainly came from the detonator and inner debris of reactor vessel.These metallic elements produced stable metallic silicates in the sample during the experiment. It can be concluded from Table 2 that the composition of spherical aggregates is complex, which may be due to the co-agglomeration of TiC, TiCx(x<1), amorphous SiO2, TiO2and metallic silicates,and the mechanism of agglomeration needs to be explored further.
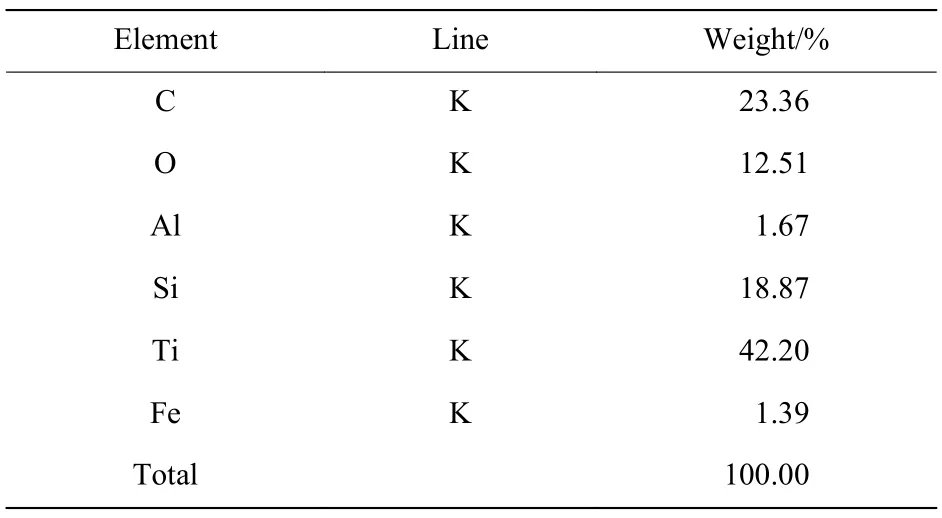
Table 1 EDS spectrum of integral sample
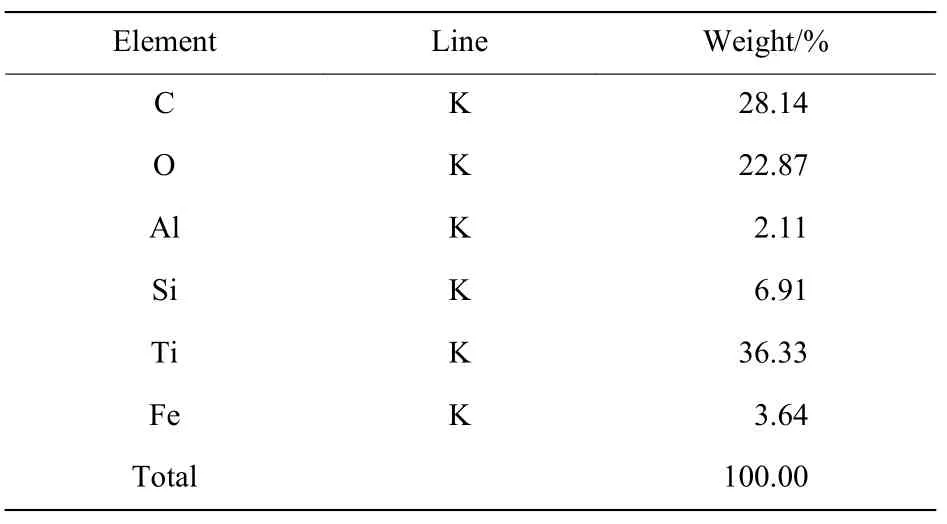
Table 2 EDS spectrum of Spherical aggregate
2.3 Analysis of mechanism
In the traditional carbothermic method to synthesis titanium carbide, the order of forming product is Ti4O7,Ti3O5, Ti2O3, TiO, TiC, and Ti with the increasing temperature[13]. The reaction of TiO to TiC has the lowest Gibbs free energy in the six reactions above, which indicates that TiC can be generated at a higher reaction temperature.Theoretical calculation shows that the reaction temperature of TiO to TiC is 1 271 ℃ at normal atmospheric pressure, and the heat required for the reaction is above 500 kJ/mol. So when the TiC powders are synthesized by detonation shock, the reaction mechanism is different because of the characteristics of high temperature, high pressure and short reaction time. In this paper, the detonation parameters of HMX possess the pressure of 30 GPa, the temperature of 3 800 K, the heat of 1 697 kJ/mol, and the action time of detonation shock wave is only a few microseconds. Combining with analysis of the XRD patterns, the synthesis process of TiC powder under detonation shock wave was discussed preliminarily. When the shock wave acts on the precursors, the products of titanium dioxide by carbothermic method does not undergo the process of Ti4O7,Ti3O5, Ti2O3, but directly to TiC and CO.This is the reason why no other forms of titanium oxides are observed in Fig.2 except the residual titanium dioxide. Since the properties of titanium carbide and titanium dioxide are similar, the purification method is the focus of work in the future.
The solid state chemical reactions undergo diffusion, formation of new crystalline phases and grain growth.First, a product layer is formed on the surface of the reactant. The subsequent reaction depends on the diffusion of the reactant in the product layer and the rate of chemical reaction of the reactants. For solid state reactions, the diffusion rate is usually very slow. So in most cases, the diffusion of particles plays a controlling role. However, the time of detonation shock wave is very short and the diffusion degree of their mutual reaction can only reach 0.1 nm when the powder is synthesized at the diffusion rate of 10−8cm2/s[17]. It is inferred that nano-TiC powders can hardly be obtained in this processing, which was not consistent to the results confirming the existence of nano-TiC. So it is clear that the reaction by shock wave follows the solid state reaction, but it is quite different from the solid state reaction under normal conditions, for it has the characteristics of faster diffusion process and shorter reaction time. According to the detonation shock wave theory, a large number of dislocations produced by shock wave in materials will cause the decrease of diffusion activation energy. In addition, the plastic flow and deformation of materials will lead to the formation of slip bands and vortices at the interface, so that the atoms diffuse following the dislocations, twinning, and slip bands, which makes diffusion coefficients of solid materials increase significantly under the action of shock waves.
It was showed that the powder synthesized produce a lot of defects and lattice distortion by detonation shock waves[18]. Theoretically, the density of lattice distortion can reach 1012to 1013cm-2by the detonation shock waves.These defects can reduce the Gibbs free energy of nucleation and accelerate the formation and growth of the nucleation. Moreover, CO gas is released, and the heat transfer rate of gas is higher than that of solid material, which improves the mixing of reactants and promotes the powder synthesized to a certain extent.
3 Conclusion
The nano-TiC and TiCx(x<1) with the particle size of less than 50 nm were synthesized by detonation shock method. It is believed that although the mechanism of synthesizing nano-TiC by detonation shock wave follows the solid state reaction, it is quite different from the traditional solid state reaction because of the high temperature,high pressure and short action time of detonation shock wave. Compared to the traditional carbothermic method,the reaction by detonation shock wave of nano-TiC does not go through the order of Ti4O7, Ti3O5, Ti2O3, but directly to TiC and CO. The effect of detonation shock wave leads to dislocation, slip band, vortex, defect and lattice distortion in the precursors, and CO gas was produced in the reaction process. These promotes the synthesis of nanometer TiC to a certain extent.
